Abstract
Cognitive reserve (CR) refers to the hypothesized capacity of an adult brain to cope with brain damage in order to minimize symptomatology. The present review is focused on the contribution of brain PET in the understanding of the influence of CR on the disassociation between cognition and degree of Alzheimer’s disease (AD) pathology. Theories for the explanation CR-related mechanism as well as PET imaging evidence for the existence of CR are described. Moreover functional imaging studies investigating specific networks for CR both in healthy subjects and AD patients are discussed. Finally implications for amyloid PET imaging are presented.
Keywords: Brain FDG PET, Alzheimer’s disease, cognitive reserve, amyloid PET, mild cognitive impairment
Introduction
Several factors may influence the relationship between cerebral amount of Alzheimer disease (AD) pathology and the clinical expression of cognitive impairment and eventually dementia [1]. Among biological factors, high relevance has been recognized to age of onset of the disease and to apolipoprotein E (ApoE) genotype [2,3]. In fact in patients with early onset AD (EOAD), a greater brain damage is required to reach the same severity of cognitive impairment as patients with late onset dementia (LOAD) [2]. Similarly, the presence of at least one epsilon 4 allele of the ApoE gene, a recognized risk factor for LOAD, has been associated with a faster cognitive decline [4,5]. These two biological actors may interact within each other but, more importantly, the effect of both of them is further influenced by the more general concept of the underlying brain cognitive reserve. Brain cognitive reserve (CR) has been defined as the capacity of an adult brain to cope with brain damage in order to minimize symptomatology [6]. CR acts as an independent mediator of the relationship between brain damage and AD expression and it also seems to be a meaningful substrate for all the biological factors contributing to the interaction between AD pathology and its outcome. In fact the greater brain damage required in EOAD than in LOAD patients to reach clinical expression of dementia, could reflect a greater functional reserve in younger than in older subjects [2]. Similarly, although a reserve mechanism has been demonstrated also in ApoE epsilon 4 carriers a trend toward a stronger reserve phenomenon has been proven in noncarriers [4]. First evidence for the existence of CR dates back to the late 80s and early 90s when it was demonstrated that at least 20% of elderly people who are cognitively normal before death have sufficient postmortem pathology to meet neuropathological criteria for AD [7]. Following studies have focused on the understanding mechanisms (related to CR) that accounts for the disjunction between the degree of AD brain damage and the clinical expression of the disease (i.e., the phenotype). The first model proposed for the interpretation of cognitive reserve phenomenon was the passive model where reserve was defined in terms of the amount of damage that can be sustained before reaching a threshold for clinical expression [8]. In this type of model anatomic measures such as brain size, head circumference or synaptic count have been initially used as effective measures for reserve [9]. However contemporary epidemiologic studies demonstrated that all these anatomic measures are influenced by demographic factors such as socioeconomic status, educational level and occupational complexity. In particular higher prevalence of AD was observed among elder populations with low levels of education [10]. Therefore, also due to its easy measurability, educational attainment has been, since then, the most straightforward used brain CR proxy. Accordingly, to fulfill CR definition, education (or other epidemiological factors) has been used as proxies for CR and neuropsychological tests have been used to measure the actual cognitive performance of a certain subject.
However, this epidemiological/neuropsychological approach lacks of anatomic localization and yields only indirect evidence about brain functional networking. Due to these limitations many groups have thus turned to neuroimaging approach (able to assess the ‘endophenotype’) for a more comprehensive understanding of CR.
PET imaging evidence for the existence of CR
The investigation of CR by means neuroimaging tools has two main advantages: 1. It can develop a proxy measure for pathology in vivo. 2. It allows a more precise anatomo-functional correlate of CR phenomenon. Several PET perfusion studies used resting regional cerebral blood flow (rCBF) measurements as a surrogate for AD pathology and proved that parietotemporal and occipital cortex perfusion has inverse correlation with education and life-time activities score in AD patients [11]. Similarly, structural studies with 3D magnetic resonance imaging (MRI) demonstrated that on one side education increases regional cortical thickness in healthy controls, on the other side years of schooling are inversely correlated with regional cortical thicknesses in temporoparietal and occipital regions in AD patients [12]. Accordingly, imaging approach supports the idea that even when pathology is more advanced in AD patients with higher education, the clinical manifestations of the disease may be comparable to those in patients with lower education and less pathology. Certainly it can be also hypothesized that a similar reserve mechanism exists in patients with amnestic mild cognitive impairment (aMCI). Garibotto and colleagues assess the impact of education and occupation on brain glucose metabolism measured with 18F-fluorodeoxyglucose (FDG) PET in aMCI and in probable AD (pAD) patients [13]. The analysis showed that both in pAD patients as well as in aMCI patients later converting to AD-dementia (so-called aMCI ‘converters’), higher reserve proxies (education or occupation) correlate with lower brain metabolism again in the posterior temporo-parietal associative areas. Accordingly, this study proved that CR-mediated mechanisms are already at work in the predementia stage of AD. This aspect is well evident on FDG PET scans also at a single patient level thus being of interest for daily reporting practice. Figure 1 shows a representative example of two patients with aMCI due to AD with similar cognitive impairment. They have the same age and ApoE genotype but different educational level and occupational attainment. In both cases the typical temporo-parietal pattern of hypometabolism is well evident, but hypometabolism is much more severe and extended in the highly educated subject.
Figure 1.
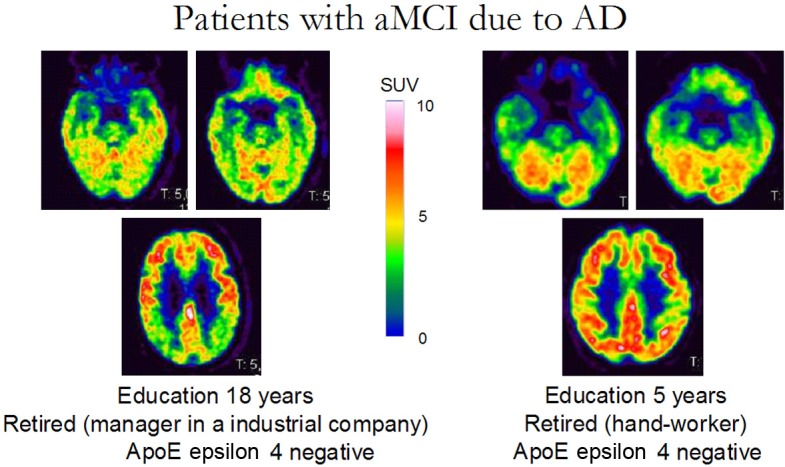
Representative example of two patients with aMCI due to AD with a similar degree of cognitive impairment (neuropsychological assessment normal except for a mild impairment in verbal memory; RAVLT delayed recall 5/15). These two patients have the same age and ApoE genotype but different educational level and occupational attainment. In both cases the typical temporo-parietal pattern of hypometabolism is already evident, but hypometabolism is much more severe and extended in the highly educated subject (left panel). aMCI, amnesic Mild Cognitive Impairment; AD Alzheimer’s Disease; MMSE, Mini-mental state examination; RAVLT, Rey Auditory Verbal Learning Test; ApoE, Apolipoprotein E.
Mechanisms underlying CR: functional neuroimaging and the active model of CR
Epidemiologic and neuroimaging data provide evidence for the existence of CR. However, these approaches cannot provide yet clues about the neural mechanisms that may mediate CR. This further issue is related to the “Active” Model of CR. To investigate mechanisms that may mediate CR, many groups historically turned to cognitive activation studies using H2O [15] PET or functional MRI (fMRI). In both cases the aim was to highlight brain activation mediated by CR phenomenon. Springer and colleagues recorded fMRI during a memory tasks in young and healthy older adults and demonstrated that while in young adults, education was negatively correlated with prefrontal cortex activity, in older adults education was positively correlated with prefrontal activity [14]. Accordingly, authors hypothesized that prefrontal cortex is engaged by older adults, particularly by the highly educated ones, as an alternative network that may be engaged to aid cognitive function. Similarly, by means of perfusion PET, difference in CR-mediated brain activation was tested during a nonverbal episodic memory task in AD subjects and elderly controls. Brain regions whose regression slopes differed between the 2 groups were searched for. The working hypothesis was that brain regions where systematic relationships between subjects’ education and brain activation differ as a function of disease status may mediate the differential ability to delay the clinical manifestations of AD [15]. The analysis showed that indeed the slopes were significantly more positive for the AD patients, specifically in the left precentral gyrus and more negative in the right fusiform and left middle temporal gyri and in occipital cortex.
By using a different approach but again in the search of CR-related mechanisms, a FDG PET multicentre study of the European Consortium of Alzheimer’s Disease (EADC) was carried out to compare brain metabolism of two age-matched groups of highly and poorly educated prodromal AD patients [16]. Education cut-off was the median of schoolings years of the whole group (in this case 12 years) and it was preliminary proved that the two groups of pAD were expressing the same level of cognitive symptoms as measured by means of an extensive neuropsychological tests battery. Both direct and with controls comparison of the two groups allowed confirming the more pronounced AD-typical posterior damage in highly educated group. However, and more importantly, the direct comparison between patients was able to highlight that an area of relatively higher metabolic levels was indeed present also in highly educated group. This cortical region substantially corresponded to the right dorsolateral prefrontal cortex (DLFC) (see Figure 2). Besides being consistent with previous activation studies, the cortical topography of this result is interestingly in keeping with other PET-derived more clinically linked evidence. In fact it has been highlighted an emerging role of the DLFC in the discrimination between those MCI patients rapidly converting to dementia of Alzheimer type and those remaining stable [17]. Similarly by means of SPECT it has been demonstrated that right DLFC perfusion is negatively correlated with hippocampal and parahippocampal MR-based atrophy in pAD patients. The paradoxical negative correlation between medial temporal lobe (MTL) volumes and prefrontal perfusion may result from recruitment of an alternative anterior temporofrontal network. Therefore this evidence suggests again a functional compensative role of DLFC in response to the lesional impairment of the MTL [18].
Figure 2.
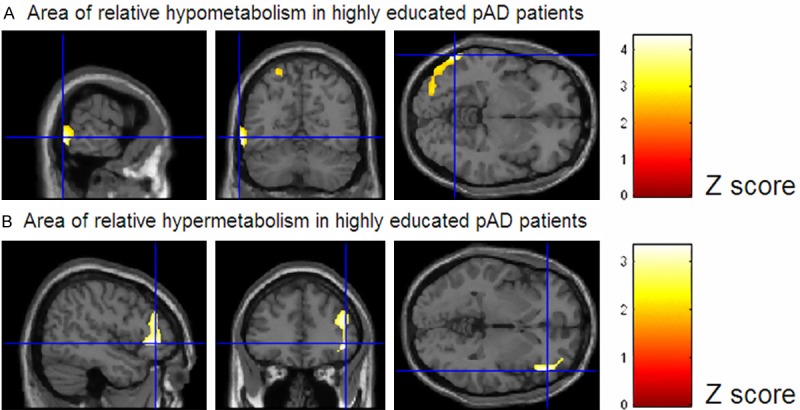
Statistical parametric mapping (SPM8) comparison between poorly (n=36) and highly (n=28) educated prodromal AD patients. Height thresholds: uncorrected p<0.001 at peak level, p<0.05 FDR-corrected at cluster level. Figure displays regions of significant difference, colour-graded in terms of Z values. Highly educated subject show the expected more pronounced AD-typical posterior damage (A); however they also show an area of relatively higher metabolic levels with respect to poorly educated AD patients (B). This cortical region substantially corresponds to the right dorsolateral prefrontal cortex. pAD, prodromal Alzheimer’s Disease patients. Adapted from reference [16]. (© by the Society of Nuclear Medicine and Molecular Imaging, Inc.).
Noteworthy, PET technology further allows to evaluate compensative mechanisms in highly educated AD patients also at receptor level. In fact by means of a voxelwise non-parametric permutation method it has been specifically evaluated the relationship between cholinergic neurotransmission (by measurement of the acetylcholinesterase enzyme, AChE) and CR proxies both in controls and AD patients [19]. This approach showed that only in the AD group significant correlation between AChE activity and CR proxies was present in structures belonging to the memory network (namely bilateral hippocampi and right posterior cingulate gyrus) while no correlation was highlighted in the healthy control group. According to this study, it can be concluded that the brain reserve in AD is associated with a preserved/stimulated cholinergic neurotransmission. Noteworthy, this evidence supports the “active model” of CR, as reserve proxies seem to specifically protect the system from decline in the damaged brain of AD patients.
A specific functional network for CR: from activation studies to PET-based metabolic connectivity
Taken all together the above-mentioned evidence suggests that compensation mechanisms are involved in CR and a specific relationship can be identified between cognitive performance and CR proxies during cognitive tasks in highly educated AD patients. However even the described activation approaches do not fully address the alternative concept of how CR is, more in general, functionally mediated. In fact, one can hypothesize that a specific CR network can work in highly educated AD patients. This view is supported by the fact that, despite the presence of brain pathology, CR helps maintain effective function across a wide range of activities [8]. Accordingly, to address this issue a different strategy undertaken by Yaakov Stern’s group was to identify a single network showing increased load-related activation as a function of CR across two tasks with different cognitive processing demands [20]. By means of fMRI they were thus able to demonstrate that a network including bilateral superior and medial frontal gyri and left middle frontal gyrus was actually common to the two administered tasks. This has been a step forward in the understanding of CR; however this approach can still be related to a limited number of tasks. By contrast, among all the functional neuroimaging techniques, FDG-PET has the unique ability to estimate the local cerebral metabolic rate of glucose consumption (CMRgl). As CMRgl maximally occurs at synaptic levels [21], FDG-PET is indeed able to provide general information on the distribution of synapse function/ dysfunction in vivo. Accordingly, analysis of FDG brain PET studies acquired in resting state might be suitable to test the question on how CR is supported at network level (‘resting-state’ ‘FDG-PET’).
In fact, by calculating correlation coefficients-or pattern of intercorrelations-between regional values of FDG uptake, it is possible to estimate the functional association between cerebral areas.
This approach was first used in the 80s by Horwitz and colleagues [22] but was recently refined and validated in the context of statistical parametric mapping by Lee et al [23]. Briefly according to this method PET images are spatially normalized within the standard Montréal neurological institute (MNI) space, and then FDG mean counts are extracted and normalized to a reference region. Finally, for inter-regional correlation analysis extracted mean regional volumetric regions of interest (VOI) counts are used as a covariate to find regions showing significant voxel-wise correlations across subjects, thus providing parametric maps of metabolic connectivity [16,23,24] (Figure 3). Therefore, taking advantage from FDG PET technology and by means of this analytical method, it has been demonstrated that only in highly educated pAD patients DLFC is involved in distributed correlations with several cortical areas in both hemispheres (frontal, temporal, occipital cortex, parahippocampal gyrus and precuneus) as well as in cerebellar hemispheres while it is substantially just autocorrelated in poorly educated AD. Accordingly, this metabolic connectivity analysis suggests explanation on how highly educated pAD patients can cope better with their more severe posterior damage (Figure 4). However, it did not completely elucidate whether these metabolic networks are due to preserved functional connections that are physiologically present in more educated subjects (i.e., the brain reserve component of cognitive reserve) or to the recruitment of alternative neural networks able to support cognitive function just in the presence of disease related damage elsewhere (i.e., the brain compensation component of CR).
Figure 3.

Schematic representation of the steps required to perform a “metabolic connectivity” analysis. (A) PET images are first spatially normalized by means of a PET template and smoothed, then (B) FDG mean counts corresponding to seed volumetric regions of interest (VOI) are extracted and normalized to a reference region (i.e. cerebellar counts). Finally (C) for interregional correlation analysis, extracted mean regional VOI counts are used as covariate to find regions showing significant voxel-wise correlations across subjects thus providing parametric maps of metabolic connectivity.
Figure 4.
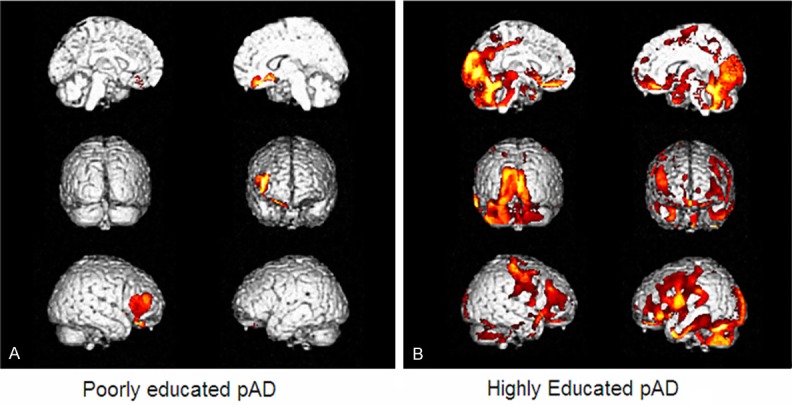
Inter-regional metabolic correlation of right dorso-lateral prefrontal cortex (DLFC) in two groups of poorly (A) and highly (B) educated prodromal AD (pAD) patients. In highly educated pAD patients DLFC is involved in distributed correlations with several cortical areas in both hemispheres (frontal, temporal, occipital cortex, parahippocampal gyrus and precuneus) while it is substantially just autocorrelated in poorly educated AD. Adapted from reference [16]. (© by the Society of Nuclear Medicine and Molecular Imaging, Inc.).
Neural reserve, neural compensation or both?
This issue was addressed using the same inter-regional correlation analysis (IRCA) approach but by repeating the analysis of DLCF in two age-matched groups of healthy controls, with high and low level of education, respectively [16]. The analysis allowed demonstrating that the DLFC-related wide metabolic network highlighted in highly educated pAD was indeed present- and topographically similar- in highly educated controls as well, but it was quantitatively less pronounced with respect to highly educated pAD patients. Accordingly (and in agreement with Stern’s definition) the connectivity analysis showed that CR in pAD patients with high education is a result of both neural reserve (preexisting brain networks more efficient or less susceptible to disruption) and neural compensation (new, compensatory brain networks active after pathology has impacted those networks typically utilized for particular tasks). Similarly, the role of neural reserve in highly educated healthy subjects was also confirmed in the context of an elegant multi-modal imaging study carried out in thirty-six healthy elders with normal cognition and a negative Amyloid (18F-florbetapir) PET scan [25] (Figure 5). Seed connectivity analyses was performed and it was demonstrated that education was positively related to the functional connectivity between the anterior cingulate cortex on one side and hippocampus, inferior frontal lobe, posterior cingulate cortex and angular gyrus on the other side. Accordingly, reinforcement in the connectivity was proved and it can be interpreted as the proof of mechanisms underlying education-related reserve also in healthy elders.
Figure 5.
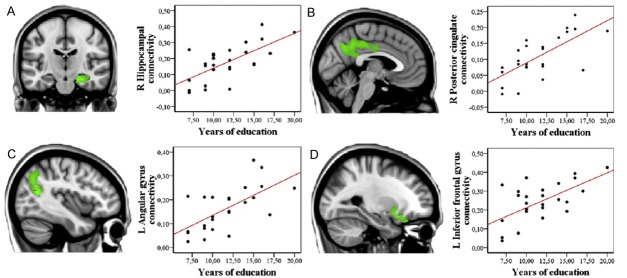
Sagittal/coronal views and scatter plots of the voxel-wise multiple regression between education and anterior cingulate functional connectivity (p uncorrected <.005). R: Right, L: Left. Seed connectivity analyses demonstrated that in healthy (Amyloid PET negative) elderly subjects education is positively related to the functional connectivity between the anterior cingulate cortex on one side and hippocampus (A), posterior cingulate cortex (B), angular gyrus (C) and inferior frontal lobe (D) on the other side. This analysis supports the presence of mechanisms underlying education-related reserve also in healthy elders. Adapted from reference [25].
Evidence and implications for amyloid PET imaging
Understanding the dis-association between cognition and pathology has historically been recognized as an important step for the success of preventive strategies targeted at neuropathology in the non-demented elderly as well as relevant for improving our understanding of AD. In the last years, development of new PET tracers has further underlined the critical and diagnostic relevance of CR.
In fact, it has been demonstrated that a percentage varying between 10% and 40% of apparently healthy, cognitively normal elderly people have a positive Amyloid PET scan [26]. This percentage is not surprising as it fully matches previously known neuropathological evidence that at least 20% of elderly people who are cognitively normal prior to death have sufficient post-mortem pathology to meet neuropathological criteria for AD [7]. Moreover groups analysis allowed to link the already known greater posterior hypometabolism present in highly educated pAD with a greater [11C] Pittsburgh compound B (PIB) uptake especially in the ventrolateral frontal cortex [27]. Although expected, this finding has important diagnostic and prognostic repercussions. To go deeply in this concept, Rowe and colleagues performed a logistic regression analysis to generate receiver operating characteristic curves with the aim of predicting accuracy of using amyloid imaging alone or together with other variables (including education) to distinguish between CTR and AD patients [5]. Curves analysis demonstrated that factors reported to influence associations between AD pathology and dementia (such as education and occupation) can improve the predictive accuracy of amyloid imaging for the identification of symptomatic AD.
Conclusion
In conclusion, highly educated AD patients have more severe hypometabolism than poorly educated patients in AD-typical posterior cortical regions. Multi-modal imaging approaches suggested that in these patients lateral frontal cortex is engaged within a specific network that counterbalance this greater posterior damage. This network corresponds to both pre-existing neural reserve but also to the recruitment of alternative compensatory neural networks. Accordingly predictive value of pathophysiological AD biomarkers cannot be resolved in the evaluation of the presence of AD pathology but can benefit from consideration of individual differences in demographic characteristics. As a final remark, CR construct seems to confirm that integration of amyloid pathology and synaptic dysfunction biomarkers (i.e. FDG PET) seems to be critical for a comprehensive understanding of the development and progression of the disease.
References
- 1.Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51(Suppl 1):S2–17. doi: 10.1212/wnl.51.1_suppl_1.s2. [DOI] [PubMed] [Google Scholar]
- 2.Kim EJ, Cho SS, Jeong Y, Park KC, Kang SJ, Kang E, Kim SE, Lee KH, Na DL. Glucose metabolism in early onset versus late onset Alzheimer’s disease: an SPM analysis of 120 patients. Brain. 2005;128:1790–801. doi: 10.1093/brain/awh539. [DOI] [PubMed] [Google Scholar]
- 3.Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, Salmon E, Baron JC, De Cristofaro MT, Padovani A, Borroni B, Franceschi M, Bracco L, Pupi A. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–40. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 4.Garibotto V, Borroni B, Sorbi S, Cappa SF, Padovani A, Perani D. Education and occupation provide reserve in both ApoE ε4 carrier and noncarrier patients with probable Alzheimer’s disease. Neurol Sci. 2012;33:1037–42. doi: 10.1007/s10072-011-0889-5. [DOI] [PubMed] [Google Scholar]
- 5.Roe CM, Mintun MA, Ghoshal N, Williams MM, Grant EA, Marcus DS, Morris JC. Alzheimer disease identification using amyloid imaging and reserve variables: proof of concept. Neurology. 2010;75:42–48. doi: 10.1212/WNL.0b013e3181e620f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 7.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–44. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 8.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perneczky R, Wagenpfeil S, Lunetta KL, Cupples LA, Green RC, Decarli C, Farrer LA, Kurz A MIRAGE Study Group. Head circumference, atrophy, and cognition: implications for brain reserve in Alzheimer disease. Neurology. 2010;75:137–42. doi: 10.1212/WNL.0b013e3181e7ca97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–10. [PubMed] [Google Scholar]
- 11.Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, Marder KS, Bell KL, Sackeim HA, Van Heertum RL, Moeller JR, Stern Y. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60:359–65. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Julkunen V, Paajanen T, Westman E, Wahlund LO, Aitken A, Sobow T, Mecocci P, Tsolaki M, Vellas B, Muehlboeck S, Spenger C, Lovestone S, Simmons A, Soininen H AddNeuroMed Consortium. Education increases reserve against Alzheimer’s disease--evidence from structural MRI analysis. Neuroradiology. 2012;54:929–38. doi: 10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, Sorbi S, Cappa SF, Padovani A, Fazio F, Perani D. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology. 2008;71:1342–1349. doi: 10.1212/01.wnl.0000327670.62378.c0. [DOI] [PubMed] [Google Scholar]
- 14.Springer MV, McIntosh AR, Winocur G, Grady CL. The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology. 2005;19:181–92. doi: 10.1037/0894-4105.19.2.181. [DOI] [PubMed] [Google Scholar]
- 15.Scarmeas N, Zarahn E, Anderson KE, Honig LS, Park A, Hilton J, Flynn J, Sackeim HA, Stern Y. Cognitive reserve-mediated modulation of positron emission tomographic activations during memory tasks in Alzheimer disease. Arch Neurol. 2004;61:73–8. doi: 10.1001/archneur.61.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morbelli S, Perneczky R, Drzezga A, Frisoni GB, Caroli A, van Berckel BN, Ossenkoppele R, Guedj E, Didic M, Brugnolo A, Naseri M, Sambuceti G, Pagani M, Nobili F. Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: a European Alzheimer disease consortium project. J Nucl Med. 2013;54:894–902. doi: 10.2967/jnumed.112.113928. [DOI] [PubMed] [Google Scholar]
- 17.Nobili F, Salmaso D, Morbelli S, Girtler N, Piccardo A, Brugnolo A, Dessi B, Larsson SA, Rodriguez G, Pagani M. Principal component analysis of FDG PET in amnestic MCI. Eur J Nucl Med Mol Imaging. 2008;35:2191–202. doi: 10.1007/s00259-008-0869-z. [DOI] [PubMed] [Google Scholar]
- 18.Guedj E, Barbeau EJ, Didic M, Felician O, de Laforte C, Ranjeva JP, Poncet M, Cozzone PJ, Mundler O, Ceccaldi M. Effects of medial temporal lobe degeneration on brain perfusion in amnestic MCI of AD type: deafferentation and functional compensation? Eur J Nucl Med Mol Imaging. 2009;36:1101–12. doi: 10.1007/s00259-009-1060-x. [DOI] [PubMed] [Google Scholar]
- 19.Garibotto V, Tettamanti M, Marcone A, Florea I, Panzacchi A, Moresco R, Virta JR, Rinne J, Cappa SF, Perani D. Cholinergic activity correlates with reserve proxies in Alzheimer’s disease. Neurobiol Aging. 2013;34:2694, e13–8. doi: 10.1016/j.neurobiolaging.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, Flynn J, Steffener J, Brown T. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18:959–67. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magistretti PJ, Pellerin L. Astrocytes Couple Synaptic Activity to Glucose Utilization in the Brain. News Physiol Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz B, Duara R, Rapoport SI. Intercorrelations of glucose metabolic rates between brain regions: application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab. 1984;4:484–99. doi: 10.1038/jcbfm.1984.73. [DOI] [PubMed] [Google Scholar]
- 23.Lee DS, Kang H, Kim H, Park H, Oh JS, Lee JS, Lee MC. Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging. 2008;35:1681–91. doi: 10.1007/s00259-008-0808-z. [DOI] [PubMed] [Google Scholar]
- 24.Morbelli S, Drzezga A, Perneczky R, Frisoni GB, Caroli A, van Berckel BN, Ossenkoppele R, Guedj E, Didic M, Brugnolo A, Sambuceti G, Pagani M, Salmon E, Nobili F. Resting metabolic connectivity in prodromal Alzheimer’s disease. A European Alzheimer Disease Consortium (EADC) project. Neurobiol Aging. 2012;33:2533–50. doi: 10.1016/j.neurobiolaging.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mézenge F, Perrotin A, Desgranges B, Bartrés-Faz D, Eustache F, Chételat G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage. 2013;83:450–7. doi: 10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 26.Rowe CC, Villemagne VL. Brain amyloid imaging. J Nucl Med. 2011;52:1733–40. doi: 10.2967/jnumed.110.076315. [DOI] [PubMed] [Google Scholar]
- 27.Kemppainen NM, Aalto S, Karrasch M, Någren K, Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63:112–8. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]


