Abstract
The metabotropic glutamate receptor type 1 (mGluR1) is a novel target protein for the development of new drugs against central nervous system disorders. Recently, we have developed 11C-labeled PET probes 11C-ITMM and 11C-ITDM, which demonstrate similar profiles, for imaging of mGluR1. In the present study, we compared 11C-ITMM and 11C-ITDM PET imaging and quantitative analysis in the monkey brain. Respective PET images showed similar distribution of uptake in the cerebellum, thalamus, and cingulate cortex. Slightly higher uptake was detected with 11C-ITDM than with 11C-ITMM. For the kinetic analysis using the two-tissue compartment model (2-TCM), the distribution volume (VT) in the cerebellum, an mGluR1-rich region in the brain, was 2.5 mL∙cm-3 for 11C-ITMM and 3.6 mL∙cm-3 for 11C-ITDM. By contrast, the VT in the pons, a region with negligible mGluR1 expression, was similarly low for both radiopharmaceuticals. Based on these results, we performed noninvasive PET quantitative analysis with general reference tissue models using the time-activity curve of the pons as a reference region. We confirmed the relationship and differences between the reference tissue models and 2-TCM using correlational scatter plots and Bland-Altman plots analyses. Although the scattergrams of both radiopharmaceuticals showed over- or underestimations of reference tissue model-based the binding potentials against 2-TCM, there were no significant differences between the two kinetic analysis models. In conclusion, we first demonstrated the potentials of 11C-ITMM and 11C-ITDM for noninvasive PET quantitative analysis using reference tissue models. In addition, our findings suggest that 11C-ITDM may be superior to 11C-ITMM as a PET probe for imaging of mGluR1, because regional VT values in PET with 11C-ITDM were higher than those of 11C-ITMM. Clinical studies of 11C-ITDM in humans will be necessary in the future.
Keywords: Central nervous system (CNS), positron emission tomography (PET), metabotropic glutamate receptor type 1 (mGluR1)
Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS), and exerts its actions on synaptic transmission by binding to the iontropic and metabotropic glutamate receptor families. Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors [1], and include eight subtypes (mGluR1-8) divided into three groups based on sequence homology, intracellular transduction pathways, and pharmacological properties [2].
Indirect glutamate-induced neurotransmissions via postsynaptic group I (mGluR1 and mGluR5) metabotropic glutamate receptors cause polyphosphoinositide hydrolysis by the formation of inositol 1,4,5-triphosphate and diacylglycerol as second messengers, with subsequent intracellular calcium release and protein kinase C activation [3-5]. Through these indirect excitatory neurotransmission pathways, mGluR1 and mGluR5 can trigger signaling cascades and modulate the activity of ion- and ligand-gated channels. Although mGluR1 and mGluR5 are highly homologous, they have distinct levels of expression, distribution, and function in the CNS [6-10]. Interestingly, inhibition of mGluR1 activation has been indicated as potentially useful for neuroprotection against various brain injuries, such as stroke [11-16], Parkinson disease [17-19], and Huntington disease [20]. As such, the ability to monitor mGluR1 as a biomarker would provide a greater understanding of its pathophysiological, physiopsychological, and biological roles.
We previously developed several promising 11C and 18F-labeled PET probes (Figure 1) for imaging of mGluR1 in the brain [21-23].Of these, N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-4-11C-methoxy-N-methylbenzamide (11C-ITMM) was modified from 4-18F-fluoro-N-[4-[6-(isopropyllamino)pyrimidin-4-yl]-N-metylbenzamide (18F-FITM), a highly selective radioligand for mGluR1 and successfully translated to human clinical studies [24,25]. Although the in vitro binding affinity of ITMM (Ki = 12.6 nM) for mGluR1 was weaker than that of the parent compound FITM (Ki = 5.4 nM), PET analysis in the rat indicated that 11C-ITMM underwent relatively moderate clearance from mGluR1 in the brain with improved brain kinetics over 18F-FITM [22]. Based on these data, 11C-ITMM was progressed to a clinical study in humans [25]; however, the kinetics of 11C-ITMM in the human brain showed slow clearance following a peak at 15 min after injection. As such, PET quantitative analysis with 11C-ITMM for estimation of its specific binding to mGluR1 remains difficult.
Figure 1.
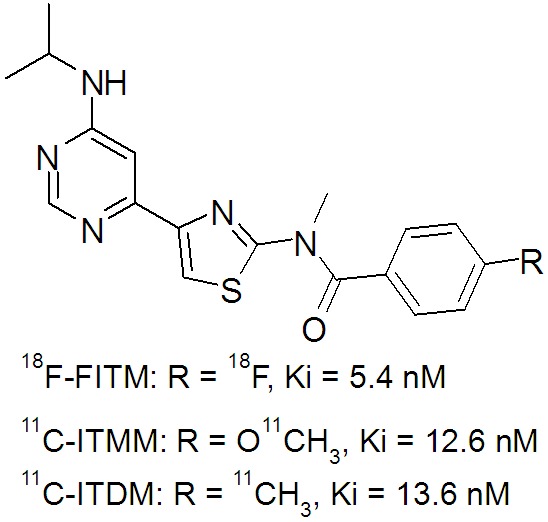
Chemical structures and in vitro binding affinities of 18F-FITM, 11C-ITMM, and 11C-ITDM for mGluR1.
We recently developed N-[4-[6-(isopropyla-mino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-methyl-4-11C-methylbenzamide (11C-ITDM) with an 11C-methyl group replacing the 18F-fluoro or 11C-methoxyl group in 18F-FITM or 11C-ITMM (Figure 1) [21]. The in vitro binding affinity of 11C-ITDM (Ki = 13.6 nM) was similar to that of 11C-ITMM, while it showed faster clearance of radioactivity in the monkey when compared with 11C-ITMM in the human [21]. In addition, the uptake of 11C-ITDM in the pons, a region with negligible mGluR1 expression, was very low, at a similar level to tTAC in the monkey brain following specific mGluR1 blockade. These data strongly suggested that 11C-ITDM PET can acquire brain uptakes without perturbing the reference region. Therefore, 11C-ITDM would be an adequate PET ligand for quantitative analysis of mGluR1.
To aid in the progression of 11C-ITDM-PET toward clinical use in humans, we first performed a PET study on monkey with 11C-ITMM and 11C-ITDM to compare their brain kinetics.
Materials and methods
Production of radioligands
11C-ITMM was synthesized by O-11C-methylation of a desmethyl precursor with 11C-iodomethane in the presence of sodium hydroxide at 70°C for 5 min in N,N-dimethylforamide [22]. At the end of synthesis, 2.4 ± 0.4 GBq of 11C-ITMM was obtained with > 99% radiochemical purity and 111.9 ± 3.1 GBq/μmol specific activity using 19.9 ± 1.2 GBq of 11C-carbon dioxide (n = 3).
11C-ITDM was synthesized by C-11C-methylation of an arylstannane precursor with 11C-iodomethane in the presence of tris(dibenzylideneacetone)dipalladium(0) and tri(o-tolyl)phosphine at 80°C for 5 min in N,N-dimethylforamide [21]. At the end of synthesis, 1.4 ± 0.1 GBq of 11C-ITDM was obtained with > 99% radiochemical purity and 70.3 ± 3.0 GBq/μmol specific activity using 20.1 ± 0.3 GBq of 11C-carbon dioxide (n = 3).
PET studies
A male rhesus monkey (5-6 kg) was purchased from the Central Institute for Experimental Animals (Kanagawa, Japan), and was maintained, handled, and used for experiments according to the recommendations of the Committee for the Care and Use of Laboratory Animals, National Institute of Radiological Sciences.
Prior to the PET assessments, magnetic resonance imaging (MRI) of the monkey’s brain was performed with a 3.0 T scanner (Signa Excite HD, GE Medical Systems, Milwaukee WI, USA)using a short time inversion recovery sequence (repetition time = 5000 ms, echo time = 80 ms, inversion time = 110 ms, field of view = 100 mm, number of slices = 52, slice thickness = 1 mm without slice gap, 512 × 384 acquisition matrix, which after reconstruction was reformatted to a 512 × 512 image matrix, number of excitations = 6, total acquisition time = 72 min).
PET scans were performed using a high-resolution SHR-7700 PET camera (Hamamatsu Photonics, Shizuoka, Japan) designed for laboratory animals.A solution containing 11C-ITMM (172 MBq, 1.8 nmol, 0.3 mL) or 11C-ITDM (185 MBq, 5.0 nmol, 0.5 mL) was injected intravenously into the monkey, which was immobilized in a homemade chamber, and dynamic tomographic scanning was performed for 90 min (30 s × 7 frames, 60 s × 7 frames, 120 s × 20 frames, and 300 s × 8 frames).
Metabolite analysis of blood samples
Arterial blood (0.5-1 mL) was manually sampled at 10, 20, 30, 40, 50 s, 1, 2, 3, 4, 5, 10, 15, 30, 60, and 90 min after injection. The radioactivity in the whole blood and plasma was counted using a 1480 Wizard autogamma scintillation counter (Perkin-Elmer, Waltham, MA, USA). Radioactivity was corrected for decay. Whole blood (0.5-1.5 mL) samples were centrifuged at 20,000 g for 1 min at 4°C to separate the plasma. The supernatant (0.5 mL) was then collected in a test tube containing acetonitrile (0.5 mL), and the resulting mixture was vortexed for 15 s and centrifuged at 20,000 g for 2 min at 4°C for deproteinization. An aliquot of the supernatant obtained from the plasma was injected into a high-performance liquid chromatography (HPLC) system with a radiation detector [26], and then analyzed using a Capcell Pack C18 column (4.6 mm i.d. × 250 mm; Shiseido, Tokyo, Japan) with a mixture of acetonitrile, water, and triethylamine (6/4/0.01 for 11C-ITMM; 7/3/0.01 for 11C-ITDM). The percentage of 11C-ITMM (retention time = 5.3 min at 1.5 mL∙min−1) or 11C-ITDM (retention time = 7.1 min at 1.0 mL∙min−1) to total radioactivity (corrected for decay) on the HPLC charts was calculated as percentage of intact = (peak area of intact/total peak area) × 100.
Kinetic analysis
All PET images were generated by averaging the uptakes between 10 and 90 min. Volumes of interest (VOIs) were placed on each brain region using PMOD version 3.2 image analysis software (PMOD Technologies, Zurich, Switzerland) with reference to the MRI template. Each PET image was manually overlain on the MRI template, and the tissue time-activity curves (tTACs) in each VOI were generated. Brain uptake was decay corrected to the injection time and expressed as the standardized uptake value (SUV), which was normalized to the injected radioactivity and body weight [27].
The plasma input function was obtained from the plasma fraction corrected by metabolite analysis using the PMOD (PMOD Technologies). All kinetic parameters in the cerebellum, thalamus, caudate, putamen, cingulate cortex, hippocampus, and pons were generated by nonlinear-least-square fitting with the two-tissue compartment model (2-TCM). The six parameters were: plasma-to-tissue influx and efflux rate constants, K1 and k2; the ligand binding rate on and off the receptors, k3 and k4; the volume of distribution, VT = (K1/k2) (1 + k3/k4); and the area under the curve (AUC). The distribution volume ratio (DVR) in each region was acquired using VT of the pons as a reference region, where mGluR1 expression is negligible [8]. The DVR of the target region was calculated as: DVR = VT(region)/VT(pons). The ability to identify the parameters was expressed by coefficients of variation (%COV). Blood volume in the monkey brain was fixed at 3% according to a previous report [28].
To validate the efficacy of the reference tissue model, the binding potential (BPND) values based on general reference tissue methods were compared with DVR-1 values based on the 2-TCM. The BPND values based on the simplified reference tissue model (SRTM) [29], Ichise’s multilinear reference tissue model (MRTM) [30], or Logan’s reference tissue model (Logan R) [31] were acquired using tTAC of the pons as a reference region. The analysis based on Logan R were performed using average k2’, which has been obtained from the k2 value of the pons based on 1-TCM.
Results
PET studies
Figure 2 shows PET images with 11C-ITMM (E-H) and 11C-ITDM (I-L) in the monkey brain. Uptake of 11C-ITDM in the brain seemed slightly higher than that of 11C-ITMM. In both PET images, the highest radioactive signal was found in the cerebellum, moderate uptake was detected in the thalamus and cingulate cortex, and low uptake was observed in the striatum and brainstem.
Figure 2.
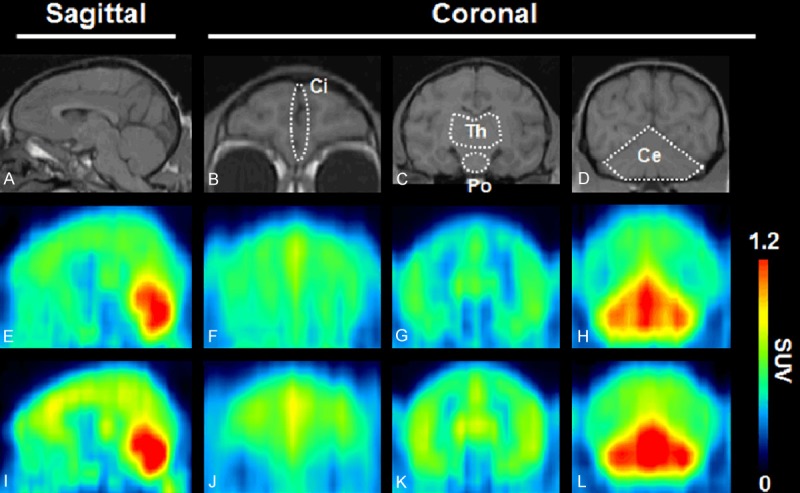
MRI and summed (10-90 min) PET images in the monkey brain. Prior to PET assessment, a monkey was scanned via high-resolution MRI (A-D). PET scans with 11C-ITMM (E-H) or 11C-ITDM (I-L) were performed on the same monkey. The tomograms were reconstructed in sagittal or coronal planes. Abbreviations: Ci, cingulate cortex; Th, thalamus; Po, pons; Ce, cerebellum.
Figure 3 shows the tTACs of 11C-ITMM (A) and 11C-ITDM (B) in various brain regions. The respective tTACs of the radioprobes peaked at 10-20 min for 11C-ITMM and 15-20 min for 11C-ITDM after a bolus injection, showing gradual clearance after that. The maximum of SUV in PET studies with 11C-ITMM or 11C-ITDM was 1.1 or 1.2 for the cerebellum, 0.7 or 0.8 for the thalamus, 0.6 or 0.6 for the caudate, 0.6 or 0.7 for the putamen, 0.8 or 0.9 for the cingulate cortex, 0.6 or 0.7 for the hippocampus, and 0.6 or 0.7 for the pons, respectively.
Figure 3.
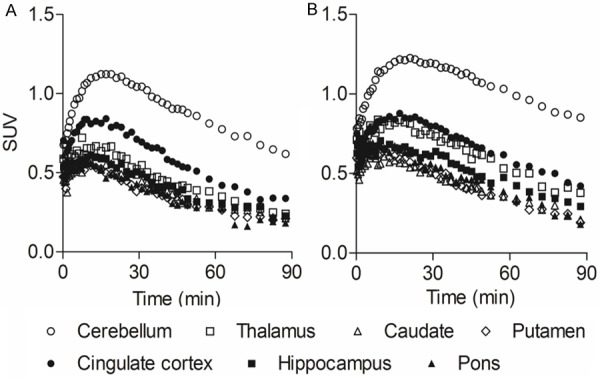
Time-activity curves of 11C-ITMM (A) and 11C-ITDM (B) in brain regions. Regions of interest were drawn on the cerebellum (open circles), thalamus (open squares), caudate (open triangles), putamen (open diamonds), cingulate cortex (solid circles), hippocampus (solid squares), and pons (solid triangles). The uptakes were expressed as SUV.
Input function in PET studies
Table 1 shows the results of metabolite analyses in the monkey plasma. 11C-ITMM and 11C-ITDM were both gradually decomposed to radiolabeled metabolites following a bolus injection. At 90 min following injection, the percentage of parent compound was approximately 30% for 11C-ITMM and 40% for 11C-ITDM. Two unknown radiolabeled metabolites were detected at much a higher polarity than their corresponding intact forms.
Table 1.
Percentage of radioactivity in monkey plasma at 1, 5, 15, 30, 60, and 90 min after injection of 11C-ITMM or 11C-ITDM
| Time after the injection (min) | 11C-ITMM | 11C-ITDM | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| % of total radioactivity | ||||||
|
| ||||||
| Metabolite 1 | Metabolite 2 | Parent | Metabolite 1 | Metabolite 2 | Parent | |
| 1 | 0.1 | 0.0 | 99.9 | 0.8 | 0.1 | 99.2 |
| 5 | 8.9 | 2.5 | 88.6 | 18.9 | 3.2 | 78.0 |
| 15 | 33.0 | 8.2 | 58.8 | 35.4 | 6.7 | 57.9 |
| 30 | 46.3 | 14.6 | 39.1 | 40.0 | 6.9 | 53.2 |
| 60 | 50.6 | 17.6 | 31.8 | 52.7 | 9.0 | 38.3 |
| 90 | 52.0 | 17.5 | 30.4 | 49.3 | 12.3 | 38.4 |
Figure 4 shows metabolite-corrected input curves of plasma with 11C-ITMM or 11C-ITDM. Both input curves showed a rapid decrease following a bolus injection. At 30 min following injection, plasma activities for both probes were one-fiftieth of their peak.
Figure 4.
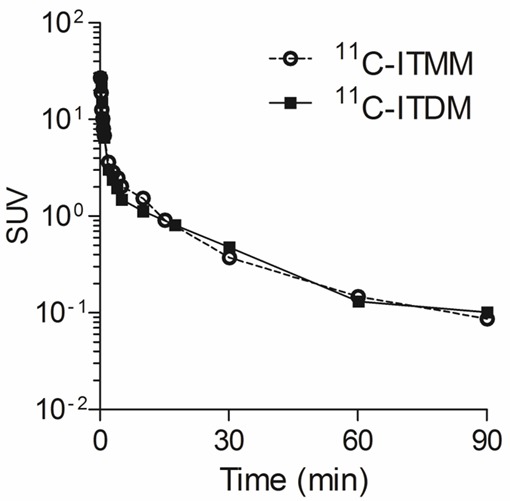
Input curves of 11C-ITMM (open circles) and 11C-ITDM (solid squares) in monkey plasma. The radioactivities were expressed as SUV.
Kinetic analysis
Tables 2 and 3 show the full kinetic parameters acquired by 2-TCM with 11C-ITMM and 11C-ITDM. The VT values corresponding to bindings of radioligand and the AUC values indicating uptake of radioactivity in 11C-ITDM PET were superior to those in 11C-ITMM PET. All of the generated fitting curves showed high confidence (r2 > 0.90).
Table 2.
Kinetic parameters obtained by 2-TCM in PET with 11C-ITMM
| Parameter | Cerebellum | Thalamus | Caudate | Putamen | Cingulate cortex | Hippocampus | Pons | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||
| Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | |
| K1 (mL∙cm-3∙min-1) | 0.052 | 3.0 | 0.061 | 7.9 | 0.043 | 6.3 | 0.042 | 4.2 | 0.106 | 13.2 | 0.092 | 11.7 | 0.168 | 18.2 |
| k2 (min-1) | 0.105 | 13.4 | 0.433 | 19.9 | 0.317 | 16.7 | 0.251 | 11.4 | 1.275 | 25.0 | 1.243 | 20.3 | 2.943 | 24.9 |
| k3 (min-1) | 0.099 | 15.1 | 0.201 | 15.2 | 0.147 | 14.9 | 0.111 | 12.2 | 0.441 | 12.6 | 0.332 | 10.9 | 0.378 | 12.2 |
| k4 (min-1) | 0.025 | 7.8 | 0.031 | 7.2 | 0.028 | 8.0 | 0.030 | 7.1 | 0.031 | 6.2 | 0.029 | 5.9 | 0.032 | 7.0 |
| VT (mL∙cm-3) | 2.5 | 3.2 | 1.0 | 3.0 | 0.9 | 3.4 | 0.8 | 2.7 | 1.3 | 2.3 | 0.9 | 2.7 | 0.7 | 3.5 |
| AUC (SUV∙min) | 88.4 | ― | 49.3 | ― | 39.2 | ― | 37.3 | ― | 60.5 | ― | 45.0 | ― | 38.4 | ― |
Table 3.
Kinetic parameters obtained by 2-TCM in PET with 11C-ITDM
| Parameter | Cerebellum | Thalamus | Caudate | Putamen | Cingulate cortex | Hippocampus | Pons | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||
| Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | Mean | %COV | |
| K1 (mL∙cm-3∙min-1) | 0.045 | 1.6 | 0.049 | 6.7 | 0.027 | 2.6 | 0.032 | 1.9 | 0.059 | 9.8 | 0.032 | 2.7 | 0.036 | 3.7 |
| k2 (min-1) | 0.030 | 12.2 | 0.312 | 29.7 | 0.054 | 13.1 | 0.075 | 8.3 | 0.825 | 32.8 | 0.071 | 15.6 | 0.095 | 17.2 |
| k3 (min-1) | 0.026 | 37.0 | 0.377 | 22.9 | 0.028 | 42.8 | 0.037 | 22.2 | 0.832 | 17.8 | 0.066 | 30.2 | 0.065 | 33.6 |
| k4 (min-1) | 0.019 | 37.8 | 0.052 | 8.6 | 0.031 | 31.6 | 0.036 | 14.8 | 0.043 | 9.9 | 0.044 | 14.4 | 0.045 | 17.4 |
| VT (mL∙cm-3) | 3.6 | 10.9 | 1.3 | 1.7 | 0.9 | 5.4 | 0.9 | 2.7 | 1.5 | 1.3 | 1.1 | 2.7 | 0.9 | 3.3 |
| AUC (SUV∙min) | 94.2 | ― | 55.0 | ― | 37.4 | ― | 37.7 | ― | 59.4 | ― | 45.5 | ― | 40.6 | ― |
Correlation between general reference tissue models and 2-TCM in PET with 11C-ITMM or 11C-ITDM
Figure 5 shows correlational scatter plots between general reference tissue model-based BPNDs and 2-TCM-based DVR-1 values in the quantitative PET analysis with 11C-ITMM or 11C-ITDM. Table 4 shows the slope of a regression line and coefficient of determination in each brain region. The slope of regression lines directly indicates under- or overestimation of BPND based on the reference tissue model when compared with DVR-1 based on the 2-TCM. As shown in Figure 5 and Table 4, BPNDs derived from SRTM in both radiopharmaceuticals showed marked underestimation (slope was 0.56 for 11C-ITMM and 0.54 for 11C-ITDM) when compared with DVR-1 values calculated by 2-TCM (Figure 5A). In the MRTM-based BPNDs, that of 11C-ITDM showed slight overestimation (slope was 1.15) against 2-TCM-based DVR-1 values, whereas that of 11C-ITMM was underestimated (slope was 0.67) (Figure 5B). BPNDs based on Logan Ref in both radiopharmaceuticals showed relatively low underestimation (slope was 0.66 for 11C-ITMM and 0.65 for 11C-ITDM) against DVR-1 values calculated by 2-TCM (Figure 5C). The majority of regression lines of scatter plots exhibited with high correlation (r2 > 0.95).
Figure 5.

Correlation between BPND based on reference tissue model and DVR-1 based on 2-TCM in PET studies with 11C-ITMM (solid circles) or 11C-ITDM (open squares). Reference tissue models were used SRTM (A), MRTM (B), and Logan R (C).
Table 4.
The slope of regression line and coefficient of determination (r2) in the correlational scatter plot of BPND based on reference tissue models against DVR-1 based on 2-TCM
| Probe | SRTM | MRTM | Logan R | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Slope | 1/Slope | r2 | Slope | 1/Slope | r2 | Slope | 1/Slope | r2 | |
| 11C-ITMM | 0.56 | 1.8 | 0.95 | 0.67 | 1.49 | 0.98 | 0.66 | 1.52 | 0.99 |
| 11C-ITDM | 0.54 | 1.85 | 0.97 | 1.15 | 0.87 | 0.98 | 0.65 | 1.55 | 0.99 |
Figure 6 shows Bland-Altman plots between reference tissue models and 2-TCM in PET studies using 11C-ITMM or 11C-ITDM. There were no significant differences between general reference tissue models and 2-TCM in PET kinetic analysis using both radiopharmaceuticals. Bias of reference tissue model for 11C-ITMM or 11C-ITDM were 39.2 ± 15.5% or 1.49 ± 40.3%, 27.0 ± 10.3% or -23.9 ± 37.0%, and 27.6 ± 10.1% or -3.8 ± 34.7% for the SRTM vs. 2-TCM, MRTM vs. 2-TCM, and Logan R vs. 2-TCM, respectively.
Figure 6.
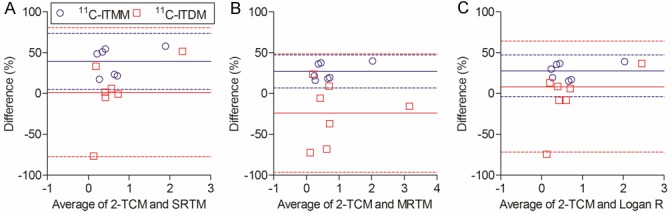
Bland-Altman plot between BPND based on reference tissue methods, which are SRTM (A), MRTM (B), and Logan R (C), and DVR-1 based on 2-TCM in PET studies with 11C-ITMM (blue circles) or 11C-ITDM (red squares).
Discussion
In this study, we compared the results of quantitative PET analysis in the monkey brain between 11C-ITMM and 11C-ITDM. In respective PET images (Figure 2), heterogeneous uptake of radioactivity was found in brain regions corresponding to mGluR1 distribution with 18F-FITM in the monkey [32], suggesting that the uptake of radioactivity in the PET images for both radiopharmaceuticals reflected their binding to mGluR1. The uptake of 11C-ITDM was slightly higher than that of 11C-ITMM, although the brain kinetics of both radiopharmaceuticals showed a similar rapid clearance following a peak uptake at approximately 15 min after injection (Figure 3). Surprisingly, although the tTACs of 11C-ITMM in a human showed a slow clearance after peaking at 15 min [25], the tTACs of 11C-ITMM in the monkey were more rapid. These differences in brain kinetics of 11C-ITMM between the monkey and human may be caused by a difference in enzymatic metabolism of 11C-ITMM in vivo. This difference is also seen in the kinetic analysis of other PET radiopharmaceuticals. For example, the metabolization rate of 11C-Ro15-4513, a selective probe for a central benzodiazepine receptor, was reported to be caused by enzymatic hydrolysis of 11C-Ro15-4513 to differing degrees in the blood of several species: mouse < rat < human < monkey [33]. In the case of 11C-ITMM, the percentage of unchanged form in the plasma at 60 min after injection was 62% in the human [25] and 31% in the monkey. Thus, differences in enzyme activity in vivo may contribute to differences in metabolization rate of 11C-ITMM in the human and monkey, resulting in different input functions in brain tissues. Indeed, the maximum uptake of 11C-ITMM in the cerebellum was 2.5 SUV for human and 1.1 SUV for monkey. In addition to differences in enzyme activity, there are also differences in mGluR1 density among different species. In a binding study with 18F-MK-1312, a radioligand for mGluR1, the density (Bmax) of mGluR1 in the cerebellum was reported to be 82 nM for human and 53 nM for monkey [34]. Considering these findings, differences in mGluR1 density in the peripheral organs are not surprising. Because of these differences, the brain uptake and clearance of 11C-ITMM in human may be higher and slower, respectively, when compared with the monkey.
Although mGluR1 density is different between human and monkey brains, the distribution pattern is very similar in both species. We previously assessed the distribution pattern of mGluR1 in rodents (mouse and rat) and primates (monkey and human) [21-23,35], and found that mGluR1 density in rodent brains was relatively high in the thalamus, striatum, and hippocampus, while high expression of mGluR1 in primate brains was highest in the cerebral cortex followed by the cerebellum, the region with the highest density of this receptor in both rodent and primate brains. Considering these profiles, quantitative PET analysis for mGluR1 in the monkey brain would be a suitable simulation for progression toward clinical studies in humans.
The variation of the VT values based on 2-TCM in brain regions was between 0.7-2.5 mL∙cm-3 for 11C-ITMM and 0.9-3.6 mL∙cm-3 for 11C-ITDM (Tables 2 and 3). However, the variation of VT values for 18F-FITM was reported to be 2.4-11.5 mL∙cm-3 in monkey brain regions [32]. Importantly, although the corresponding VT value for both radioligands was lower than that of 18F-FITM, their VT values in the pons, a mGluR1-negligible region, were similar to the reference rather than the plasma input function for 18F-FITM; i.e., the VT in the pons was 0.7 mL∙cm-3 for 11C-ITMM, 0.9 mL∙cm-3 for 11C-ITDM, and 2.4 mL∙cm-3 for 18F-FITM. These data strongly suggest that tTACs in the pons of 11C-ITMM and 11C-ITDM would be suitable as the reference. In fact, the pons is often used as a reference region instead of the plasma input function in quantitative PET analysis of neuroreceptors [36,37].
To validate the usefulness of the reference tissue model using tTAC of the pons, we confirmed the relationship between BPNDs based on several reference tissue models and DVR-1 based on 2-TCM in PET studies with 11C-ITMM or 11C-ITDM. Among the scattergrams (Figure 5), all of the slopes of regression lines for 11C-ITMM showed underestimations of reference tissue models against 2-TCM, whereas the slope of the regression line of 11C-ITDM with MRTM showed slight overestimation, despite the slopes of the regression lines of 11C-ITDM with SRTM or Logan R indicating underestimation similar to that with 11C-ITMM. Although BPNDs based on reference tissue models using alternative PET probes were under-or overestimated compared with 2-TCM, there were no significant differences between the two kinetic models. Thus, noninvasive PET quantitative analysis using a reference tissue for both radioprobes is feasible, and the blood sampling method is not required.
Conclusion
We performed quantitative PET analysis of 11C-ITDM binding to mGluR1 in the monkey. We validated that PET with 11C-ITMM or 11C-ITDM can use reference tissue models instead of the blood sampling method. Therefore, noninvasive kinetic analysis using reference tissue models in 11C-ITDM PET is a candidate for clinical studies using human subjects.
Acknowledgements
We thank the staff at the National Institute of Radiological Sciences for their support with the cyclotron operation, radioisotope production, radiosynthesis, and animal experiments.
Disclosure of conflict of interest
The authors declare no conflict of interest.
References
- 1.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferraguti F, Crepaldi L, Nicoletti F. Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacol Rev. 2008;60:536–581. doi: 10.1124/pr.108.000166. [DOI] [PubMed] [Google Scholar]
- 3.Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- 4.Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- 5.Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998;2731:5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- 6.Battaglia G, Bruno V, Pisani A, Centonze D, Catania MV, Calabresi P, Nicoletti F. Selective blockade of type-1 metabotropic glutamate receptors induces neuroprotection by enhancing gabaergic transmission. Mol Cell Neurosci. 2001;17:1071–1083. doi: 10.1006/mcne.2001.0992. [DOI] [PubMed] [Google Scholar]
- 7.Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999;59:55–79. doi: 10.1016/s0301-0082(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 8.Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, Snyder SH, Dawson TM. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisani A, Calabresi P, Centonze D, Bernardi G. Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- 11.De Vry J, Horvath E, Schreiber R. Neuroprotective and behavioral effects of the selective metabotropic glutamate mGlu(1) receptor antagonist BAY 36-7620. Eur J Pharmacol. 2001;428:203–214. doi: 10.1016/s0014-2999(01)01296-1. [DOI] [PubMed] [Google Scholar]
- 12.Kohara A, Takahashi M, Yatsugi S, Tamura S, Shitaka Y, Hayashibe S, Kawabata S, Okada M. Neuroprotective effects of the selective type 1 metabotropic glutamate receptor antagonist YM-202074 in rat stroke models. Brain Res. 2008;1191:168–179. doi: 10.1016/j.brainres.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Moroni F, Attucci S, Cozzi A, Meli E, Picca R, Scheideler MA, Pellicciari R, Noe C, Sarichelou L, Pellegrini-Giampietro DE. The novel and systemically active metabotropic glutamate 1 (mGlu1) receptor antagonist 3-MATIDA reduces post-ischemic neuronal death. Neuropharmacology. 2002;42:741–751. doi: 10.1016/s0028-3908(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 14.Murotomi K, Takagi N, Takayanagi G, Ono M, Takeo S, Tananaka K. mGluR1 antagonist decreases tyrosine phosphorylation of NMDA receptor and attenuates infarct size after transient focal cerebral ischemia. J Neurochem. 2008;105:1625–1634. doi: 10.1111/j.1471-4159.2008.05260.x. [DOI] [PubMed] [Google Scholar]
- 15.Szydlowska K, Kaminska B, Baude A, Parsons CG, Danysz W. Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur J Pharmacol. 2007;554:18–29. doi: 10.1016/j.ejphar.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, Xu W, Liao G, Bi X, Baudry M. Neuroprotection against neonatal hypoxia/ischemia-induced cerebral cell death by prevention of calpain-mediated mGluR1alpha truncation. Exp Neurol. 2009;218:75–82. doi: 10.1016/j.expneurol.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull. 2006;69:318–326. doi: 10.1016/j.brainresbull.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Kaneda K, Tachibana Y, Imanishi M, Kita H, Shigemoto R, Nambu A, Takada M. Down-regulation of metabotropic glutamate receptor 1 alpha in globus pallidus and substantia nigra of Parkinsonian monkeys. Eur J Neurosci. 2005;22:3241–3254. doi: 10.1111/j.1460-9568.2005.04488.x. [DOI] [PubMed] [Google Scholar]
- 19.Ossowska K, Wardas J, Pietraszek M, Konieczny J, Wolfarth S. The striopallidal pathway is involved in antiparkinsonian-like effects of the blockade of group I metabotropic glutamate receptors in rats. Neurosci Lett. 2003;342:21–24. doi: 10.1016/s0304-3940(03)00221-0. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro FM, Paquet M, Ferreira LT, Cregan T, Swan P, Cregan SP, Ferguson SS. Metabotropic glutamate receptor-mediated cell signaling pathways are altered in a mouse model of Huntington’s disease. J Neurosci. 2010;30:316–324. doi: 10.1523/JNEUROSCI.4974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujinaga M, Yamasaki T, Maeda J, Yui J, Xie L, Nagai Y, Nengaki N, Hatori A, Kumata K, Kawamura K, Zhang MR. Development of N-[4-[6-(isopropylamino)pyrimidin-4-yl] -1,3-thiazol-2-yl] -N-methyl-4-11C-methylbenzamide for positron emission tomography imaging of metabotropic glutamate 1 receptor in monkey brain. J Med Chem. 2012;55:11042–11051. doi: 10.1021/jm301597s. [DOI] [PubMed] [Google Scholar]
- 22.Fujinaga M, Yamasaki T, Yui J, Hatori A, Xie L, Kawamura K, Asakawa C, Kumata K, Yoshida Y, Ogawa M, Nengaki N, Fukumura T, Zhang MR. Synthesis and evaluation of novel radioligands for positron emission tomography imaging of metabotropic glutamate receptor subtype 1 (mGluR1) in rodent brain. J Med Chem. 2012;55:2342–2352. doi: 10.1021/jm201590g. [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki T, Fujinaga M, Yoshida Y, Kumata K, Yui J, Kawamura K, Hatori A, Fukumura T, Zhang MR. Radiosynthesis and preliminary evaluation of 4-18F-fluoro-N-[4-[6-(isopropy-lamino)pyrimidin-4-yl] -1,3-thiazol-2-yl] -N-me-thylbenzamide as a new positron emission tomography ligand for metabotropic glutamate receptor subtype 1. Bioorg Med Chem Lett. 2011;21:2998–3001. doi: 10.1016/j.bmcl.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 24.Toyohara J, Sakata M, Fujinaga M, Yamasaki T, Oda K, Ishii K, Zhang MR, Moriguchi Jeckel CM, Ishiwata K. Preclinical and the first clinical studies on 11C-ITMM for mapping metabotropic glutamate receptor subtype 1 by positron emission tomography. Nucl Med Biol. 2013;40:214–220. doi: 10.1016/j.nucmedbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Toyohara J, Sakata M, Oda K, Ishii K, Ito K, Hiura M, Fujinaga M, Yamasaki T, Zhang MR, Ishiwata K. Initial human PET studies of metabotropic glutamate receptor type 1 ligand 11C-ITMM. J Nucl Med. 2013;54:1–6. doi: 10.2967/jnumed.113.119891. [DOI] [PubMed] [Google Scholar]
- 26.Takei M, Kida T, Suzuki K. Sensitive measurement of positron emitters eluted from HPLC. Appl Radiat Isot. 2001;55:229–234. doi: 10.1016/s0969-8043(00)00392-4. [DOI] [PubMed] [Google Scholar]
- 27.Keyes JW Jr. SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–1839. [PubMed] [Google Scholar]
- 28.Ohya T, Okamura T, Nagai Y, Fukushi K, Irie T, Suhara T, Zhang MR, Fukumura T, Kikuchi T. Effect of radiolabeled metabolite elimination from the brain on the accuracy of cerebral enzyme activity estimation using positron emission tomography with substrate tracers. Neuroimage. 2011;56:105–1110. doi: 10.1016/j.neuroimage.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 30.Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to 11C-DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- 31.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Yamasaki T, Fujinaga M, Maeda J, Kawamura K, Yui J, Hatori A, Yoshida Y, Nagai Y, Tokunaga M, Higuchi M, Suhara T, Fukumura T, Zhang MR. Imaging for metabotropic glutamate receptor subtype 1 in rat and monkey brains using PET with 18F-FITM. Eur J Nucl Med Mol Imaging. 2012;39:632–641. doi: 10.1007/s00259-011-1995-6. [DOI] [PubMed] [Google Scholar]
- 33.Kida T, Noguchi J, Zhang MR, Suhara T, Suzuki K. Metabolite analysis of 11C-Ro15-4513 in mice, rats, monkeys and humans. Nucl Med Biol. 2003;30:779–784. doi: 10.1016/s0969-8051(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 34.Hostetler ED, Eng W, Joshi AD, Sanabria-Bohorquez S, Kawamoto H, Ito S, O’Malley S, Krause S, Ryan C, Patel S, Williams M, Riffel K, Suzuki G, Ozaki S, Ohta H, Cook J, Burns HD, Hargreaves R. Synthesis, characterization, and monkey PET studies of 18F-MK-1312, a PET tracer for quantification of mGluR1 receptor occupancy by MK-5435. Synapse. 2011;65:125–135. doi: 10.1002/syn.20826. [DOI] [PubMed] [Google Scholar]
- 35.Fujinaga M, Maeda J, Yui J, Hatori A, Yamasaki T, Kawamura K, Kumata K, Yoshida Y, Nagai Y, Higuchi M, Suhara T, Fukumura T, Zhang MR. Characterization of 1-(2-18F-fluoro-3-pyridyl)-4-(2-isopropyl-1-oxo-isoindoline-5-yl)-5-methyl-1H-1,2,3-triazole, a PET ligand for imaging the metabotropic glutamate receptor type 1 in rat and monkey brains. J Neurochem. 2012;121:115–124. doi: 10.1111/j.1471-4159.2011.07348.x. [DOI] [PubMed] [Google Scholar]
- 36.Geeraerts T, Coles JP, Aigbirhio FI, Pickard JD, Menon DK, Fryer TD, Hong YT. Validation of reference tissue modelling for 11C-flumazenil positron emission tomography following head injury. Ann Nucl Med. 2011;25:396–405. doi: 10.1007/s12149-011-0480-4. [DOI] [PubMed] [Google Scholar]
- 37.Pearl PL, Gibson KM, Quezaso Z, Dustin I, Taylor J, Trzcinski S, Schreiber J, Forester K, Reeves-Tyer P, Liew C, Shamim S, Herscovitch P, Carson R, Butman J, Jacobs C, Theodore W. Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology. 2009;73:423–429. doi: 10.1212/WNL.0b013e3181b163a5. [DOI] [PMC free article] [PubMed] [Google Scholar]


