Abstract
As progression and outcome of relapsed hepatocellular carcinoma (HCC) are distinct from those of a second primary tumor, clonal analyses of initial and recurrent HCCs are thus clinically useful. Although several studies in Japan and Taiwan had shown that the multicentric origin (MO) recurrences were more common than intrahepatic metastases (IM), a recent report from China indicated that IM cases outnumber MO recurrences. In managing HCC cases, both tumor malignancy and background liver function are important considerations (and which we characterize as tumor factors and background liver factors, respectively); they indicate both appropriate treatment, and likely post-surgical outcome. In this editorial, we explain why the report had shown such a different conclusion. We also discuss current management of HCC.
Keywords: Tumor factors, background liver factors, hepatocellular carcinoma (HCC), intrahepatic metastases (IM), multicentric origin (MO)
Introduction
One of the most common tumors worldwide is hepatocellular carcinoma (HCC). Although early HCC may be cured by surgical resection, the central concern of treating this fatal disease is that it is prone to multicentric occurrence. As progression and outcome of truly relapsed HCC are distinct from second primary tumors, clonal analyses of initial and recurrent HCC are clinically significant. Although several studies have shown multicentric origin (MO) recurrences to be more common than intrahepatic metastases (IM) (1-5), an article by a Chinese group concluded that IM-type recurrences were more common and had poorer prognosis than MO-type recurrences (6).
The technique of determining tumor clonality is well-tested from a previous investigation of loss of heterozygosity (LOH) loci using many microsatellites (7). As their figures and tables show, this method of assessing LOH and its frequency at each locus was suitable to their experiments. However, their results differed from those of other researchers, which necessitated consideration of all aspects. Regrettably, the authors did not address this in their discussion.
In this editorial, we would like to explain our view of HCC management, and discuss this divergent result.
Tumor factors and background liver factors
When considering appropriate therapy for HCC, we must consider factors of both the tumor itself and the background liver. Tumor factors include tumor size, differentiation, existence of a portal or venous invasion, AFP value etc. and clinical stage (which is determined by tumor factors). Background liver factors include existence of liver cirrhosis, prothrombin time (PT), serum albumin value, Child-Pugh classification, etc. Both tumor factors and background liver factors help determine appropriate treatment, and indicate likely post-surgical outcomes.
A meta-analysis of overall and disease-free survival following resection for HCC found in multivariate analyses that the strongest predictors of adverse prognosis were clinical stage of the tumor and vascular invasion, both of which are tumor factors (8). However, liver background factors, including poor Child-Pugh score and existence of cirrhosis, were also associated with worse prognoses.
Among cancers with apparent recurrences, in cases where tumor factors indicate high malignancy, we can suppose that the original tumor would tend to generate IM recurrences (Figure 1), whereas MO type recurrences would be produced in other portions of the liver, in environments with poor background liver factors (Figure 2); IM recurrences are more common than MO recurrences, but the backgrounds of the examined cases differ greatly.
Figure 1.
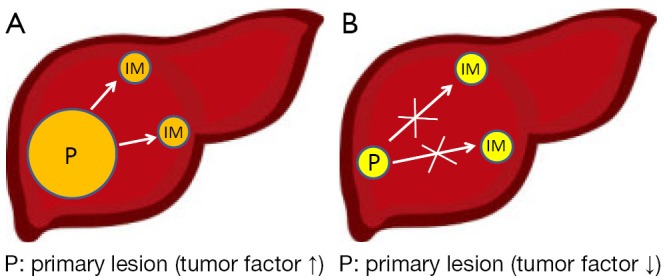
Possibility of intrahepatic metastatic (IM) recurrences with different tumor factors. (A) Tumor factor grade indicates high malignancy; the tumor would tend to make IM recurrences; (B) Primary lesion was resected in early stage; IM recurrences would be less likely to occur.
Figure 2.
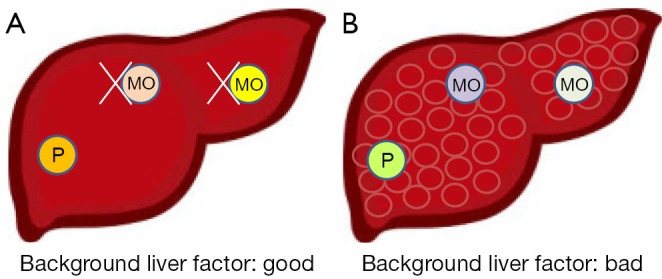
Possibility of secondary [multicentric origin (MO)] recurrences with different background liver factors. (A) As background liver factors were well conserved; the liver is less likely to generate MO-type recurrences; (B) In a liver with poorly conserved background function, MO recurrences in other parts of the liver are more likely to occur.
HCC of HBV or HCV origin
Tables 1 and 2 show background liver factors and tumor factors, respectively, of 320 patients who underwent liver resections in our department. The clinicopathological features of these patients as a group do not seem to differ greatly from other Japanese patients with HCC, and HCC from chronic non-B, non-C hepatitis, such as non-alcoholic steato-hepatitis (NASH), is similarly increasing in Western countries. In examining clonal origins of recurrent tumors (4,5), we examined 19 cases (14 of HCV origin, 3 of HBV origin, and 2 of non-B, non-C hepatitis), whereas the study from China examined 38 cases (37 with HBV infection and 1 non-B, non-C hepatitis).
Table 1. Background liver factors.
| Variables | Value |
|---|---|
| Age (years), mean ± SD (range), (n=320) | 63.4±10.1 [21-84] |
| Sex, (n=320) | |
| Male | 261 (81.6%) |
| Female | 59 (18.4%) |
| Viral infection, (n=319) | |
| HBV | 78 (24.5%) |
| HCV | 177 (55.5%) |
| HBV/HCV | 4 (1.2%) |
| Non-HBV/non-HCV | 60 (18.8%) |
| Albumin (mg/dL), mean ± SD, (n=317) | 3.84±0.51 |
| Total bilirubin (mg/dL) mean ± SD, (n=317) | 0.81±0.50 |
| PT (%) mean ± SD, (n=316) | 87.4±15.5 |
| Liver cirrhosis, (n=309) | |
| (+) | 130 (42.1%) |
| (–) | 179 (57.9%) |
| Child-Pugh classification, (n=315) | |
| A | 293 (93.0%) |
| B | 22 (7.0%) |
| Liver damage score, (n=308) | |
| A | 237 (77.0%) |
| B | 70 (22.7%) |
| C | 1 (0.3%) |
PT, prothrombin time.
Table 2. Tumor factors.
| Variables | Value |
|---|---|
| Tumor size (mm), mean ± SD (range), (n=311) | 45.1±32.0 (0.8-175) |
| Tumor number, solitary/multiple (n=320) | 235 (73.4%)/85 (26.6%) |
| AFP (ng/mL), <100/≥100 (n=311) | 211 (67.8%)/100 (32.2%) |
| Portal vein or hepatic vein invasion, +/– (n=312) | 85 (27.2%)/227 (72.8%) |
| Differentiation, (n=311) | |
| Well | 66 (21.2%) |
| Moderate | 220 (70.7%) |
| Poor | 25 (8.1%) |
| Growth form, expansive/infiltrative (n=311) | 257 (82.6%)/54 (17.4%) |
| Formation of capsule, +/– (n=316) | 215 (68.0%)/101 (32.0%) |
| Infiltration to capsule, +/– (n=315) | 174 (55.2%)/141 (44.8%) |
| Septal formation, +/– (n=310) | 205 (66.1%)/105 (33.9%) |
| Serosal infiltration, +/– (n=277) | 66 (23.8%)/211 (76.2%) |
| Surgical margin, +/– (n=288) | 59 (20.5%)/229 (79.5%) |
| Japanese staging | |
| I | 34 (10.8%) |
| II | 163 (51.7%) |
| III | 77 (24.4%) |
| IVa | 38 (12.1%) |
| IVb | 3 (1.0%) |
AFP, alpha-fetoprotein.
A report from Japan compared HCC of HBV origin with HCC of HCV origin (9), and found that, although the AFP level of HBV-based tumors was higher, other tumor factors, such as size or TNM stage, were not different from HCV-based tumors. Patients with HCC based on either HBV or HCV were probably periodically screened as candidates for HCC because of these virus infections. Liver functions, such as albumin levels, were worse in patients with HCV than HBV, and patients with HBV-based HCC had longer overall survival and disease-free survival.
A report from the United States (10) found that patients with HBV were more likely to develop HCC at young age than patients with HCV, with greater serum AFP production and larger tumors, but without cirrhosis. Conversely, patients with HCV were more likely to develop HCC in association with multiple co-morbidities including cirrhosis, and at older ages. Thus, we supposed that HCC outcomes would vary with these viral causes.
Malignancy of the tumor factors
When we looked more closely at the backgrounds of cases that had more IM-type recurrences, their tumors showed vascular invasion, which is the strongest adverse prognostic factor—as high as 65% in IM type. Moreover, the average tumor diameter was also as large as 65 mm, which indicated that tumor was highly malignant and the TNM stage was advanced. Naturally, cancer in advanced stages shows a poor prognosis.
Frequency of vascular invasion among our cases in Japan was about 27%; average tumor diameter was 45 mm (Table 1)—almost equivalent to the MO type cases. In our earlier investigations, the average diameter of primary tumors was 41±27 mm (4, 5).
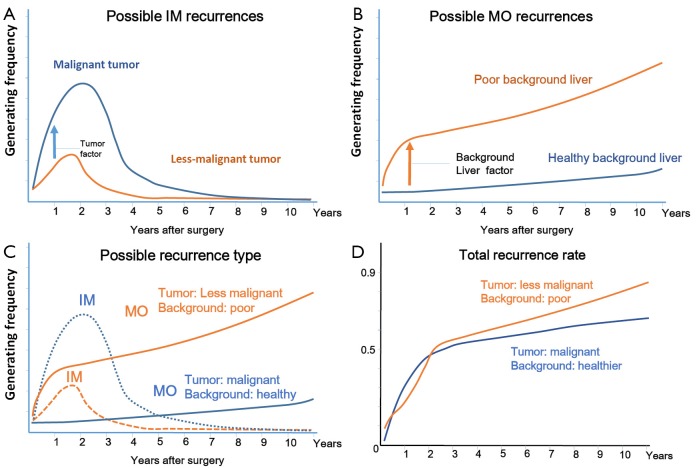
We predicted the probability of each recurrence of IM and MO. Figure 3A compares a case with malignant tumor factors with a less-malignant case in IM type recurrence, and Figure 2B compares a case with poor background liver factors with a case with healthier background liver factors in MO-type recurrence (Figure 3B). Figure 3C shows the probability of total recurrence, comparing cases with malignant tumors but relatively healthy background livers (blue solid line: MO, blue dotted line: IM), to cases with less malignant tumors but poor background livers (orange solid line, MO; orange dotted line, IM). Although the total recurrence rate is not so different in the first few years after surgery, in the later years, cases with poorer background livers would show higher recurrence rates (Figure 3D).
Figure 3.
(A) Possible IM recurrences: lines indicate a liver with malignant tumor factors (blue line) and a less malignant case (orange line) with IM type recurrences. IM recurrences happened more in malignant tumors, especially in the first few years after surgery; (B) Possible MO recurrences: a liver with poor background factors would have increased probability of MO-type recurrences, especially in late years after surgery; (C) Probability of total recurrence when a patient had a malignant tumor and a healthy background liver (blue solid line: MO, blue dotted line: IM), or a less malignant tumor and a poor background liver (orange solid line: MO, orange dotted line: IM); (D) Total recurrence rate after liver resection in each case: although the two lines were not so different in early years after operation, in later years, patients with poorer background liver would show a higher recurrence rate. IM, intrahepatic metastases; MO, multicentric origin.
Thus, we thought that metastatic recurrences increased after surgery because tumor factors of their cases were more malignant. Moreover, in our cases, we considered that background livers were more damaged by HCV, which implied that generating secondary tumors occurred more readily.
Recurrence-free survival rate in HCC cases
Unlike other cancers, the recurrence-free survival rate of HCC must include both IM and MO elements, either of which might recur in any HCC case. A trial to identify which tumor factors and background liver factors were most associated with IM and MO (respectively) might be interesting, and could plausibly allow prediction of recurrence by analyzing a resected tumor. If, for example, the change in percentage of risk for IM and MO recurrence at two years after surgery could be found, we think it will be an epoch-making trial.
Over-all survival rate in HCC cases
The components of overall survival are even more complex. After the initial tumor resection, survival rate changes with the grade of tumor factors of recurrent HCCs, and by their methods of therapy. Moreover, for HCCs, the specific cause of death also varies (e.g., cancer progression, liver failure, etc.), and thus affects overall survival. This would be difficult to predict at initial resection, even if the treatment methods and rule of observation were standardized and causes of death was examined in detail.
How to manage HCCs for better survival
Physicians who manage HCC cases should be mindful of the following things. IM recurrence risk mainly depends on tumor factor malignancy. To avoid aggravating tumor factors, periodical screening of patients with HCC is important, particularly those with chronic hepatitis.
After the surgery for the primary lesion, recurrences are best discovered at the earliest stage possible to prevent exacerbating tumor factors of the recurrent lesion.
To reduce risk of MO recurrences, anti-viral therapy should be recommended, and should probably be used as a postoperative adjunct therapy.
Surgical managements of HCC
Intraoperative factors that affect prognosis also strongly influence IM recurrence. Surgeons must take care to leave clean surgical margins, decrease blood loss, etc. In recurrences found after surgery, we should examine both tumor and background liver factors. If the former shows high malignancy, the recurrence is probably due to IM. However, poor background liver factors indicate likely MO; adjuvant anti-viral therapy should therefore be started promptly.
Conclusions
The significance of new information and recognition of clinical patterns in management of HCC should be deeply considered as we strive to improve patient outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Yamamoto T, Kajino K, Kudo M, et al. Determination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNA. Hepatology 1999;29:1446-52 [DOI] [PubMed] [Google Scholar]
- 2.Chen YJ, Yeh SH, Chen JT, et al. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology 2000;119:431-40 [DOI] [PubMed] [Google Scholar]
- 3.Ochiai T, Urata Y, Yamano T, et al. Clonal expansion in evolution of chronic hepatitis to hepatocellular carcinoma as seen at an X-chromosome locus. Hepatology 2000;31:615-21 [DOI] [PubMed] [Google Scholar]
- 4.Nomoto S, Yamashita K, Koshikawa K, et al. Mitochondrial D-loop mutations as clonal markers in multicentric hepatocellular carcinoma and plasma. Clin Cancer Res 2002;8:481-7 [PubMed] [Google Scholar]
- 5.Nomoto S, Kinoshita T, Kato K, et al. Hypermethylation of multiple genes as clonal markers in multicentric hepatocellular carcinoma. Br J Cancer 2007;97:1260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang B, Xia CY, Lau WY, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg 2013;217:1054-62 [DOI] [PubMed] [Google Scholar]
- 7.Tsuda H, Oda T, Sakamoto M, et al. Different pattern of chromosomal allele loss in multiple hepatocellular carcinomas as evidence of their multifocal origin. Cancer Res 1992;52:1504-9 [PubMed] [Google Scholar]
- 8.Morris-Stiff G, Gomez D, de Liguori Carino N, et al. Surgical management of hepatocellular carcinoma: is the jury still out? Surg Oncol 2009;18:298-321 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, Yamada T, Tanaka H, et al. Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg 2006;244:771-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiotis SP, Rahbari NN, Villanueva GA, et al. Hepatitis B vs. hepatitis C infection on viral hepatitis-associated hepatocellular carcinoma. BMC Gastroenterol 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]