Abstract
Background: Tremendous advances have been made in the medical application of the laser in the past few decades. Many diseases in the dermatological field are now indications for laser treatment that qualify for reimbursement by many national health insurance systems. Among laser types, the carbon dioxide (CO2) laser remains an important system for the dermatologist.
Rationale: The lasers used in photosurgery have wavelengths that differ according to their intended use and are of various types, but the CO2 laser is one of the most widely used lasers in the dermatology field. With its wavelength in the mid-infrared at 10,600 nm, CO2 laser energy is wellabsorbed in water. As skin contains a very high water percentage, this makes the CO2 laser ideal for precise, safe ablation with good hemostasis. In addition to its efficacy in ablating benign raised lesions, the CO2 laser has been reported to be effective in the field of esthetic dermatology in the revision of acne scars as well as in photorejuvenation. With the addition of fractionation of the beam of energy into myriad microbeams, the fractional CO2 laser has offered a bridge between the frankly full ablative indications and the nonablative skin rejuvenation systems of the 2000s in the rejuvenation of photoaged skin on and off the face.
Conclusions: The CO2 laser remains an efficient, precise and safe system for the dermatologist. Technological advances in CO2 laser construction have meant smaller spot sizes and greater precision for laser surgery, and more flexibility in tip sizes and protocols for fractional CO2 laser treatment. The range of dermatological applications of the CO2 laser is expected to continue to increase in the future.
Keywords: CO2 laser, laser ablation, fractional laser, nevus, verruca, photorejuvenation
Introduction
Tremendous advances have been made in the use of the laser in medicine and surgery since the 1990s, and in the field of dermatology efficacy is now recognized in the treatment of angiomas, nevi of Ota, nevus spilus, telangiectasia, and so on, as well as against superficial pigmented lesions, including chloasma, ephelides, and senile lentigines: in fact, the laser is now the treatment of choice for many of these indications. As a benchmark of the acceptance of laser surgery and medicine, some diseases are now indications for the laser that qualify for reimbursement by the Japanese National Health Insurance System. The laser has also been recognized as effective in the treatment of common skin diseases, including but not limited to verruca vulgaris, verruca plantaris, verruca senilis, soft fibromas, condylomata acuminata, syringomas, common warts, vitiligo, leukoplakia, skin ulcers, ingrown toenails, and psoriasis vulgaris. Laser treatment is also used in the field of esthetic dermatology to treat alopecia and acne, and many devices designed for facial cosmesis to treat photo- and chronologically-aged skin, including wrinkles and sagging, have become available.
The intended indications for the lasers used to treat these diseases vary with laser wavelength. Although there are various types of laser, in this article we will describe the fundamentals and clinical applications of the carbon dioxide (CO2) laser, which is the one of the most widely used lasers in the field of dermatology.
Mechanisms of laser surgery and medicine 1
Everyone reading this article has probably heard the following many hundreds, if not thousands of times. The word “LASER” is an acronym for “Light Amplification by Stimulated Emission of Radiation.” Although repeated so often, the actual meaning behind the phrase is vitally important to the understanding of laser/tissue reaction: it is in fact the distillation of what makes laser energy unique among other types of light energy, and so what the well-quoted acronym actually stands for bears careful consideration.
Lasers generate light energy in the form of a beam of photons emitted from the laser medium, which usually gives the laser its name and determines the precise wavelength produced by the laser. For example a ruby crystal gives the ruby laser its name, and emits at 694.3 nm: the CO2 laser has carbon dioxide gas as its medium, and emits energy at 10,600 nm. Current medical lasers emit wavelengths from the ultraviolet to the mid-infrared portions of the spectrum. The medium is activated, or “pumped”, with some form of energy, which is usually either light from a flashlamp, or electricity. The stimulated emission of photons occurs in the medium, which are then are amplified in the laser cavity, consisting of the medium bounded in the front and rear by mirrors, and emitted from the front mirror in a beam of unique light energy: the beam is more or less parallel, known as collimation; the photons are precisely in step temporally and spatially, known as phase; and the photons are absolutely identical, all one color, known as monochromaticity. The sum of these three components is coherence, and it is coherence which gives a laser beam its uniquely high photon intensity, and allows a laser beam to be focused to very small spots.
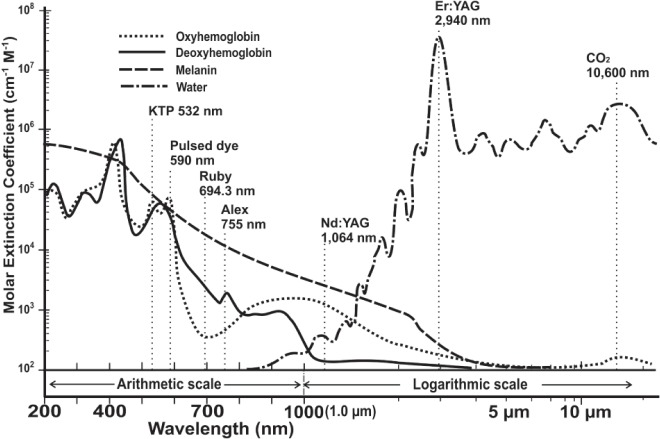
The main biological targets such as blood, melanin and water, absorb light energy very differently and have optimum absorption spectra depending on the wavelength of the incident photon energy (Figure 1). For visible light lasers and some near-infrared lasers, the main target chromophores are hemoglobin (consisting of oxy- and deoxy hemoglobin) and melanin, the former being found in vascular lesions and the latter in melanogesic lesions. For the CO2 laser at 10,600 nm, the only chromophore is water, as is also the case with the Er: YAG laser.
Fig. 1:
Wavelength is sown on the x-axis (200–400 nm, UV; 400 - 7 - nm, visible light; 700 – 2000 nm, nearinfrared; > 2000 nm, mid-infrared) with an arithmetic scale from 200 – 1000 nm (1.0 µm) and a logarithmic scale from 1.0 µm and longer. The y-axis denotes the coefficient of molar extinction in logarithmic units. This can be thought of as denoting the degree of penetration into tissue: the lower the value on the y-axis, the better the penetration of light at the given wavelength. The absorption spectra are for the laser tissue chromophores comprising the major biological pigments (oxy- and deoxyhemoglobin and melanin) and water. Note the high absorption in water at 10,600 nm, the wavelength of the CO2 laser.
When lasers are used in surgery and medicine, especially for the removal of any kind of pigment, the optimum approach is based on the principle put forward by Anderson and Parrish, known as ‘selective photothermolysis (SPTL)’ 2) which means using laser energy at high peak powers and short pulse widths to destroy the intended target alone while inflicting as little damage as possible on the surrounding tissue. Three key conditions must be met to achieve this: 1) The target must contain chromophores that absorb a specific laser wavelength; 2) These chromophores should not be found in the surrounding tissue; and 3) Minimization of damage to the surrounding tissue is achieved through the high peak powers and short pulse widths of the laser energy in the millisecond, microsecond or nanosecond domain, the latter being achieved by Q-switching the laser beam. The CO2 laser does not meet the criteria for SPTL because its chromosphere, water, exists uniformly in soft tissue, not just in the target zone. CO2 laser surgery could therefore be described as tissue-selective since only the target tissue is ablated with minimal involvement of surrounding normal tissue. The advantage of this is that the CO2 laser is not pigment-selective, and so both pigmented and non-pigmented skin lesions can be targeted with the CO2 wavelength.
Fundamentals of the CO2 laser 3
The CO2 laser was one of the earliest of the laser systems to appear. It was first developed in 1964 by Patel and colleagues working Bell Labs in the USA. It was quickly recognized as an ideal surgical laser because of its high water absorption, and many indications were pioneered by the late Professor Isaac Kaplan and others. 3)
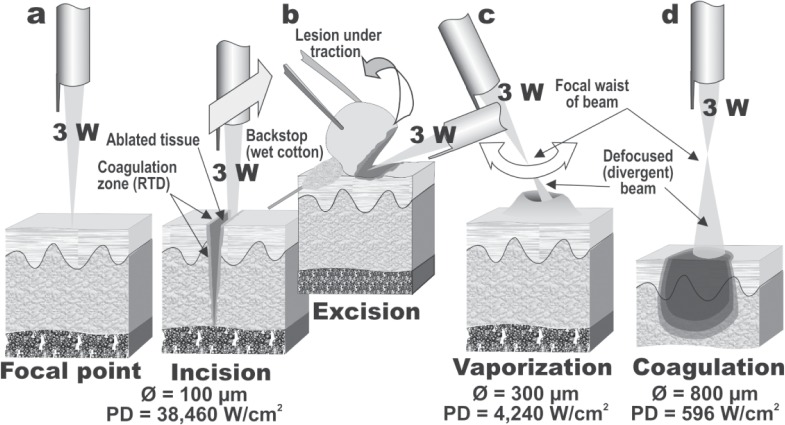
If a focusing lens is fitted to the handpiece as shown in Figure 2a, it will bring the collimated beam to a fine spot, concentrating all the photon energy of the beam into that area. The CO2 laser operates in the invisible infrared waveband, and an aiming beam is required to see where the treatment beam will impact tissue more recently from a visible red diode laser. The clinician can use the aiming beam to position the CO2 laser beam at the point of focus on the tissue. Focusing the laser produces an extremely high irradiance or power density, for example 3 W of CO2 laser energy focused to a 100 µm spot will have an irradiance of over 38 kW/cm2. This is sufficient for instant vaporization and ablation and if the handpiece is moved linearly, the target tissue will be cleanly incised, thus giving the CO2 laser its reputation as a laser scalpel. As an important point regarding the safety of the clinician, patient and all in the operating room when a CO2 laser is being used, this explosive reaction disperses a laser plume into the air consisting of steam and particulate matter, so a dedicated laser smoke evacuator is always recommended when using the CO2 laser.
Fig. 2:
Manipulation of the biological effect of the laser beam through moving the handpiece towards or away from the focal point of the beam, shown in 2a. (2b): Linear motion of the handpiece can give laser incision. Note the zone of RTD adjacent to the ablated tissue. The focused beam can also be used to excise tissue en bloc, with traction being applied to the lesion as indicated. Note the use of a wet cotton bud as a backstop to prevent damage to the normal tissue behind the lesion. (2c): By slightly defocusing the beam, and reducing the irradiance, bulk vaporization of tissue is achieved, again leaving a zone of RTD around the ablated tissue. (2d): By moving the handpiece further away from the tissue, a dramatic drop in irradiance is achieved which will result in nonablative coagulation of tissue. This is useful for swift hemostasis of small bleeding or oozing vessels.
The irradiance of a laser beam decreases in an inverse square ratio to the beam diameter, as the area of a laser beam is calculated using the formula π·r2, where π (Greek letter pi) is the constant 3.142, and r is the radius, or one-half of the diameter, of the spot size. Accordingly, simply by moving the handpiece away from the tissue and defocusing the beam, the clinician can make dramatic changes in the irradiance and instantly change from incision/excision mode (Figure 2b) to bulk vaporization (Figure 2c) to coagulation (Figure 2d) while leaving the output power of the laser unaltered.
When we consider the theoretical side of the CO2 energy/tissue reaction, the absorption coefficient of the 10,600 nm CO2 laser in water is around 5 × 102 cm−1 (reciprocal: 0.2 × 10−2 cm), and the theoretical depth of penetration is therefore from 2 × 10−5 m (20 µm). In other words, this means that when the carbon dioxide laser is used on biological tissue in vivo, all of the incident energy is absorbed in the tissue water down to a specific depth, thus making the CO2 laser a comparatively “safe” system, since the water in tissue quenches the beam and prevents deeper tissue damage. The actual depth reached and the damage volume (irradiated area × depth) will depend primarily on the irradiance and secondarily on the energy density, the latter being calculated in J/cm2 as irradiance (W/cm2) × exposure time (s).
The boiling point of water, which comprises about 70% of soft tissue, is 100°C. When the aqueous component of tissue is raised to 100°C by the incident CO2 laser energy, it is immediately converted to water vapor and gasifies, the volume expands, and as a result the skin (or other target) with its connective tissue, cells, cell membranes and intracellular organelles, is removed by fragmentation, vaporization, and ablation. There is always some degree of residual thermal damage at the borders of a CO2 laser ablated zone caused by decreasing levels of photothermal reaction in the tissue. Thermocoagulation occurs in this zone, at least to some extent, and small blood vessels are cauterized together with the surrounding tissue, achieving hemostasis and therefore a dry field. Larger blood vessels may not be cauterized, however, and hemorrhage will occur. In this case the quickest method is to use electrocautery to control the bleeding, because, even with defocusing, all of the CO2 energy will be absorbed in the water at the surface of the blood and will not reach the cut wall of transected vessel to coagulate it. As has been pointed out by Ohshiro in his laser apple concept, 4) all of the incident energy is not quenched in ablation of the tissue, and there is a series of temperature-dependent bioreactions in tissue associated with each laser shot which become less destructive as decreasing levels of the photons penetrate into the tissue from the point of impact (Figure 3). At the very perimeter of the beam, there is insufficient photon intensity to raise the temperature of the tissue, and this is the athermal photoactivation zone, also referred to as the simultaneous LLLT zone, 5) occuring concomitantly with the photodestructive effects. It is this zone which accounts for the beneficial results of laser surgery compared with the cold scalpel, such as less pain, less inflammation and good wound healing.
Fig. 3:
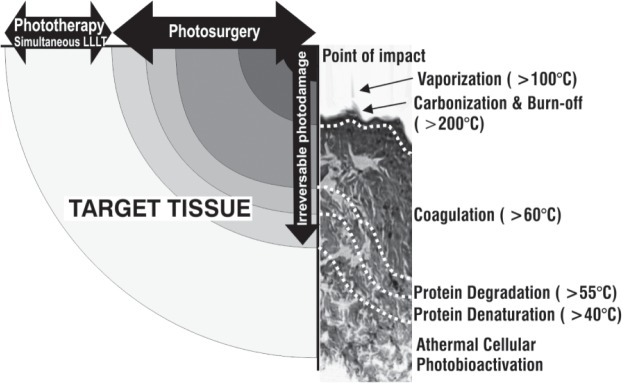
Temperature-dependent bioreaction zones from a surgical CO2 laser impact illustrated schematically with a typical histological pattern shown alongside the schematic. Please see the text for details.
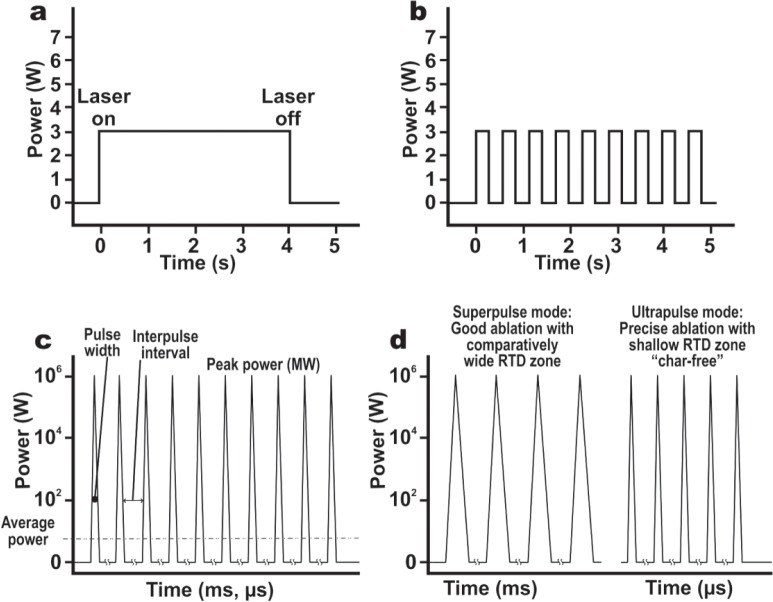
The CO2 laser has a number of temporal beam modes, each of which induces a different reaction in the tissue. The simplest mode is continuous wave (CW), in which the laser beam is emitted, operated for a specific time, and turned off, producing a pattern as in Figure 4a. A CW beam can be mechanically or electronically gated or chopped to provide a series of square waveforms as shown in Figure 4b. The output power is measured in watts and the irradiation time in seconds. More recent CO2 lasers have trains of very short high peak power pulses with a very long interpulse interval: this is referred to as quasi-CW, because the tissue ‘sees’ the average power, measured in W, although the peak power of each pulse might be in megawatts (MW) (Figure 4c). Quasi-CW has the advantage of allowing cleaner incision or bulk vaporization with less charring because each pulse is shorter than the thermal relaxation time of the target tissue (1 ms in the case of skin), so that optimum ablation is achieved with minimum heat build-up in the adjacent tissue. Quasi-CW can also be referred to as “superpulsing” or “ultrapulsing”, depending on the peak power, pulse width and interpulse interval.
Fig. 4:
Temporal mode of a laser beam illustrated schematically. (4a): Continuous wave (CW). (4b): Switched, chopped or gated CW beam, producing a series of square waveforms. These are not, strictly speaking, “pulses”, because a pulsed beam has a pulsewidth of 1 ms or less. (4c): A train of laser pulses with pulsewidths of from ms tons, with a long interpulse interval, is seen by the tissue the average power of the peak power and zero watts during the long interpulse interval. This known as quasi-CW. (4d): Quasi-CW can also be delivered as so-called “superpulsed” or “ultrapulsed” modes as illustrated. The ultrapulse dmode is also known as the char-free mode because it produces less charring due to the very short pulsewidths, much less than the thermal relaxation time of the target tissue.
Safety with the CO2 Laser
Safety with lasers is paramount for the well-being of the patient and all in the operating room. This is particularly so for the CO2 laser given its range of use in deliberate destructive indications. As the eye is the most vulnerable target for optical energy, eye protection is mandatory for all in the treatment room. However clear glass or plastic will stop the CO2 beam completely: this is why normal quartz fibers cannot be used as a beam transmission system for the CO2. Ordinary prescription lenses can be used, but dedicated glasses or goggles incorporating lateral shields are always recommended. The 10,600 nm beam is also a “safe” wavelength for the eye, or at least the retina, because all of the energy will be absorbed in the water in the cornea at the front of the eye. This makes the CO2 laser marginally safer than the visible and nearinfrared lasers, because the latter will pass straight through the cornea, be focused by the lens, and severely damage the macula/fovea complex in the retina.
Protecting the patient is also a priority, and for the CO2 laser this means draping the skin around the target area with damp drapes or gauze, and keeping these materials damp throughout the procedure because the CO2 energy is absorbed in water. If a beam of CO2 energy strikes dry gauze or cotton, the potential for starting a fire is very high. When using the CO2 laser to excise tissue held under traction, a backstop of some kind should be employed, either a dampened wooded tongue depressor or a damp cotton bud depending on the site. Finally, as already discussed, the laser plume created by the CO2 laser can contain potentially harmful substances such as viable viral particles, 7) so the use of a dedicated smoke evacuator is extremely important. The use of masks alone will not suffice.
Indications of the carbon dioxide laser
The CO2 laser has many indications, and if one listened to the late Professor Isaac Kaplan, the CO2 laser could (and should, in his opinion) be used for almost anything surgical. 6) Some of the main indications will be covered in this subsection.
Skin lesions
The clinical indications of the CO2 laser comprise a case-by-case combination of the incision, vaporization and coagulation techniques, the latter also accomplishing hemostasis and ensuring a dry field. As an example, Figure 5a shows a raised nevus cell nevus or mole on the cheek of 23-year-old female. After local injection anesthesia, the CO2 laser (SmartXide laser, DEKA, Italy) was applied with a small spot size to vaporize and flatten the lesion (2.4 W, 50 Hz) (Figure 5b). The final result 10 weeks after treatment is seen in Figure 5c with removal of the lesion and acceptable cosmesis. In the case of any such lesion, a definitive histopathological diagnosis must be made including dermoscopic evaluation to rule out the possibility of the target nevus being a malignant melanoma, for example, and to confirm the lesion is benign.
Fig. 5:
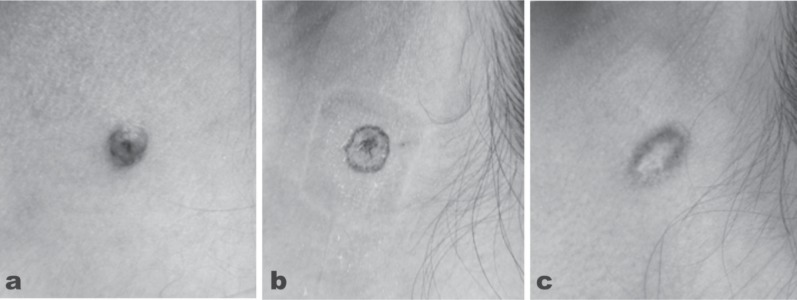
A case of nevus cell nevus on the left posterior cheek of a 23-year old female treated with CO2 laser vaporization. (5a): Baseline findings. The lesion was determined to be benign. (5b): The findings immediately after treatment with the slightly defocused CO2 laser in quasi-CW, output power of 2.4 W, frequency 50 Hz. (5c): The area of interest 10 weeks after treatment with removal of the lesion and good cosmesis.
(SmartXide laser, DEKA, Italy)
Another ideal indication of the CO2 laser is seborrheic keratosis, which can be selectively and precisely vaporized to remove the melanotic cornification of this type of lesion in controlled layers. With topical anesthesia, such as EMLA cream or Penles (lidocaine) tape, papular pedunculated lesions such as skin tags present a very good target for the CO2 laser. In CO2 treatment of skin tags traction is applied to the tag and the precisely focused beam of the CO2 laser is used to excise the lesion at its base. During treatment, an assistant should hold a moistened cotton bud or similar item at the back of the tissue being excised to act as a backstop. If spotty bleeding occurs, the blood is wiped off with dry gauze and the area painted with the defocused CO2 beam to achieve hemostasis.
Syringomas and xanthelasmas around the eye are yet another skin lesion which can also be removed under topical anesthesia with CO2 laser treatment. Even some malignant tumors are treatment indications for the CO2, such as the basal cell epithelioma in which there is little tendency to metastasize. Open surgery has long been one of the options for this condition, and can be replaced with the CO2 laser giving the advantage of good hemostasis once the lesion is removed, compared with removal with the cold scalpel.
Warts
The common wart, verruca vulgaris, has a predilection to appear on the fingers and toes, particularly of children. The causative virus is a member of the human papilloma virus (HPV) family, most commonly types 2 and 4, but others may be involved. The structure of the wart is distinctive with hyperkeratosis of the stratum corneum, thickening of the stratum granulosum and stratum spinosum (acanthosis) and elongation of the papillae at the dermoepidermal junction giving an almost columnar appearance. An active blood supply is found at the dermoepidermal junction, and because Verruca vulgaris is infectious, removal by incision very often resulted in reinfection along the path of the incision. Cryotherapy with liquid nitrogen is often used, but many treatments are required. The CO2 laser provided a very good answer, under local injection anesthesia, and could either be used to ablate and vaporize the central portion of the wart till normal tissue architecture was seen, then with a slightly more defocused beam the outer ‘crater’ was then flattened. 8) An alternative method was to apply traction to the wart and excise it en bloc from the skin, finishing off by vaporizing the ‘root’ till normal skin architecture appeared. 9) High efficacy rates with less recurrence were reported for both methods, but it a dedicated smoke evacuator to suction away the laser plume is essential when using the CO2 laser to remove any kind of wart. Active viral DNA has been identified in the plume, 7) and this has the potential to infect anyone with whom the plume comes in contact, such as the surgeon and the patient. Verruca plantaris is another type of wart (mainly HPV 1) particularly associated with the soles of the feet and toes. These are often extremely recalcitrant, but respond well to CO2 vaporization and ablation. Figure 6a shows the result immediately after CO2 laser ablation of typical multiple plantar warts in a 28-year-old male (SmartXide laser, DEKA, Italy: quasi-CW, 10 W, 100 Hz) with a very good result 3 weeks after the treatment (Figure 6b). There has been no recurrence at the time of writing, 6 months after the single treatment session.
Fig. 6:
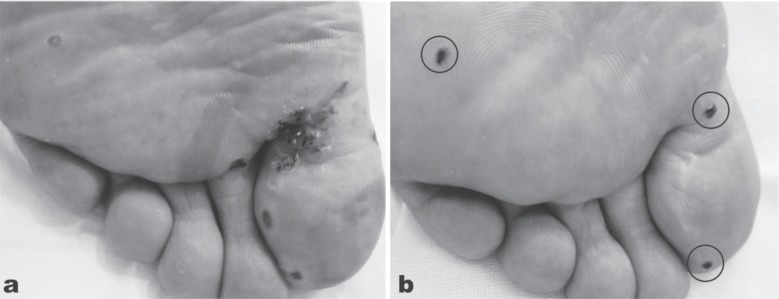
Multiple planar warts in a 28-year-old male treated with CO2 laser ablation. (6a): Immediately after irradiation the warts have been vaporized down into normal tissue architecture using the slightly defocused beam of the CO2 laser in quasi-CW, 10 W output power, frequency of 100 Hz. (6b): The result 3 weeks after the treatment. Some small touch-up treatments were required (circles) but otherwise with no sign of recurrence. The patient is wart-free some 6 months post-treatment. (SmartXide laser, DEKA, Italy)
The major advantage of using the CO2 laser over conventional approaches is the minimized charring with faster healing which increases patients' motivation for treatment, but caution is necessary with regard to recurrence if the virus persists in the surrounding tissue, and to the fact that extensive ablation may result in scarring. The CO2 laser was recognized very early on as being very effective in treating the more disturbing anogenital warts (condyloma acuminatum, mainly HPV 6 and 11), with high efficacy rates, lower recurrence rates compared with conventional approaches, and high patient satisfaction. 10) Because anogenital warts have a predilection for the external genitalia, patients sometimes are reluctant to make frequent hospital visits so swift treatment with the carbon dioxide laser under local anesthesia is preferable.
Toenail disease
An indication which has attracted attention recently is the use of the CO2 lasers on nails, in particular toenails. Diseases of the toenail, such as onychodystrophy, 11) and the often extremely painful ingrown toenail 12, 13) lend themselves well to CO2 laser treatment, especially considering the ample blood supply to the toes and in particular, the big toe. Onychodystrophy can be treated by irradiating the nail bed, and ingrown toenails with a wedge resection, removing the outer edges of the ingrowing toenail and the involved tissue en bloc. Conventional approaches usually involved removal of the entire nail, and the postoperative hemorrhage and pain were extensive. In CO2 laser wedge resection, the focused beam is applied to perform the wedge resection, defocusing as required to control any bleeding.
Antiaging
Skin rejuvenation has recently become a hot topic, and very much a patient-driven procedure as prospective patients learn through the media of new approaches to rejuvenate intrinsically- and extrinsically-aged skin. In the early 1990s the CO2 laser was hailed as a major advance on existing conventional methods to ablate the epidermis, thereby inducing regrowth of a young-looking epidermis and stimulating collagenesis and remodeling in the dermis. The development of pulsed CO2 lasers used with a computerized scanner was another large step forward, and results were very good to excellent. 14,15) The aim of full face ablative resurfacing was to ablate the epidermis cleanly off the dermis and leave a zone of partially-coagulated tissue in the dermis, which would kick-start the wound healing process and lead to collagenesis followed by remodeling. The coagulation could not be too complete as that would simply lead to necrotic sloughing rather than wound healing. This zone was known as residual thermal damage (RTD) and became the holy grail of laser rejuvenation through resurfacing. 16) In fact, ablative full-face laser resurfacing is still regarded as the gold standard for facial rejuvenation of severely photoaged skin. 17) However, this application of the CO2 laser came at the price of severe and unsightly oozing and crusting in the first few days after the procedure followed by the potential unwanted sequelae of prolonged erythema, infection, scarring and herpes simplex lesions 18) and a very long patient downtime.
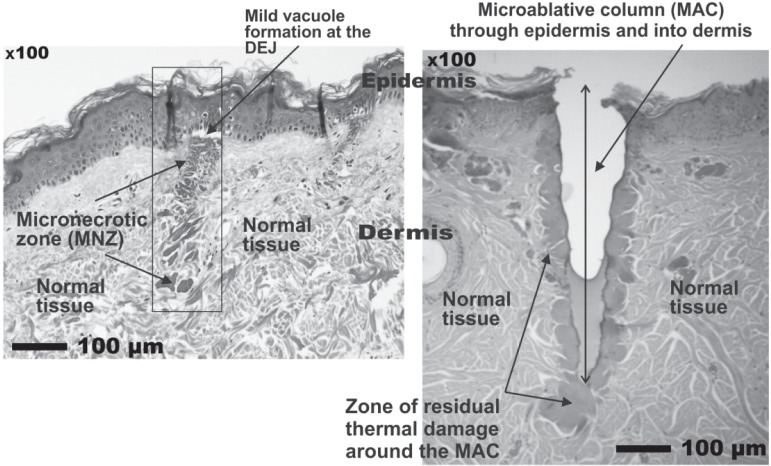
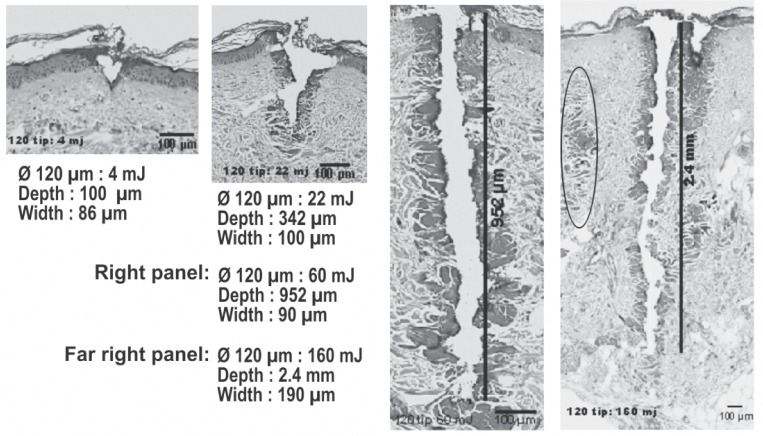
In the 2000s, the problem of the side effects following laser ablative resurfacing was addressed by the launching of a number of so-called nonablative skin rejuvenation lasers (not including the CO2 laser), which were based on the reasonable theory of delivering controlled dermal damage to achieve wound healing and remodeling under a cooled and intact epidermis to eliminate downtime. Sadly the theory was not borne out in clinical practice, and even though dermal histology was shown to be well-improved, it was not echoed by improvement of the epidermis: patient satisfaction was generally very low. 19) To meet this problem, the technology of fractionating the laser beam into a myriad of microbeams via a scanner was developed as a bridge between the frankly ablative and completely nonablative approaches and fractional laser rejuvenation made its first appearance. 20) To minimize downtime the first fractional systems used a nonablative approach with 1540 nm Er:glass lasers which produced micronecrotic zones (MNZs) of coagulation in the dermis under a more or less intact epidermis. However, after the initial excitement associated with the promise of the new technology, patient satisfaction was found to be much less than anticipated. This was probably because the fractional nonablative approach left the epidermis intact, and it became recognized that some damage to the epidermis was required for epidermal renewal. The next step was therefore the introduction of ablative fractional technology with the CO2 laser, and the treatment wheel had turned 360° back to ablation of the epidermis. 21) Fractional CO2 laser rejuvenation can be delivered with a variety of microbeam sizes and densities via a scanner system to meet the individual requirements of each indication on a patient-by-patient basis. However, unlike full face ablation, the microablative columns (MACs), which involved both the epidermis and the dermis, were surrounded by normal undamaged skin so that reepithelization could be achieved in 2–3 days, and wound healing could occur more rapidly under the protection of the new skin. The histology in Figure 7 illustrates in histological specimens the difference between the immediately post-treatment condition of the MNZs from the nonablative approach and the MACs of the ablative approach. Looking at the histology, the reader can understand why the nonablative approach has less downtime than the more aggressive ablative approach, but may also understand why the ablative approach is superior for the more severe examples of the conditions it is used to treat, including rhytides and other sequelae of aging skin, and additionally for scar revision including acne scars. 22) The CO2 laser is usually associated with limited penetration into tissue due to the high absorption in water of its 10,600 nm wavelength. However, by increasing the irradiance with a very small spot size in the fractional approach, such as 120 µm, and then using higher or lower pulse energies, MAC depths of over 2 mm can be easily achieved (Figure 8). The different depths have different indications, with the shallow MACs giving good results for very superficial skin conditions, and the deep MACs being very effective to reach the deep reticular ermis to help in scar remodeling. This is how the clinician can make science work for him or her to achieve the desired clinical effect, rather than being controlled by the preconceived ideas surrounding a particular laser system. The layer of RTD surrounding all the MACs is also clear, as is the unharmed dermal and epidermal tissue between the MACs. Figure 9 shows the good results of a fractional CO2 treatment in a 32 year-old acne scar patient (SmartXide laser, DEKA, Italy: Fractional mode handpiece, 20 W, microbeam spacing of 600 µm, triple multipulse) 3weeks after the 3 treatment sessions.
Fig. 7:
Histological specimens (hematoxylin and essoin stain) showing the differences between the nonablative fractional effect in tissue (left panel) and the ablative effect (right panel). The zones of normal tissue between the MNZs and MACS is clearly seen in both specimens. Histology courtesy of Prof. WS Kim, Department of Dermatology, Sungkyunkwan University, Seoul, South Korea, scale bars as shown. (eCO2 system, Lutronic. Goyang, South Korea)
Fig. 8:
Despite the high absorption in tissue water, the CO2 microbeams are capable of a MAC deeper than 2 mm in human tissue in vivo (hematoxylin and eosin staining). With all other parameters remaining the same, changing the pulse energy alters the penetration depth achieved. from the left, the pulse energies were 4 mJ, 22 mJ. 60 mJ and 160 mJ with MAC depths ranging from 100 µm to 2.4 mm. The zone of RTD, however, does not change as dramatically as the depth, maintaining the advantages of fractionation of the beam while still inducing active wound repair and tissue remodeling. The 160 mJ specimen is interesting, because it actually shows two MACs in 3-dimensions, one behind and to the right of the main MAC in this 2-dimensional specimen. Only the very top of the ablative zone is seen, but the RTD is clearly visible extending down into the dermis. Another MAC-associated RTD zone appears to the left of the main MAC, oval marking. Histology courtesy of Prof. WS Kim, Department of Dermatology, Sungkyunkwan University, Seoul, South Korea, scale bars as shown. Note that the right hand image has been reduced in size to fit the illustration, as can be seen from the size of the 100 µm scale bar. (eCO2 system, Lutronic. Goyang, South Korea)
Fig. 9:
(9a): Baseline appearance of acne scars on the right cheek of a 32-year-old female patient. (9b): Result 3 weeks after 3 treatments separated by 4 weeks with a fractional CO2 laser, output power of 20 W, microbeam spacing of 600 µm. The re areas are typical of the post-acne redness seen especially in Asian skin types III/IV following treatment of acne scars, and are part of the wound-healing process where active revascularization is more clearly seen under an immature epidermis. (SmartXide laser, DEKA, Italy)
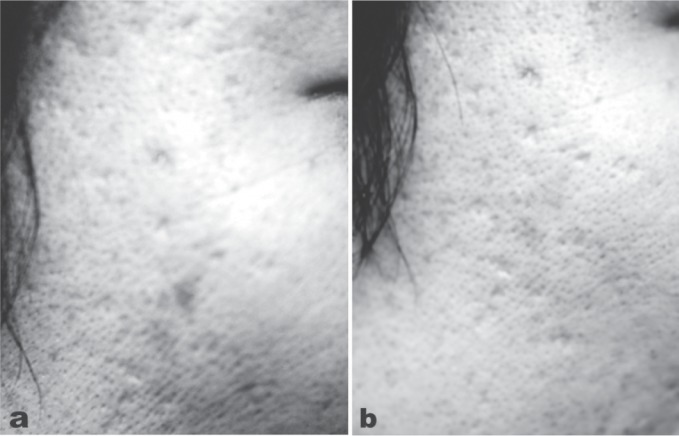
A major limitation of the full laser ablative approach was that off-face use was at the very least limited owing to the comparative paucity of pilosebaceous units in other areas of the body. No such limitation exists for the fractional CO2 laser and as an example, rejuvenation of the hand and arm with a fractional CO2 system in a 72-year-old female is shown in Figure 10. (eCO2 system, Lutronic, Goyang, South Korea: 300 µm diameter microbeam, pulse energy 12 mJ/cm2). Furthermore, in a controlled study the fractional CO2 laser has additionally shown promise in prophylaxis against hypertrophic scar formation in early application after a thyroidectomy, 23) so it can be used both to remodel existing scars, and help prevent unwanted scar formation in new wounds.
Fig. 10:
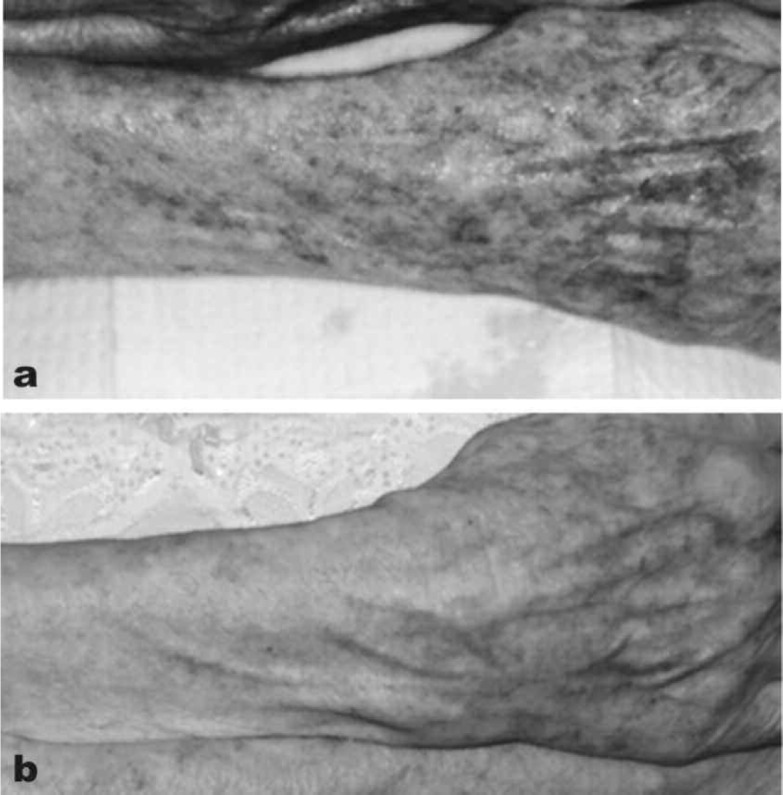
Fractional CO2 laser applied off the face in the rejuvenation of the arms of this 72-year-old lady. (10a): Baseline appearance of the right forearm and back of the hand. (10b): 4 weeks after 1 session of fractional CO2. (eCO2 system, Lutronic, Goyang, South Korea: Photography courtesy of Mark Rubin MD, Los Angeles, CA, USA)
Conclusions
The CO2 laser remains a workhorse across many specialties. In the hands of an appropriately trained practitioner, the CO2 laser offers a very large range of dermatological indications, with great precision for procedures involving incision, excision, vaporization and coagulation. The residual thermal damage deposited by the CO2 laser beam helps to ensure a dry field, limits blood loss and swiftly induces the wound healing and remodeling process. The high water content of soft tissue both makes it an excellent target for the 10,600 nm wavelength, and also offers a degree of inherent safety by preventing deeper penetration at appropriate parameters. Furthermore, the addition of fractional technology has opened up aesthetic dermatological indications to the CO2 laser practitioner, offering proven applications in rejuvenation of photoaged skin both on and off the face, and scar revision especially for acne scars. Technological advances which continue in CO2 laser design and delivery will ensure that the role for this useful wavelength in dermatological practice will continue to expand.
References
- 1: Itzkan I, Drake EH: History of lasers in medicine. In: Arndt KA, Dover JS, Olbricht SM, eds. Lasers in Cutaneous and Aesthetic Surgery. Philadelphia: Lippincott-Raven, Inc. 1997: 3-10 [Google Scholar]
- 2: Anderson RR, Parrish JA: Selective photothermolysis, precise microsurgery by selective absorption of pulsed radiation. Science, 1983; 220: 524-527 [DOI] [PubMed] [Google Scholar]
- 3: Kaplan I. The CO2 surgical laser. Photomed Laser Surg, 2010; 28: 847-848 [DOI] [PubMed] [Google Scholar]
- 4: Ohshiro T: The laser apple: a new graphic representation of medical laser applications. Laser Therapy, 1995; 8: 185-190 [Google Scholar]
- 5: Ohshiro T: Auto-simultaneous laser treatment: a new effect-based classification. Laser Therapy, 2005; 14: 11-17 [Google Scholar]
- 6: Kaplan I: The CO2 laser as a versatile surgical modality. Laser Therapy, 2007; 16: 25-38 [Google Scholar]
- 7: Sloan K, Haberman H, Lynde CW: Carbon dioxide laser-treatment of resistant verrucae vulgaris: retrospective analysis. J Cutan Med Surg, 1998. 2: 142-145 [DOI] [PubMed] [Google Scholar]
- 8: Oni G, Mahaffey PJ: Treatment of recalcitrant warts with the carbon dioxide laser using an excision technique. J Cosmet Laser Ther, 2011; 13: 231-236 [DOI] [PubMed] [Google Scholar]
- 9: Ferenczy A, Bergeron C, Richart RM: Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol, 1990; 75: 114-118 [PubMed] [Google Scholar]
- 10: Kryger-Baggesen N, Falck Larsen J, Hjortkjaer Pedersen P: CO2 laser treatment of condylomata acuminata. Acta Obstet Gynecol Scand, 1984; 63: 341-343 [DOI] [PubMed] [Google Scholar]
- 11: Lim EH, Seo YJ, Lee JH, Im M: Onychodystrophy treated using fractional carbon dioxide laser therapy and topical steroids: new treatment options for nail dystrophy. Dermatol Surg, 2013; 39: 1931-1933 [DOI] [PubMed] [Google Scholar]
- 12: Omi T, Honda M, Kawana S, Hata M, Yamamoto K: Ultra Pulse CO2 laser for ingrown nails. Japanese Journal of Pediatric Dermatology, 2000. 19 121-124 [Google Scholar]
- 13: Lin YC, Su HY: A surgical approach to ingrown nail: partial matricectomy using CO2 laser. Dermatol Surg, 2002; 28: 578-580 [DOI] [PubMed] [Google Scholar]
- 14: Waldorf HA, Kauvar AN, Geronemus RG: Skin resurfacing of fine to deep rhytides using a charfree carbon dioxide laser in 47 patients. Dermatol Surg, 1995; 21: 940-946 [DOI] [PubMed] [Google Scholar]
- 15: Fitzpatrick RE, Goldman MP, Satur NM, Tope WD: Pulsed carbon dioxide laser resurfacing of photoaged facial skin. Arch Dermatol. 1996. April; 132(4):395-402 [PubMed] [Google Scholar]
- 16: Ross EV, Barnette DJ, Glatter RD, Grevelink JM: Effects of overlap and pass number in CO2 laser skin resurfacing: a study of residual thermal damage, cell death, and wound healing. Lasers Surg Med, 1999; 24: 103-112 [DOI] [PubMed] [Google Scholar]
- 17: Chwalek J, Goldberg DJ: Ablative skin resurfacing. Curr Probl Dermatol, 2011; 42: 40-47 [DOI] [PubMed] [Google Scholar]
- 18: Ragland HP, McBurney E: Complications of resurfacing. Semin Cutan Med Surg, 1996; 15: 200-207 (Review). [DOI] [PubMed] [Google Scholar]
- 19: Goldberg DJ: Nonablative dermal remodeling: does it really work? Arch Dermatol, 2002; 138: 1366-1368 [DOI] [PubMed] [Google Scholar]
- 20: Geronemus RG: Fractional photothermolysis: current and future applications. Lasers Surg Med, 2006; 38: 169-176 [DOI] [PubMed] [Google Scholar]
- 21: Alexiades-Armenakas MR, Dover JS, Arndt KA: Fractional laser skin resurfacing. J Drugs Dermatol, 2012; 11:1274-1287 [PubMed] [Google Scholar]
- 22: Omi T, Kawana S, Sato S, Bonan P, Naito Z: Fractional CO2 laser for the treatment of acne scars. J Cosmet Dermatol, 2011; 10: 294-300 [DOI] [PubMed] [Google Scholar]
- 23: Jung JY, Jeong JJ, Roh HJ, Cho SH, Chung KY, et al. : Early postoperative treatment of thyroidectomy scars using a fractional carbon dioxide laser. Dermatol Surg. 2011; 37: 217-223 Epub 2011 Jan 27. [DOI] [PubMed] [Google Scholar]