Abstract
Objectives
We investigated whether lower 25-hydroxyvitamin D and higher serum parathyroid hormone concentrations are associated with incident hypertension.
Background
Disturbances in vitamin D metabolism are plausibly related to hypertension.
Methods
The Multi-Ethnic Study of Atherosclerosis is a community-based prospective cohort with baseline measurements obtained between 2000 and 2002. We studied 3,002 men and women free of prevalent cardiovascular disease and hypertension, aged 45–84 years at baseline. Serum 25-hydroxyvitamin D and intact parathyroid hormone were measured from previously frozen baseline samples using liquid chromatography-mass spectroscopy and a 2-site immunoassay, respectively. We used a complementary log-log model with interval censoring to estimate hazard ratios and 95% confidence intervals for 25-hydroxyvitamin D and parathyroid hormone concentrations with incident hypertension through 2010.
Results
During a median follow-up of 9.0 years, 41% of the cohort (n=1,229) developed hypertension. Mean serum 25-hydroxyvitamin D was 26.3±11.2 ng/ml and mean parathyroid hormone was 41.2±17.3 pg/ml. Compared with 25-hydroxyvitamin D ≥30 ng/mL, 25-hydroxyvitamin D <20 ng/ml was associated with a greater hypertension risk 1.28 (1.09–1.50), although the association was attenuated and not statistically significant after adjusting for potential confounders 1.13 (0.96–1.33). Compared with parathyroid hormone <33 pg/ml, parathyroid hormone ≥65 pg/ml was associated with a significantly greater risk of hypertension 1.27 (1.01–1.59), after adjusting for potential confounders.
Conclusions
Lower 25(OH)D concentrations were not associated with a greater risk of incident hypertension. Higher serum PTH concentrations showed a significant, but statistically marginal, relationship to the development of hypertension. These findings will require further confirmation.
Keywords: vitamin D, parathyroid hormone, incident hypertension, prospective study
Introduction
Hypertension is a major risk factor for cardiovascular disease with established complications of stroke, myocardial infarction and heart failure (1). Despite the high worldwide prevalence of hypertension and known clinical consequences, the underlying causes of hypertension are not fully elucidated. Identifying novel risk factors for hypertension is important for understanding the etiology of and suggesting possible new treatment targets to reduce the high burden of morbidity and mortality.
Disturbances in vitamin D and parathyroid hormone (PTH) metabolism are plausibly related to hypertension through diverse mechanisms. Primary hyperparathyroidism is long recognized for its association with prevalent hypertension, and more recent prospective studies demonstrate associations of greater serum PTH concentrations with higher blood pressure in men (2) and with incident hypertension in the general population (3,4). Vitamin D deficiency promotes secondary hyperparathyroidism, increases the secretion of aldosterone and stimulates the renin-angiotensin system (5). Lower serum 25-hydroxyvitamin D (25(OH)D) concentrations are associated with higher blood pressure and hypertension risk (6,7). Furthermore, disturbances in both serum 25(OH)D and PTH are related to arterial stiffness and vascular dysfunction, which are strong determinants of future hypertension (8).
Despite intriguing potential physiologic connections of biomarkers of vitamin D and PTH metabolism with blood pressure, few studies have prospectively evaluated associations of serum 25(OH)D and PTH concentrations with the initial development of hypertension (3,4,6,7,9,10). Previous studies have assessed single biomarkers, used self-reported hypertension, did not investigate both genders, or were conducted among racially homogenous populations. Understanding prospective associations of vitamin D and PTH with the onset of hypertension would provide insight into disease mechanisms and may be important for developing future preventive strategies.
We measured serum 25(OH)D and PTH concentrations in 3,002 individuals from a community-based, middle-aged multi-ethnic cohort, free of clinical hypertension and cardiovascular diseases. We hypothesized that lower serum 25(OH)D and higher serum PTH concentrations would be associated with incident hypertension during long-term follow-up.
Methods
Design and sample
The Multi Ethnic Study of Atherosclerosis (MESA) is a prospective study of cardiovascular disease among 6,814 community-dwelling residents aged 45 to 84 years who were free of cardiovascular disease at baseline. Between 2000 and 2002, participants were recruited from 6 centers across the USA with different self-reported ethnical backgrounds (11). Participants with any previous history of cardiovascular diseases were excluded at baseline as described previously (11). MESA included 4 follow-up examinations at approximately 18 months, 36 months, 4 years, and 10 years from the baseline examination between 2002–2010 along with periodic telephone contacts every 6–12 months.
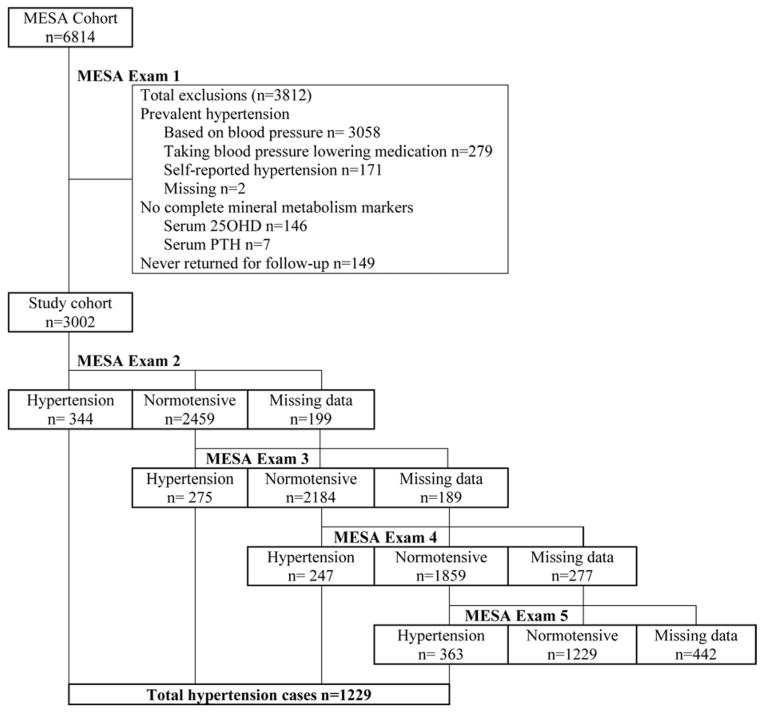
We excluded MESA participants who had baseline hypertension, defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg (n=3,058), or use of any antihypertensive medication (n=279). We further excluded for self-reported hypertension (n=171), missing data for anti-hypertension medication use (n=2), or inadequate sample volume for measuring 25(OH)D or PTH (n=153). None of the remaining participants used lithium medication or loop diuretics, which may alter calcium metabolism. Finally, we excluded participants who did not return for any follow-up MESA examinations (n=149) leaving a final analytic sample of 3,002 participants, free of hypertension at baseline with complete serum 25(OH)D and PTH measurements (Figure 1). Compared with participants excluded, included participants were younger (58.6 vs. 64.9 years) and less likely to be Black (19.6% vs. 34.2%). Study protocols were approved by the institutional review board at each participating institution and all participants granted informed consent.
Figure 1.
Flow diagram of incident hypertension ascertainment for each MESA examination
Measurements of vitamin D and PTH
We measured serum 25(OH)D and PTH concentrations in baseline serum samples that were collected in the morning after an 8–12-hour overnight fast. Samples were stored at −80°C and thawed before analysis in 2011. We measured total serum 25(OH)D (25OHD2 + 25OHD3) using liquid chromatography-mass spectroscopy on a Quattro Micro mass spectrometer (Waters, Milford, USA) (inter-assay CV<3.4%). We verified calibration of serum 25(OH)D concentrations using SRM 972 from the National Institutes of Standards and Technology. We measured intact serum PTH concentrations using an automated 2-site immunoassay (Beckman-Coulter, Inc., Brea, USA) (inter-assay CV between 3.4–6.1%) (12).
Determination of Incident hypertension
MESA study personnel assessed blood pressure and antihypertensive medications at each examination. During each examination, study personnel obtained 3 blood pressure measurements 5 minutes apart with the participant seated using an automated Dinamap sphygmomanometer (Critikon, General Electric, Madison, Wisconsin). We calculated the mean of the second two measurements for analysis. MESA study personnel instructed participants to bring all of their medications to each examination, and assessed medication use by the inventory method. We defined incident hypertension as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or the use of any antihypertensive medication during any of the follow-up examinations.
Covariates
Study personnel collected detailed data regarding demographics, co-morbidities, and medication use at enrollment (11). We measured serum phosphate and calcium to incorporate other important factors of the mineral metabolism in our analyses. We measured serum phosphate using a timed-rate colorimetry reaction and serum calcium by indirect ion selective electrode (12). Participants completed questionnaires to determine race/ethnicity, smoking status, alcohol consumption, physical activity, income and attained education (11). The highest level of attained education was categorized into 3 categories: some high school or less, completed high school, or completed college or more. Body mass index (BMI kg/m2) categories were based on measured height and weight, defined as normal weight (<25 BMI), overweight (25–30 BMI) and obese (≥ 30 BMI). Type 2 diabetes was defined as a reported history of diabetes, use of any diabetes medication, or a blood glucose level ≥7 mmol/l (≥ 126 mg/dl). Impaired fasting glucose was defined by a glucose level of 5.6–7.0 mmol/l (100–125 mg/dl). Estimated glomerular filtration rate (eGFR) was calculated from serum calibrated creatinine and cystatin C concentrations using the CKD-EPI 2012 equation. We defined chronic kidney disease (CKD) as eGFR <60 ml/min/1.73m2. The Laboratory for Clinical Biochemistry Research measured high-sensitivity C-reactive protein (CRP) levels by using the BN II nephelometer.
Statistical Analysis
We adjusted serum 25(OH)D concentrations for season using previously established methods in MESA (13). We examined Spearman’s correlations between serum 25(OH)D and PTH concentrations to describe univariate associations. We tabulated baseline participant characteristics by widely used categories of 25(OH)D (<20 ng/ml; ≥20–30 ng/ml; and ≥30 ng/ml) and by PTH categories that combined tertiles with a threshold value of 65 pg/ml (<33 pg/ml; ≥33–44.2 pg/ml; ≥44.2–65 pg/ml; ≥65 ng/ml). The PTH value of 65 pg/ml represents the upper limit of normal for this assay based on the central 95% of values from 43 normal laboratory personnel who had normal 25(OH)D concentrations in March 2005 (12). We defined primary hyperparathyroidism as a serum PTH concentration ≥65 pg/ml plus a serum calcium concentration >10.2 mg/dl (14).
We calculated risk time for incident hypertension as elapsed time from the baseline examination until the first examination at which incident hypertension was diagnosed, the last MESA examination, or the last examination before loss to follow-up, whichever came first. We calculated unadjusted hypertension rates as the number of events per 100 person-years.
We used a complementary log-log model to estimate hazard ratios for 25(OH)D and PTH with incident hypertension (15). This model is very familiar to a Cox proportional hazards model, but is able to account for the time between the discrete time points since the exact date of the development of hypertension is unknown. To account for the time that hypertension could occur between the discrete time intervals, we used interval-censored analyses to estimate hazard ratios, under the assumption that the hazard is constant within each interval but can vary across intervals. This assumption of piecewise-constant hazards is preferred for discrete time intervals since risk estimates can change over time. We used a Wald-test to calculate P-values and 95% confidence intervals. Based on visual inspection of the hazard functions by Schoenfeld residuals, we found no evidence to contradict the proportionality assumption.
We adjusted serial nested models for demographic variables and known hypertension risk factors. In the first model we adjusted for age, sex, race, and clinic site. In the second model we added adjustment for smoking (yes/no), education (3 categories), total family income (5 categories), diabetes status (3 categories), BMI, low density lipoprotein (LDL)-cholesterol, non-steroidal anti-inflammatory medication and cyclooxygenase-2 inhibitor use, and physical activity. We further adjusted models of PTH for serum 25(OH)D (ng/ml) because vitamin D deficiency is a known risk factor for hyperparathyroidism. To separately study potential confounding by kidney function, we adjusted the final model for urinary albumin creatinine ratio (ACR) and eGFR. We analyzed age, BMI, LDL-cholesterol, physical activity, eGFR and ACR as continuous variables in the multivariate models. Finally, we assessed dose-response relations of 25(OH)D and PTH with incident hypertension using restricted cubic spline models adjusted for covariates specified in model 3.
We looked for potential effect modification by race, sex, BMI, CKD and interaction by 25(OH)D with PTH using interaction terms and present combined results for the total study sample if estimates were similar. To test the stability of the estimates we conducted several sensitivity analyses: 1) excluding participants with incident hypertension diagnosed solely by incident use of antihypertensive medications because medication maybe prescribed to participants with diabetes but without hypertension, 2) excluding participants with borderline hypertension at baseline, defined as systolic blood pressure ≥130 mmHg and diastolic blood pressure ≥85 mmHg at baseline, for a meaningful change in blood pressure. We conducted analyses using IBM SPSS 20.0 for Windows 07 (SPSS Inc., Chicago, USA).
Results
Study sample
Among the 3,002 MESA participants, mean age was 59±9.7 years and 53% were female. After completing the baseline examination, 2,258 MESA participants completed all 4 subsequent examinations, 487 participants completed 3, 151 participants completed 2, and 106 participants completed 1 follow-up examination. Approximately 15% of participants were current smokers, 6% had type 2 diabetes, 6% had CKD and <1% (n=17) had primary hyperparathyroidism. Excluded participants who did not attend any follow-up visit (n=149) were older (61 vs. 59 years), more likely to be male (53 vs. 47%), had higher systolic blood pressure (116 vs. 114 mmHg), higher prevalence of diabetes (9 vs. 6%) were less likely to be White (28 vs. 43%), and more likely to be Hispanic (40 vs. 23%).
Description of serum 25(OH)D and PTH concentrations
The distribution of serum 25(OH)D and PTH concentrations were both unimodal and approximately symmetric with mean values of 26.3±11.2 ng/ml and 41.2±17.3 pg/ml. Serum 25(OH)D concentrations were inversely correlated with serum PTH concentrations (correlation coefficient −.34). Lower serum 25(OH)D concentrations were related to younger age, Black and Hispanic race, lower attained education, higher prevalence of type 2 diabetes, current smoking, higher BMI, lower HDL cholesterol, higher PTH, eGFR and CRP concentrations (Table 1). Higher serum PTH concentrations were associated with older age, Black and Hispanic race, higher BMI, systolic blood pressure, and CRP concentrations, and lower 25(OH)D and phosphate concentrations.
Table 1.
Characteristics of 3,002 participants by serum 25-hydroxyvitamin D and parathyroid hormone concentrations
| Variable | Serum 25OHD (ng/ml)* | Serum PTH (pg/ml)* | |||||
|---|---|---|---|---|---|---|---|
| <20 | ≥20–30 | ≥30 | <33 | ≥33–44.2 | ≥44.2–65 | ≥65 | |
|
| |||||||
| Demographic data | |||||||
| N | 922 | 1028 | 1052 | 1056 | 912 | 789 | 245 |
| Age (years) | 57±9.4 | 59±9.7 | 60±9.8 | 58±9.4 | 58±9.8 | 60±9.7 | 60±10.0 |
| Females (%) | 497(54) | 511 (50) | 572 (53) | 566 (54) | 463 (51) | 410 (52) | 141 (58) |
| Race (%) | |||||||
| White | 204(22) | 441 (43) | 661 (63) | 540 (51) | 407 (45) | 287 (36) | 72 (29) |
| Black | 391 (43) | 139 (14) | 58 (5) | 125 (12) | 173 (19) | 208 (26) | 82 (34) |
| Chinese | 94 (10) | 187 (18) | 135 (13) | 193 (18) | 131 (14) | 83 (11) | 9 (4) |
| Hispanic | 233 (25) | 261 (25) | 198 (19) | 198 (19) | 201 (22) | 211 (27) | 82 (33) |
| Highest level of education | |||||||
| Some high school or less | 141 (15) | 170 (17) | 141 (13) | 147 (14) | 142 (16) | 121 (15) | 42 (17) |
| Completed high school | 386 (42) | 390 (38) | 369 (35) | 401 (38) | 331 (36) | 311 (40) | 102 (42) |
| Completed college or more | 393 (43) | 466 (45) | 541 (52) | 506 (48) | 437 (48) | 357 (45) | 100 (41) |
| Type 2 diabetes (%) | 75 (8) | 67 (7) | 40 (4) | 62 (6) | 54 (6) | 53 (7) | 13 (5) |
| IFG (%) | 131 (14) | 112 (11) | 77 (7) | 91 (9) | 98 (11) | 95 (12) | 36 (15) |
| CKD (%) | 38 (4) | 58 (6) | 95 (9) | 61 (6) | 55 (6) | 55 (7) | 20 (8) |
| Cigarette smokers (%) | 185 (20) | 132 (13) | 124 (12) | 171 (16) | 145 (16) | 100 (13) | 25 (10) |
| Physical activity (MET-min/wk) | 6703±6455 | 5692±5509 | 6484±6052 | 6254±5853 | 6203±5859 | 6326±6339 | 6577±6321 |
| BMI (kg/m2) | 29±5.9 | 27±4.6 | 26±4.2 | 25.9±4.3 | 27.3±5.0 | 28.1±5.1 | 30.0±6.3 |
| SBP (mmHg) | 115±12.3 | 114±12.9 | 114±13.3 | 112±12.9 | 114±13.0 | 116±12.7 | 117±12.1 |
| DBP (mmHg) | 70±8.3 | 69±8.6 | 68±8.8 | 68±8.6 | 69±8.8 | 69±8.5 | 69±8.7 |
| Metabolic variables | |||||||
| 25OHD (ng/ml) | 14±3.8 | 25±2.8 | 38±8.4 | 30±11.3 | 27±10.3 | 23±10.3 | 19±10.1 |
| PTH (pg/ml) | 49±20.8 | 40±15.0 | 35±12.9 | 26±4.8 | 38±3.2 | 52±5.6 | 81±21.6 |
| Calcium (mg/dl) | 9.6±0.4 | 9.6±0.4 | 9.7±0.4 | 9.7±0.4 | 9.6±0.4 | 9.6±0.4 | 9.6±0.5 |
| Phosphate (mg/dl) | 3.7±0.5 | 3.7±0.5 | 3.7±0.5 | 3.8±0.5 | 3.7±0.5 | 3.6±0.5 | 3.5±0.5 |
| LDL cholesterol (mg/dl) | 120±34.1 | 119±29.7 | 118±30.1 | 119±29.3 | 119±32.0 | 121±31.5 | 118±34.0 |
| eGFR (ml/min/1.732) | 93±15.4 | 90±15.1 | 87±15.0 | 90±14.6 | 90±15.2 | 90±16.0 | 89±16.4 |
| Triglycerides (mg/dl) | 125±91.0 | 132±104 | 123±73.7 | 127±75.8 | 131±118 | 123±75.7 | 119±66.7 |
| CRP (mg/l) | 3.9±5.6 | 2.9±4.7 | 2.9±4.8 | 2.9±5.3 | 3.1±4.6 | 3.5±4.7 | 3.9±6.2 |
| Urinary ACR (mg/g) † | 5.2±2.4 | 5.0±2.3 | 4.8±2.2 | 4.6±2.1 | 5.0±2.4 | 5.4±2.4 | 6.0±2.5 |
| Medication use | |||||||
| Thyroid agents (%) | 46 (5) | 56 (6) | 68 (7) | 70 (7) | 41 (5) | 46 (6) | 13 (5) |
| NSAID (%) | 139 (15) | 170 (17) | 205 (20) | 207 (20) | 148 (16) | 122 (16) | 37 (15) |
| COX-2 inhibitor (%) | 42 (5) | 37 (4) | 51 (5) | 43 (4) | 32 (4) | 43 (6) | 12 (5) |
Values are mean±standard deviation or N (%).
To convert 25OHD values into mmol/L multiply by 2.5, to convert PTH values into pmol/L divide by 9.4.
Geometric mean.
IFG: impaired fasting glucose, CKD: chronic kidney disease, MET: metabolic equivalent of task, BMI: body mass index, SBP: systolic blood pressure, DPB: diastolic blood pressure, 25OHD: 25-hydroxyvitamin D, PTH: parathyroid hormone, LDL: low density lipoprotein, eGFR: estimated glomerular filtration rate, CRP: C-reactive protein, ACR: albumin creatinine ratio, NSAID: non-steroidal anti-inflammatory drug, COX-2 inhibitor: cyclooxygenase-2 inhibitor use.
Incident hypertension
During a median follow-up of 9.0 years (range 1.2–11.1 years) 1,229 participants (41%) developed incident hypertension. The determination of incident hypertension was made by blood pressure measurements alone in 349 participants, anti-hypertensive medication use alone in 249 participants, and both blood pressure and medication use in 631 participants.
Associations of 25(OH)D with incident hypertension
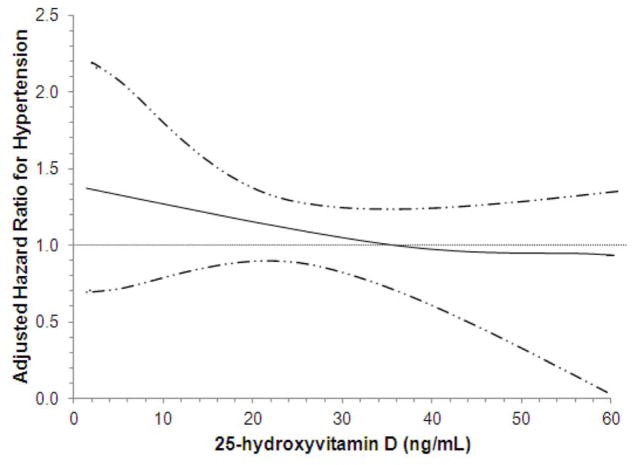
Lower serum 25(OH)D categories were associated with greater unadjusted incident hypertension rates during follow-up (Table 2). Associations of 25(OH)D with hypertension persisted after minimally adjustments; however, further adjustment for potential confounders plus measures of kidney function progressively attenuated these associations, which were no longer statistically significant HR 1.13 (0.96–1.33). Fully adjusted spline models of continuous serum 25(OH)D concentrations showed a tendency toward greater hypertension risks with lower 25(OH)D concentrations although not statistically significant (Figure 2A).
Table 2.
Associations of serum 25-hydroxyvitamin D and parathyroid hormone with incident hypertension among 3,002 participants
| Mineral metabolism markers | Adjusted hazard ratios (95% CI) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Cases | Incidence rates per 100 person-years | Model 1 | Model 2 | Model 3 | |
| 25OHD < 20 (ng/ml) | 922 | 414 (44.9%) | 6.7 | 1.28 (1.09, 1.50) | 1.17 (0.99, 1.38) | 1.13 (0.96, 1.33) |
| 25OHD ≥ 20–30 (ng/ml) | 1028 | 428 (41.6%) | 6.2 | 1.23 (1.06, 1.41) | 1.14 (0.98, 1.32) | 1.14 (0.98, 1.31) |
| 25OHD > 30 (ng/ml) | 1052 | 387 (36.8%) | 5.2 | Ref | Ref | Ref |
| P-for trend | <0.001 | 0.003 | 0.140 | 0.199 | ||
| PTH < 33 (pg/ml) | 1056 | 384 (36.4%) | 5.1 | Ref | Ref | Ref |
| PTH ≥ 33–44.2 (pg/ml) | 912 | 361 (39.6%) | 5.7 | 1.03 (0.90, 1.19) | 1.02 (0.87, 1.19) | 1.00 (0.86, 1.17) |
| PTH ≥ 44.2–65 (pg/ml) | 789 | 359 (45.5%) | 6.9 | 1.16 (1.00, 1.35) | 1.11 (0.95, 1.30) | 1.09 (0.94, 1.28) |
| PTH ≥65 (pg/ml) | 245 | 125 (51.0%) | 8.1 | 1.36 (1.10, 1.68) | 1.30 (1.03, 1.64) | 1.27 (1.01, 1.59) |
| P-for trend | <0.001 | 0.016 | 0.124 | 0.150 | ||
Values are hazard ratios with 95% confidence intervals
Ref: reference category, 25OHD: 25-hydroxyvitamin D, PTH: parathyroid hormone.
Model 1: adjusted for age, sex, race, and clinic site
Model 2: Model 1 + smoking, education, income, diabetes status, body mass index, low density lipoprotein, non-steroidal anti-inflammatory drug use, cyclooxygenase-2 inhibitor use, physical activity (in case of PTH, adjusted for 25(OH)D)
Model 3: Model 2 + albumin creatinine ratio, estimated glomerular filtration rate
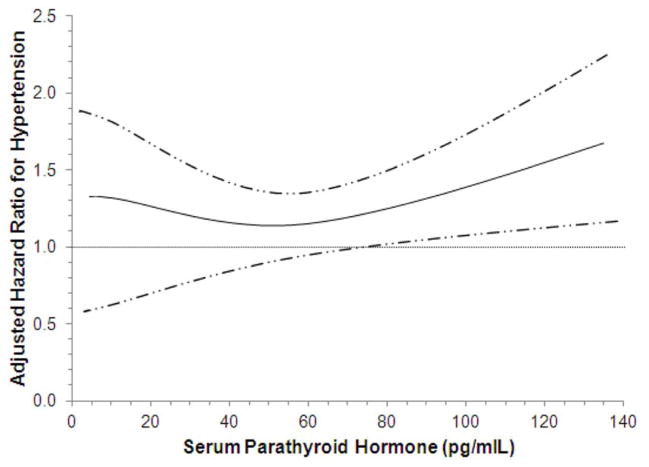
Figure 2. Continuous Associations of 25(OH)D (A) and PTH (B) Concentrations with Incident Hypertension.
Adjusted dose-response relations of serum 25-hydroxyvitamin D (A) and parathyroid hormone (B) concentrations with incident hypertension.
Associations of 25(OH)D and hypertension were similar across subgroups defined by age, sex, race, CKD and PTH (P-for interaction >.10). We found evidence for an interaction between 25(OH)D categories and BMI categories in relation to incident hypertension (P-interaction <.001) with greater and statistically significant risk among obese participants (BMI range ≥ 30–52) (Figure 3A). However, when adjusting for BMI as a continuous variable –since associations may be driven by the severity of BMI within the BMI categories– the results attenuated notably, and were no longer significant within BMI strata, 1.17 (0.92–1.42 for overweight and 1.32 (0.88–1.75) for obese participants.
Figure 3. Associations of 25(OH)D (A) and PTH (B) with Incident Hypertension by BMI-categories.
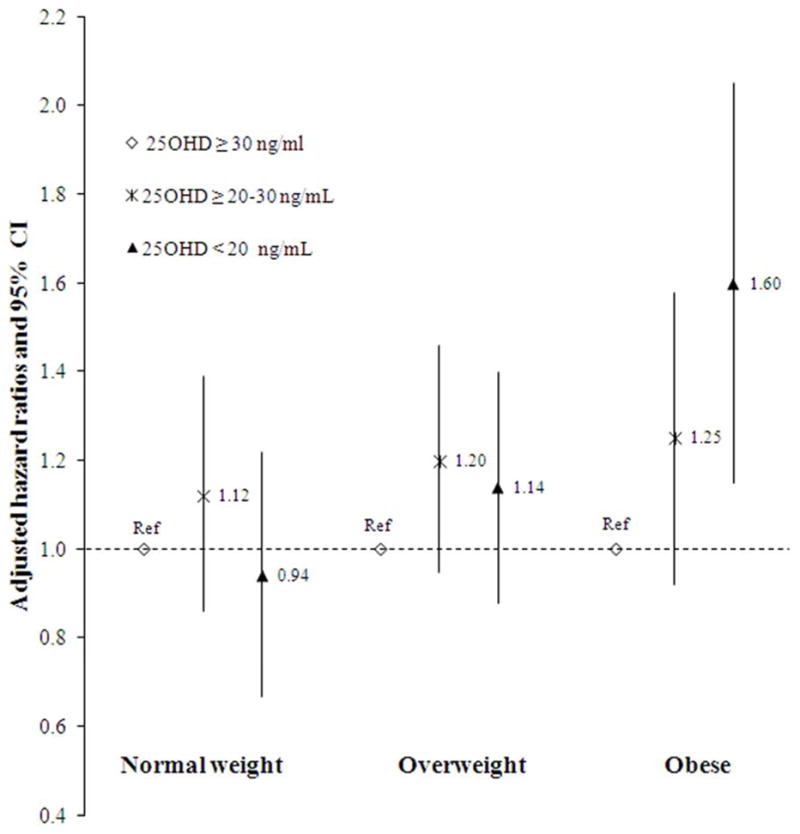
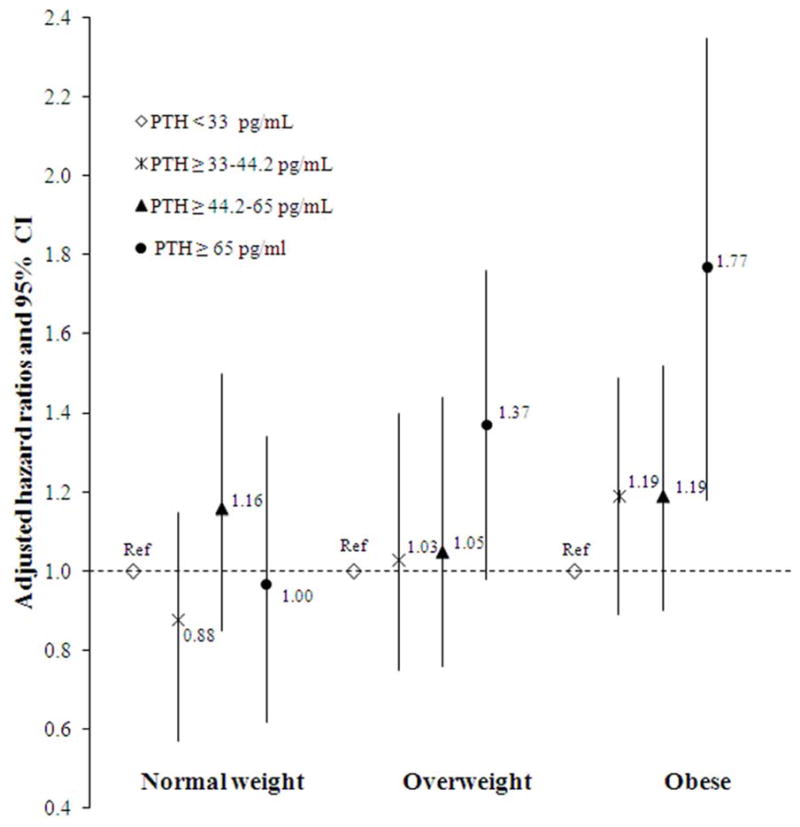
Associations between categories of 25-hydroxyvitamin D (A) and parathyroid hormone (B) with incident hypertension within three different BMI categories.
Associations of PTH with incident hypertension
In unadjusted, and demographic adjusted models, higher serum PTH concentrations were associated with greater rates of incident hypertension (Table 2). Further adjustment for potential confounders, including 25(OH)D and different measures of kidney function attenuated this trend across PTH categories. However, serum PTH concentrations ≥65 pg/ml remained associated with incident hypertension in fully adjusted models HR 1.27 (1.01–1.59). The adjusted spline models were consistent with this observation, demonstrating greater hypertension risks for serum PTH concentrations greater than about 70 pg/ml (Figure 2B).
For PTH we also found significant interaction with BMI categories in relation to hypertension (P-interaction <.001) with a greater risk for obese participants (Figure 3B). After adjusting for BMI as continuous variable the associations were no longer statistically significant: 1.23 (0.83–1.63) for overweight and 1.38 (0.92–1.74) for obese participants. Associations of PTH with incident hypertension were similar across subgroups of age, sex, race, CKD and 25(OH)D. Adjustment for mineral metabolism markers including calcium and phosphate did not appreciably change the observed associations.
Sensitivity analyses
After excluding 248 participants who had incident hypertension diagnosed solely by use of antihypertensive medications, the adjusted associations of 25(OH)D and PTH with hypertension were numerically similar. Similarly, excluding the 444 participants who had borderline hypertension at baseline or the 17 participants who had evidence of primary hyperparathyroidism did not appreciably change these associations.
Discussion
In this study we report associations of serum 25(OH)D and PTH with incident hypertension in a large multi-ethnic cohort during 9 years of follow-up. Lower 25(OH)D was associated with greater hypertension risk, although the association became non-significant after adjusting for potential confounders. In contrast, higher serum PTH remained significantly associated with a greater risk of incident hypertension after adjustments. These findings suggest that higher PTH concentrations might play a role in the pathogenesis of developing hypertension. These associations may underlie the increased risk for cardiovascular disease among participants with high PTH (16).
Previous studies that investigated circulating 25(OH)D concentrations and hypertension risk are sparse and showed inconsistent results. In a case-control study among young women, lower plasma 25(OH)D was significantly associated with self-reported hypertension risk, (6) although the sample excluded obese women and almost two thirds of the women was vitamin D deficient. Another study showed that plasma 25(OH)D was inversely associated with self-reported incident hypertension in a subsample of older men and women, and the risk estimates for predicted 25(OH)D concentrations showed stronger estimates in the larger sample (7). It should be noted that most participants had only predicted 25(OH)D values, the study did not include measured blood pressure, and confidence intervals were wide. Differences in study design, ascertainment of hypertension, and gender differences may explain the observed differences compared with our study.
Our study confirms previous work that showed weak inverse relationships between circulating 25(OH)D concentrations and measured incident hypertension risk among a Norwegian sample that included a broad age spectrum of men and women, (17) a large cohort of postmenopausal women, (9) and Finnish middle-aged male smokers (10). The inconsistent results for 25(OH)D and incident hypertension indicate that the relationship between 25(OH)D and hypertension risk is less clear. Intervention trials that investigated vitamin D supplementation and blood pressure are scarce. A study among post-menopausal women, reported that daily supplementation of calcium (1200mg), and calcium and vitamin D (800 IU) for 8 weeks reduced systolic blood pressure by 8 mmHg, (18) In a dose-response study in blacks, 3 months of vitamin D supplementation (1000/2000/4000 IU) significantly lowered systolic blood pressure in a dose dependent manner (19). Others did not observe effects of vitamin D on blood pressure among different populations (20–22). These clinical trials of vitamin D supplementation have been limited by inadequate vitamin D dosage, insufficient duration of therapy, and the use of “bolus therapy” to replete vitamin D. Moreover, our results should be interpreted in the growing recognition of heterogeneity of response to vitamin D deficiency according to variation in vitamin D metabolism genes (23,24).
Previous studies that investigated PTH and hypertension risk showed similar risk estimates as ours (3,4). Among older men, plasma PTH concentrations were positively associated with hypertension risk (3). Anderson et al, reported that higher PTH was borderline associated with incident hypertension in an older population HR 1.50 (0.99–2.24) (4).
Taken together, our study provides further evidence for an association between higher PTH concentrations and future hypertension risk in different ethnic groups. Hypertension contributes to the development of vascular aging and is a major risk factor for cardiovascular diseases. Moreover, in the MESA study serum PTH was cross-sectionally associated with endothelial dysfunction and arterial stiffness and may partially explain associations of elevated serum PTH with hypertension risk (12).
Although we can only speculate on potential mechanisms, several important observations regarding the biologic actions of PTH warrant discussion. The fact that serum PTH was associated with greater hypertension risk after adjustment for 25(OH)D suggests that PTH, independent of 25(OH)D, contributes to the development of hypertension. Multiple mechanisms might explain how PTH might influence the vasculature. Recent evidence suggests interaction between aldosterone and PTH by modulating angiotensin signaling (5). Furthermore, circulating PTH may affect intracellular signaling causing intracellular calcium overload leading to severe cell injury and death (25,26). Also, PTH stimulates the renin-angiotensin system (27). Among 123 ambulatory older adults undergoing 24-hour blood pressure monitoring, higher PTH levels were independently related with higher blood pressure (28). Among 85 women selected from a population-based study, higher PTH levels were related with both higher systolic and diastolic blood pressure (29). One potential mechanisms is that PTH excess, by precipitating increases in blood pressure, might predispose individuals to a higher risk of hypertension and eventually CVD (30). The complexity of the mineral metabolism makes it hard to fully disentangle the individual contribution of these factors on the vasculature and warrants further investigation. In our study we found significant interaction of 25(OH)D and PTH with BMI categories. In obese participants, but not in overweight and normal weight persons, lower 25(OH)D, and higher PTH concentrations were associated with incident hypertension (Figure 3). However, after adjustment for BMI as continuous variable within each BMI stratum, the association in obese persons attenuated and was no longer significant, suggesting residual confounding due to BMI in this group. Intervention studies have shown a lower impact of vitamin D supplementation on serum 25(OH)D concentrations in obese versus normal weight individuals among different age and ethnic groups (31,32) and increased concentrations after weight loss (33). These trials indicate that vitamin D concentrations are likely to be dependent on adiposity and could explain the observed associations with higher BMI categories due to a greater fat mass. This could be due to changes in serum leptin, FGF-23, or bone alkaline phosphatase concentrations (34). Adjusting for BMI led to significant attenuation of the associations, suggesting that BMI is a very important factor, even within BMI strata, that should be taken into account when studying these relationships. Strengths of our study are the use of a large, community-based cohort without pre-existing cardiovascular or prevalent hypertension at baseline and a considerable length of follow-up. This is to our knowledge the first study that assessed simultaneously the relationship of serum 25(OH)D and PTH with incident hypertension. These findings add new knowledge to underlying subclinical cardiovascular disease mechanisms and strengthen the case for disturbances in high PTH as cardiovascular risk factor. We studied a multi-ethnic cohort which allows generalization to other ethnicities; although generalization to younger age groups should be done with caution since the pathophysiology may differ between age groups. Additionally, we used standardized methods to assess serum 25(OH)D and PTH, hypertension risk factors, several measures of kidney function, and co-morbid conditions, therein minimizing the possibility of residual confounding.
Our study also has important limitations. These include possible misclassification of hypertension, ascertainment of hypertension at discrete time points, and potential for confounding by measurement error in available characteristics. These concerns are to some extent mitigated by sensitivity analyses demonstrating robust associations with only minor changes in the magnitude between serum 25(OH)D and PTH in relation to incident hypertension. The observational design does not confirm causal relationships with incident hypertension and residual confounding by unmeasured characteristics cannot be excluded. Exposure markers –serum 25(OH)D and PTH– were assessed at baseline (2000–2002), and changes in exposure over time can lead to misclassification, however, the prospective design favors non-differential misclassification, which would most likely dilute the observed associations. Future studies should confirm whether the observed associations are similar for younger individuals.
In summary, we report associations of higher PTH concentrations with greater incident hypertension in a community-based, middle-aged multi-ethnic cohort. Vitamin D deficiency and PTH excess are common findings among the general population and a better understanding of the clinical implications of the pathogenesis of hypertension may help to promote cardiovascular health.
Acknowledgments
Sources of funding: This research was supported by grant R01-HL-096875 from the National Heart, Lung and Blood Institute (NHLBI), and contracts N01-HC-95159 through N01-HC-95169 from NHLBI, and grants UL1-RR-024156 and UL1-RR-025005 from National Center for Research Resources.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- PTH
parathyroid hormone
- eGFR
estimated glomerular filtration rate
- BMI
body mass index
- MESA
Multi Ethnic Study of Atherosclerosis
- CRP
C-reactive protein
Footnotes
Conflict of interest: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 2.Jorde R, Svartberg J, Sundsfjord J. Serum parathyroid hormone as a predictor of increase in systolic blood pressure in men. J Hypertens. 2005;23:1639–44. doi: 10.1097/01.hjh.0000179764.40701.36. [DOI] [PubMed] [Google Scholar]
- 3.Taylor EN, Curhan GC, Forman JP. Parathyroid hormone and the risk of incident hypertension. J Hypertens. 2008;26:1390–4. doi: 10.1097/HJH.0b013e3282ffb43b. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Vanwoerkom RC, Horne BD, et al. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am Heart J. 2011;162:331–9. doi: 10.1016/j.ahj.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Tomaschitz A, Ritz E, Pieske B, et al. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res. 2012;94:10–9. doi: 10.1093/cvr/cvs092. [DOI] [PubMed] [Google Scholar]
- 6.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–32. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 8.Al Mheid I, Patel R, Murrow J, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–92. doi: 10.1016/j.jacc.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis KL, Martin LW, Ray RM, et al. A prospective study of serum 25-hydroxyvitamin D levels, blood pressure, and incident hypertension in postmenopausal women. Am J Epidemiol. 2012;175:22–32. doi: 10.1093/aje/kwr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke L, Graubard BI, Albanes D, et al. Hypertension, Pulse, and Other Cardiovascular Risk Factors and Vitamin D Status in Finnish Men. Am J Hypertens. 2013;26:951–6. doi: 10.1093/ajh/hpt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Bosworth C, Sachs MC, Duprez D, et al. Parathyroid hormone and arterial dysfunction in the Multi-Ethnic Study of Atherosclerosis. Clinical endocrinology. 2013;79:429–36. doi: 10.1111/cen.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97:1243–51. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eastell R, Arnold A, Brandi ML, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. The Journal of clinical endocrinology and metabolism. 2009;94:340–50. doi: 10.1210/jc.2008-1758. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett NR. A survival model for a wood preservative trial. Biometrics. 1978;34:673–679. [Google Scholar]
- 16.Kestenbaum B, Katz R, de Boer I, et al. Vitamin d, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58:1433–41. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. 2010;55:792–8. doi: 10.1161/HYPERTENSIONAHA.109.143990. [DOI] [PubMed] [Google Scholar]
- 18.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. The Journal of clinical endocrinology and metabolism. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 19.Forman JP, Scott JB, Ng K, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–85. doi: 10.1161/HYPERTENSIONAHA.111.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7:e36617. doi: 10.1371/journal.pone.0036617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margolis KL, Ray RM, Van Horn L, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative Randomized Trial. Hypertension. 2008;52:847–55. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witham MD, Price RJ, Struthers AD, et al. Cholecalciferol Treatment to Reduce Blood Pressure in Older Patients With Isolated Systolic Hypertension: The VitDISH Randomized Controlled Trial. JAMA internal medicine. 2013 doi: 10.1001/jamainternmed.2013.9043. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M, Yoshioka M, Hashimoto M, et al. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013;97:1004–13. doi: 10.3945/ajcn.112.051664. [DOI] [PubMed] [Google Scholar]
- 24.Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA: the journal of the American Medical Association. 2012;308:1898–905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Law CS, Grigsby CL, et al. Cardiomyocyte-specific deletion of the vitamin d receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–47. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osto E, Fallo F, Pelizzo MR, et al. Coronary microvascular dysfunction induced by primary hyperparathyroidism is restored after parathyroidectomy. Circulation. 2012;126:1031–9. doi: 10.1161/CIRCULATIONAHA.111.081307. [DOI] [PubMed] [Google Scholar]
- 27.Tomaschitz A, Pilz S, Ritz E, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411:1354–60. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Morfis L, Smerdely P, Howes LG. Relationship between serum parathyroid hormone levels in the elderly and 24 h ambulatory blood pressures. J Hypertens. 1997;15:1271–6. doi: 10.1097/00004872-199715110-00011. [DOI] [PubMed] [Google Scholar]
- 29.Jorde R, Sundsfjord J, Haug E, Bonaa KH. Relation between low calcium intake, parathyroid hormone, and blood pressure. Hypertension. 2000;35:1154–9. doi: 10.1161/01.hyp.35.5.1154. [DOI] [PubMed] [Google Scholar]
- 30.van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. Am Heart J. 2013;165:655–664. doi: 10.1016/j.ahj.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. The Journal of clinical endocrinology and metabolism. 2010;95:4584–91. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156:425–37. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 33.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 2012;36:387–96. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 34.Grethen E, Hill KM, Jones R, et al. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. The Journal of clinical endocrinology and metabolism. 2012;97:1655–62. doi: 10.1210/jc.2011-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]