Abstract
This paper reviews the foundation for a role of the human anterior insular cortex (AIC) in emotional awareness, defined as the conscious experience of emotions. We first introduce the neuroanatomical features of AIC and existing findings on emotional awareness. Using empathy, the awareness and understanding of other people’s emotional states, as a test case, we then present evidence to demonstrate: 1) AIC and anterior cingulate cortex (ACC) are commonly coactivated as revealed by a meta-analysis, 2) AIC is functionally dissociable from ACC, 3) AIC integrates stimulus-driven and top-down information, and 4) AIC is necessary for emotional awareness. We propose a model in which AIC serves two major functions: integrating bottom-up interoceptive signals with top-down predictions to generate a current awareness state and providing descending predictions to visceral systems that provide a point of reference for autonomic reflexes. We argue that AIC is critical and necessary for emotional awareness.
INDEXING TERMS: anterior insular cortex, emotional awareness, empathy, fMRI, meta-analysis, top-down, bottom-up, predictive coding
NEUROANATOMICAL FEATURES OF THE INSULAR CORTEX
The human insular cortex was first described by Johann-Christian Reil in 1796 and has since been known as the island of Reil (for review see Binder et al., 2007). It lies in the depth of the lateral sulcus and can be directly observed only by removal of the overlaying frontal and temporal lobes (Naidich et al., 2004). The insula has widespread connections with other parts of the brain (Saper, 2002). In rats, the insular cortex is interconnected with the autonomic system as well as limbic and frontal regions (Saper and Loewy, 1980; Saper, 1982; Allen et al., 1991) and has been shown to contain a viscerotopic map (Cechetto and Saper, 1987). It also receives projections from the glossopharyngeal nerve in rabbits (Yamamoto and Kawamura, 1975). Insular neurons respond to stimulation of the cervical vagus nerve in squirrel monkeys (Radna and MacLean, 1981). In humans, the insula has bidirectional connections with the frontal, parietal, and temporal lobes; the cingulate gyrus; and subcortical structures such as the amygdala, brainstem, thalamus, and basal ganglia (Flynn et al., 1999). These connections serve as the anatomical foundation for the integration of autonomic, viscerosensory, visceromotor, and limbic functions in the insular cortex.
Cytoarchitecturally, the insular cortex is roughly divided into an anterior agranular portion (anterior insula, AIC), a middle dysgranular portion (middle insula), and a posterior granular portion (posterior insula) in both humans (Flynn et al., 1999; Butti and Hof, 2010; Bauernfeind et al., 2013; Butti et al., 2013) and macaque monkeys (Mesulam and Mufson, 1982a), although more subdivisions have been revealed in monkeys recently (Gallay et al., 2012; Evrard H, Logothetis NK, Craig AD, in press). Each subdivision has its own unique connectivity and functional features. The posterior insula receives spinal lamina I afferents via the brainstem and thalamic nuclei and is largely linked to brain region involved in somato-motor functions; the agranular AIC is connected predominantly with allocortical areas and integrates autonomic and interoceptive information (Flynn et al., 1999). The insula, and especially the agranular AIC, is also among the most differentially expanded neocortical regions in humans compared with other primate species (Bauernfeind et al., 2013). Another distinguishing feature of AIC is that it contains a special group of large, bipolar, spindle-shaped neurons referred to as von Economo neurons (VENs; von Economo, 1926; Seeley et al., 2012). To date, VENs have been found to exist only in humans and great apes (Nimchinsky et al., 1995, 1999; Allman et al., 2010), macaque monkeys (Evrard et al., 2012), cetaceans and a number of their related terrestrial herbivore species (Hof and Van der Gucht, 2007; Butti et al., 2009, 2013; Butti and Hof, 2010), and elephants (Hakeem et al., 2009). They are most abundant in humans and are found primarily in layer Vb in the anterior cingulate cortex (ACC; Nimchinsky et al., 1995) and in the junction of the posterior orbitofrontal cortex and AIC known as the frontoinsular cortex (Allman et al., 2010). VENs are projection neurons approximately 4.6 times the size of neighboring pyramidal neurons and are considered well-suited for rapid, long-distance integration of information (Allman et al., 2005, 2010).
EMOTIONAL AWARENESS: EXISTING FINDINGS AND THEORIES
Conscious vs. unconscious emotional processes
We propose that the insula serves a critical role in emotional awareness. Emotion, as a multiconstrual concept, is usually considered to consist of a physiological–biological component, an experiential–psychological component, and an expressive–social component (Lane and Schwartz, 1987; Dolan, 2002). Lane and Schwartz defined five levels of emotional awareness as the awareness of bodily sensations, the body in action, individual feelings, blends of feelings, and blends of blends of feelings (Lane and Schwartz, 1987). In the current review, we simplify this definition as the conscious experience of emotions (the experiential or “feeling” domain of emotion); operationally, emotional awareness occurs during the supraliminal processing of affective stimuli (Pessoa, 2005). Compelling evidence shows that emotional perception, evaluation, and behavior can be processed with or without conscious awareness (see, e.g., Ohman and Soares, 1994) and that emotional awareness is a necessary, but not a sufficient, condition for successful emotional processing. However, it has been suggested that only coarse affective properties can be registered without awareness (Pessoa, 2005) and that the capacity to experience emotions fully significantly increases the likelihood of one to make an appropriate action or decision (Lane and Schwartz, 1987).
Interoception and emotional awareness
Interoception is the sense of the physiological condition of the body (Craig, 2002, 2003). The ongoing discussion on the relationship between interoception and emotional awareness can be dated back to the era of William James (1884) and Carl Lange (1885), if not earlier. Lange considers cardiovascular responses as a basis for emotional awareness, whereas James extends this view by including autonomic functions other than cardiovascular responses. Their ideas, usually mentioned together as the James-Lange theory, was challenged by the Cannon-Bard theory (Bard, 1928; Cannon, 1932), which argues that bodily responses are the result, not the cause, of emotions and that a central nervous system is needed to generate emotional feelings. The self-perception theory, derived from radical behaviorism, supports the notion that emotional feelings follow behavior, although the extent differs among individuals (Bem, 1967; Laird, 1974). More recently, it has been proposed that reactivation of bodily and neural responses involved in lower-level sensorimotor processes contributes to subjective awareness (Thompson and Varela, 2001; Niedenthal, 2007; Harrison et al., 2010; Gray et al., 2012; Oosterwijk et al., 2012; Pollatos et al., 2012). Such embodiment of high-level emotional feelings is sometimes termed the “somatic marker,” which captures the physical aspect of subjective awareness (Damasio, 1996). By incorporating ideas from theoretical neurobiology, it has recently been suggested that predictive coding of interoceptive information is important in awareness (Seth et al., 2011). This implies that emotion can be viewed as a form of interoceptive inference; that is, subjective feelings are based on the active interpretation of changes in the physiological conditions of the body (Seth et al., 2011). These new developments support an inseparable relationship between interoception and emotional awareness.
Brain mechanisms of emotional awareness
Although several other brain regions such as ACC, amygdala, and ventromedial prefrontal cortex are commonly implicated (Lane et al., 1998; LeDoux, 2000; Cohen et al., 2001; Adolphs, 2002; Ochsner and Gross, 2005; Phelps, 2006; Duncan and Barrett, 2007; Lieberman, 2007), the insular cortex has been singled out as a critical neural substrate for interoceptive and emotional awareness (Craig, 2009, 2010, 2011; Singer et al., 2009; Jones et al., 2010; Seth et al., 2011). The posterior insular cortex has been commonly associated with somatotopic representations of bodily states such as itch, pain and temperature, and touch (Damasio et al., 2000; Craig, 2002, 2009; Harrison et al., 2010), whereas AIC participates in a wide range of functions, including and beyond bodily representations. Neuroimaging studies consistently show that AIC activation is associated with cardiovascular functions (King et al., 1999; Henderson et al., 2002), respiration (Banzett et al., 2000; Henderson et al., 2002), pain (Treede et al., 1999; Wager et al., 2004), touch (Keysers et al., 2004; Lindgren et al., 2012), thermosensory awareness (Craig et al., 2000), disgust (Phillips et al., 1997; Wicker et al., 2003; Calder et al., 2007), interoceptive awareness (Critchley et al., 2004; Zaki et al., 2012), general emotional processing (Davidson and Irwin, 1999; Zaki et al., 2012), cognitive control (Eckert et al., 2009; Menon and Uddin, 2010), empathy (Singer et al., 2004; Gu and Han, 2007a; Gu et al., 2010, 2012, 2013; Lamm et al., 2010; Ebisch et al., 2011), intuition (Kuo et al., 2009), unfairness (Sanfey et al., 2003; Kirk et al., 2011), risk and uncertainty (Preuschoff et al., 2008; Bossaerts, 2010; Ullsperger et al., 2010; Bach and Dolan, 2012), trust and cooperation (King-Casas et al., 2008), and norm violations (Montague and Lohrenz, 2007; Xiang et al., 2013). It has also been observed that patients with focal epileptic seizures that arise from the AIC report heightened emotional awareness and enhanced wellbeing (Picard, 2013), further supporting a role of AIC in emotional awareness.
A posterior-to-anterior gradient in the insular cortex has been proposed, in which physical features of interoception are processed in the posterior insula and the integration of interoception with cognitive and motivational information in the AIC, and the right AIC serves a more dominant role than the left AIC (Craig, 2009, 2010, 2011). Recent work further suggests that, as a critical neural correlate in interoceptive predictive coding, AIC serves a computational role in emotional awareness (Seth et al., 2011). The insular cortex is therefore considered to form an interoceptive image of one’s physiological states and consequently to relay internal needs to subjective awareness of feelings (Craig, 2002; Harrison et al., 2010).
It is noteworthy that the insular cortex works closely with a network of regions, including the ACC (Critchley, 2004; Critchley et al., 2004; Medford and Critchley, 2010; Fan et al., 2011; Denny et al., 2012; Lindquist et al., 2012), somatosensory cortex (Gu et al., 2013), and amygdala (Etkin and Wager, 2007). It has been pointed out that patients with bilateral insular damage still preserve certain aspects of emotional awareness, suggesting that emotional feelings might first emerge from the brainstem and hypothalamus, which are later enriched and refined by the insula (Damasio et al., 2013).
Clinical significance: alexithymia and related disorders
Deficit in emotional awareness, termed as alexithymia (Taylor, 2000), is commonly seen in conditions associated with neuropathological degeneration of the VENs and functional deficits of the AIC, such as behavioral variant frontotemporal dementia (Seeley et al., 2006; Seeley, 2010; Kim et al., 2012), callosal agenesis (Kaufman et al., 2008), and autism (Santos et al., 2011; Butti et al., 2013). Among the popular tools to measure trait alexithymia is the 20-item Toronto Alexithymia Scale (TAS-20), which assesses three aspects of emotional deficits: difficulty in identifying emotions, difficulty in describing emotions, and externally oriented thinking style (Taylor et al., 2003). As assessed by TAS-20, the prevalence of alexithymia is approximately 10% in the general population (Kokkonen et al., 2001) and is remarkably high in patients with autism spectrum disorders (85%; Hill et al., 2004). In autism, lower AIC activations are correlated with higher TAS-20 scores (Bird et al., 2010). Patients with frontotemporal dementia are more alexithymic than matched controls, and such deficits have been associated with abnormalities in pregenual ACC (Sturm and Levenson, 2011) and AIC (Seeley, 2010). Alexithymia is also observed in individuals with depersonalization syndrome (Simeon et al., 2009). Even in the absence of psychiatric or neurological disorders, alexithymia is very common among elderly people (34%; Joukamaa et al., 1996). This suggests that emotional awareness is important to mental health and that impaired emotional awareness interferes with normal social function in both clinical and nonclinical populations. Diminished ability to integrate information rapidly among spatially distinct regions may underlie functional deficits in these conditions and, ultimately, in the inability to make quick and intuitive judgments regarding uncertain and rapidly changing social contexts (Allman et al., 2005).
EMPATHY AS A TEST CASE FOR EMOTIONAL AWARENESS
Next we review evidence supporting a critical role of AIC in emotional awareness using empathy as a test case. Empathy refers to the awareness and understanding of the sensory and emotional states of other people (Gu et al., 2012). In experimental settings, empathetic emotions are externally generated by visual or auditory affective stimuli, in contrast to self-generated emotions induced by instructions. Empathy is closely related to, yet different from, emotional contagion, in that the latter is merely passive, whereas empathy also involves active and top-down components such as perspective taking and social understanding (Preston and de Waal, 2002; Decety and Jackson, 2004). A substantial portion of work on emotion involves visual stimuli depicting another person’s emotions (e.g., facial expressions; for reviews see Davidson and Irwin, 1999; Adolphs, 2002; Phelps, 2006; Pessoa and Adolphs, 2010) because of the advantage of allowing specific yet flexible experimental manipulations (e.g., compared with somatosensory stimuli). In such studies, empathy is often involved but not explicitly discussed. In the following paragraphs, we consider four lines of evidence to support the notion that AIC is critical for empathetic emotions: 1) AIC and ACC are commonly coactivated as revealed by a meta-analysis, 2) AIC is functionally dissociable from ACC, 3) AIC integrates stimulus-driven and top-down information, and 4) AIC lesions are associated with deficits in emotional awareness.
Coactivation of AIC and ACC: a meta-analysis on empathy
To overcome the heterogeneity in experimental methods and achieve an unbiased quantification of neural substrates underlying empathy, we first conducted a quantitative meta-analysis on 47 functional magnetic resonance imaging (fMRI) studies (see Table 1) that examined brain activations related to empathy in healthy adults using the coordinate-based meta-analysis (Salimi-Khorshidi et al., 2009) of activation likelihood estimation (ALE) approach (Turkeltaub et al., 2002; Laird et al., 2005; Eickhoff et al., 2009). This algorithm treats activated foci of brain regions as three-dimensional Gaussian probability distributions centered at the given coordinates instead of points (Laird et al., 2005; Eickhoff et al., 2009) and incorporates the size of the probability distributions by taking into account the sample size of each study and by utilizing random-effect rather than fixed-effect inference by testing the above-chance clustering between experiments/contrasts rather than the above-chance clustering between foci (Eickhoff et al., 2009).
TABLE 1.
Demographics of Studies Included in the Meta-Analysis1
| References | Sample size | Stimulus valence | Stimulus modality | Task |
|---|---|---|---|---|
| Akitsuki and Decety (2009) | 26 | Pain | Visual/image | Valence rating |
| Botvinick et al. (2005) | 12 | Pain | Visual/video | Observation |
| Carr et al. (2003) | 11 | Happy/sad/angry/surprise/disgust/afraid | Visual/image | Imitation; observation |
| Chakrabarti et al. (2006) | 25 | Happy/sad/angry/disgust/neutral | Visual/video | Button press |
| Cheetham et al. (2009) | 16 | Pain | Visual/video | Button press |
| Costantini et al. (2008) | 13 | Pain | Visual/video | Observation |
| Decety et al. (2010) | 22 | Pain | Visual/video | Observation |
| Derntl et al. (2010) | 24 | Happy/sad/anger/fear/disgust | Visual/image | Perspective taking |
| Gu and Han (2007a) | 12 | Pain | Visual/image | Valence rating |
| Gu et al. (2010) | 18 | Pain | Visual/image | Valence rating |
| Han et al. (2009) | 24 | Pain | Visual/video | Valence rating |
| Hennenlotter et al. (2005) | 12 | Happy | Visual/image | Imitation |
| Hooker et al. (2008) | 20 | Mixed | Visual/image | Empathizing; emotion inference |
| Hooker et al. (2010) | 15 | Mixed | Visual/image | Empathizing |
| Immordino-Yang et al. (2009) | 13 | Mixed | Auditory/Visual | Imitation |
| Jabbi et al. (2007) | 18 | Disgust/pleasant | Visual/video | Observation |
| Jackson et al. (2005) | 15 | Pain | Visual/image | Valence rating |
| Jackson et al. (2006) | 34 | Pain | Visual/image | Valence rating |
| Kim et al. (2009) | 21 | Sad | Visual/image | Empathizing; observation |
| King et al. (2006) | 12 | Pain | Visual/video | Empathetic decision making |
| Kramer et al. (2010) | 17 | Anger/sadness/pain/anxiety | Visual/image | Button press |
| Lamm et al. (2007a) | 17 | Pain | Visual/video | Perspective taking |
| Lamm et al. (2007b) | 18 | Pain | Visual/image | Valence rating |
| Lamm and Decety (2008) | 18 | Pain | Visual/image | Valence rating |
| Lamm et al. (2010) | 24 | Pain | Visual/image | Valence rating |
| Leslie et al. (2004) | 15 | Smile/frown | Visual/video | Observation; imitation |
| Mathur et al. (2010) | 28 | Pain | Visual/image | Valence rating |
| Morrison et al. (2004) | 11 | Pain | Visual/video | Observation |
| Morrison et al. (2007) | 14 | Pain | Visual/video | Observation |
| Morrison and Downing (2007) | 16 | Pain | Visual/image | Hit/miss judgment |
| Newman-Norlund et al. (2009) | 22 | Mixed | Visual/video | Hit/miss judgment |
| Nummenmaa et al. (2008) | 10 | Threat/harm | Visual/image | Empathizing |
| Ochsner et al. (2008) | 13 | Pain | Visual/video | Observation |
| Olsson et al. (2007) | 14 | Pain | Visual/video | Observation |
| Osborn and Derbyshire (2010) | 15 | Pain | Visual/image | Valence rating |
| Saarela et al. (2007) | 12 | Pain | Visual/image | Observation |
| Schulte-Ruther et al. (2007) | 26 | Angry/fearful/sad/disgusted/happy | Visual/image | Perspective taking |
| Schulte-Ruther et al. (2008) | 26 | Angry/fearful/sad/happy | Visual/image | Perspective taking |
| Singer et al. (2004) | 16 | Pain | Visual/image | Observation |
| Singer et al. (2006) | 16 | Pain | Visual/image | Observation |
| Ushida et al. (2008) | 15 | Pain | Visual/video | Observation |
| van der Gaag et al. (2007) | 17 | Happy/disgust/fear | Visual/video | Observation; imitation |
| Vollm et al. (2006) | 13 | Mixed | Visual/image | Empathizing |
| Wicker et al. (2003) | 14 | Disgust/pleasure | Visual/video | Observation |
| Xu et al. (2009) | 33 | Pain | Visual/video | Valence rating |
| Zaki et al. (2007) | 13 | Pain | Visual/video | Observation |
| Zaki et al. (2009) | 21 | Mixed | Visual/video | Valence rating |
All references are given in the Literature Cited.
A literature search was carried out in PubMed and Web of Science (through August, 2010, the time at which we began the meta-analysis) using any of the following indexing terms: “empathy,” “empathetic,” “altruism,” “sympathy,” “emotional contagion,” “compassion,” in combination with “fMRI” by four researchers independently (X.G. and three research assistants). All of the resulting 158 articles were pooled into a database, and redundant entries were eliminated. We then excluded articles that 1) were review articles or other nonempirical studies; 2) did not report results in Talairach or Montreal Neurological Institute (MNI) space; 3) used tasks unrelated to empathetic processes and with no measurement of trait empathy; 4) were based on structural analyses only; 5) were based on region of interest (ROI) analysis (e.g., using anatomical masks or coordinates from other studies), principal component analysis, or functional or effective connectivity analysis only; 6) were based on special populations (e.g., children, aging adults, psychiatric patients, medical physicians, Buddhist meditators); and 7) focused on the cognitive aspects of understanding others’ mental states (i.e., theory of mind). These criteria resulted in 47 articles with 2,029 foci, 194 contrasts, and 3,411 subjects in the final data set (see Table 1). We further conducted analyses on foci from studies or contrasts based on empathy for pain, empathy for negative emotions, and empathy for positive emotions. For example, the following contrasts were identified as empathy for pain: “main effect of pain: pain>no pain” (Akitsuki and Decety, 2009) and “other’s pain>self pain” (Ochsner et al., 2008). The following contrasts were identified as empathy for negative emotions: “compassionate–sad>zcompassionate–neutral” (Kim et al., 2009), and “overlap between observing and feeling disgust” (Wicker et al., 2003). This category included 192 foci from 20 contrasts and 403 subjects. This category included 948 foci from 98 contrasts with 1,598 subjects. The following contrasts were identified as empathy for positive emotions: “overlap between sucrose–saliva and pleasant faces–neutral faces” (Jabbi et al., 2007) and “correlation of happy > neutral contrast with EQ” (Chakrabarti et al., 2006). This yielded 70 foci from nine contrasts with 157 subjects.
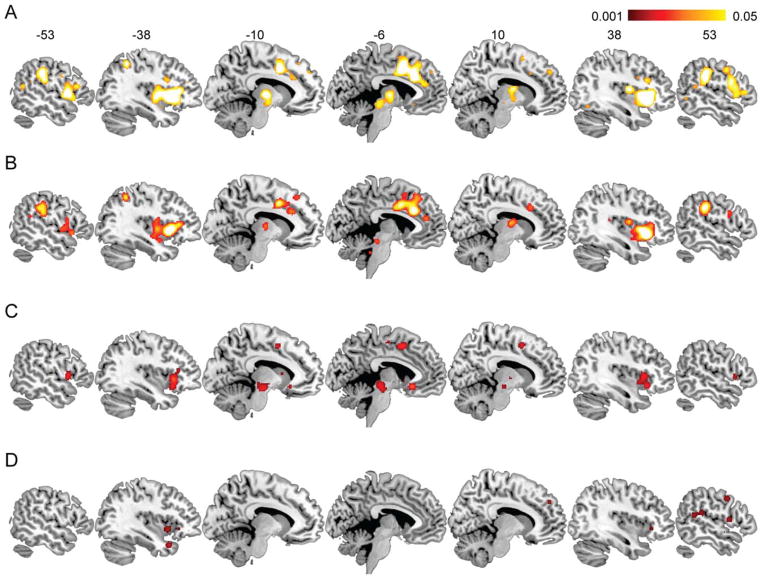
For the main analysis on all three emotional categories, we identified multiple regions involved in empathetic processing. These regions include AIC, ACC, middle and superior temporal gyri, somatosensory cortices (SI/SII), dorsal frontoparietal regions, medial prefrontal cortex (MPFC), amygdala, thalamus, and midbrain structures (substantia nigra and red nucleus; Fig. 1A, Table 2). Meta-analysis on empathy for pain also showed most consistent activations in bilateral insula and ACC (Fig. 1B, Table 3). Other regions include lateral PFC (LPFC), MPFC, SI, middle occipital gyrus, fusiform gyrus, inferior parietal lobule, amygdala, globus pallidus, claustrum, thalamus, and cerebellum. Empathy for negative emotions also reliably activated AIC and ACC (Fig. 1C, Table 4), in addition to LPFC, MPFC, red nucleus, substantia nigra, and putamen/caudate nucleus. Brain regions that showed consistent activation for empathy for positive emotions include AIC, SI, superior temporal gyrus, MPFC, LPFC, and inferior parietal lobule (Fig. 1D, Table 5). This category yielded fewer brain regions than previous categories, probably because of the smaller number of studies/contrasts for positive emotions. These regions largely, but not fully, overlap with the brain areas involved in empathy for pain and negative emotion.
Figure 1.
Meta-analysis of fMRI studies of empathy processing. A: Empathy for all three emotions examined (pain, negative, positive). B: Empathy for pain. C: Empathy for negative emotions. D: Empathy for positive emotions. Color intensity represents ALE value. Note that AIC bilaterally are commonly involved in all conditions (x = −38 and 38) and that ACC is involved in all but empathy for positive emotions. See Tables (2–5) for details regarding coordinates and ALE values.
TABLE 2.
Empathy-Related Brain Regions (All Coordinates)1
| Voxel | ALE | x | y | z | Area | Label |
|---|---|---|---|---|---|---|
| 9188 | 0.117 | −40 | 14 | 0 | L anterior insula | |
| 0.111 | 38 | 20 | −2 | R anterior insula | ||
| 0.100 | −46 | 8 | 4 | L anterior insula | ||
| 0.070 | 18 | 8 | 6 | R putamen | ||
| 0.068 | −12 | −14 | 4 | L thalamus | ||
| 0.063 | 34 | −6 | 12 | R claustrum | ||
| 0.060 | 22 | −2 | −12 | R amygdala | ||
| 0.058 | −20 | 6 | 4 | L putamen | ||
| 0.058 | 46 | 6 | 30 | 9 | R inferior frontal gyrus | |
| 0.055 | −28 | −4 | −14 | L amygdala | ||
| 0.053 | 4 | −20 | −8 | R red nucleus | ||
| 0.053 | −16 | −8 | −10 | L amygdala | ||
| 0.052 | 0 | −28 | −14 | Red nucleus | ||
| 0.051 | −42 | 10 | 26 | 9 | L inferior frontal gyrus | |
| 0.049 | 48 | 2 | 42 | 6 | R middle frontal gyrus | |
| 0.048 | 10 | −6 | 12 | R thalamus | ||
| 0.045 | −50 | 20 | 16 | 45 | L inferior frontal gyrus | |
| 0.038 | −48 | −2 | 30 | 6 | L precentral gyrus | |
| 2676 | 0.089 | −2 | 24 | 32 | 32 | L ACC/RCZa |
| 0.081 | −8 | 6 | 42 | 32 | L ACC/RCZp | |
| 0.063 | 0 | 0 | 38 | 24 | ACC/CCZ | |
| 0.051 | −2 | 40 | 20 | 9 | L MPFC | |
| 0.041 | 6 | 48 | 36 | 6 | R MPFC | |
| 0.034 | −10 | 50 | 36 | 8 | L superior frontal gyrus | |
| 0.033 | 2 | −6 | 58 | 6 | R MPFC | |
| 585 | 0.064 | 52 | −32 | 30 | 40 | R inferior parietal lobule |
| 0.034 | 50 | −46 | 16 | 13 | R superior temporal gyrus | |
| 522 | 0.066 | −54 | −28 | 36 | 40 | L inferior parietal lobule |
| 0.034 | −42 | −36 | 40 | 40 | L inferior parietal lobule | |
| 264 | 0.055 | 44 | −50 | −12 | 37 | R fusiform gyrus |
| 0.040 | 48 | −60 | −2 | 19 | R inferior temporal gyrus | |
| 0.036 | 42 | −66 | −12 | 19 | R fusiform gyrus | |
| 0.032 | 50 | −62 | 10 | 37 | R mid temporal gyrus | |
| 157 | 0.053 | −38 | −48 | 50 | 40 | L inferior parietal lobule |
| 0.031 | −26 | −60 | 40 | 7 | L superior parietal lobule | |
| 140 | 0.039 | −44 | −68 | −8 | 19 | L fusiform gyrus |
| 0.032 | −44 | −70 | 6 | 37 | L mid temporal gyrus | |
| 87 | 0.047 | 28 | 38 | 34 | 9 | R mid frontal gyrus |
| 85 | 0.047 | 32 | −84 | 0 | 18 | R mid occipital gyrus |
| 55 | 0.035 | 26 | −52 | 44 | 7 | R superior parietal lobule |
| 36 | 0.037 | −50 | −58 | 16 | 22 | L superior temporal gyrus |
PFDR < 0.05, k > 30, voxel size = 2 × 2 × 2 mm.
ACC, anterior cingulate cortex; RCZa, anterior rostral cingulate zone; RCZp, posterior rostral cingulate zone; CCZ, caudal cingulate zone; MPFC, medial prefrontal cortex; L, left; R, right. RCZa, RCZp, and CCZ are defined as by Fan et al. (2008).
TABLE 3.
Brain Regions Involved in Empathy for Pain1
| Voxel | ALE | x | y | z | Area | Label |
|---|---|---|---|---|---|---|
| 2405 | 0.084 | −40 | 14 | 0 | L anterior insula | |
| 0.041 | −30 | −4 | −14 | L amygdala | ||
| 0.036 | −16 | −8 | −8 | L globus pallidus | ||
| 0.033 | −38 | −2 | 4 | L claustrum | ||
| 0.029 | −48 | 8 | 8 | 44 | L precentral gyrus | |
| 0.028 | −46 | 0 | 24 | 9 | L inferior frontal gyrus | |
| 0.026 | −38 | −2 | 16 | L posterior insula | ||
| 2298 | 0.084 | 40 | 20 | −4 | 47 | R inferior frontal gyrus |
| 0.032 | 46 | 28 | 6 | 13 | R inferior frontal gyrus | |
| 0.032 | 26 | −2 | −14 | R amygdala | ||
| 1999 | 0.075 | −2 | 24 | 32 | 32 | L ACC/RCZa |
| 0.057 | 0 | −2 | 36 | 24 | ACC/CCZ | |
| 0.050 | −8 | 6 | 40 | 32 | L ACC/RCZp | |
| 0.031 | 0 | 10 | 54 | 6 | L MPFC | |
| 506 | 0.053 | 52 | −30 | 34 | 2 | R SI |
| 408 | 0.047 | −54 | −28 | 34 | 40 | L inferior parietal lobule |
| 0.020 | −44 | −38 | 38 | 40 | L inferior parietal lobule | |
| 302 | 0.033 | 12 | −6 | 12 | R thalamus | |
| 154 | 0.041 | −2 | 42 | 18 | 9 | L MPFC |
| 150 | 0.028 | −42 | −68 | −8 | 19 | L fusiform gyrus |
| 0.027 | −44 | −72 | 6 | 19 | L mid occipital gyrus | |
| 143 | 0.038 | −38 | −48 | 50 | 40 | L inferior parietal lobule |
| 131 | 0.040 | 36 | −4 | 14 | R posterior insula | |
| 117 | 0.027 | 48 | 4 | 30 | 9 | R inferior frontal gyrus |
| 0.024 | 44 | 2 | 42 | 6 | R mid frontal gyrus | |
| 55 | 0.026 | −10 | −12 | 6 | L thalamus | |
| 40 | 0.028 | 30 | −82 | 2 | 18 | R mid occipital gyrus |
| 35 | 0.027 | −30 | −64 | −26 | L cerebellum |
PFDR < 0.05, k > 30, voxel size = 2 × 2 × 2 mm. ACC, anterior cingulate cortex; RCZa, anterior rostral cingulate zone; RCZp, posterior rostral cingulate zone; CCZ, caudal cingulate zone; SI, primary somatosensory cortex; MPFC, medial prefrontal cortex; L, left; R, right. RCZa, RCZp, and CCZ are defined as by Fan et al. (2008).
TABLE 4.
Brain Regions Involved in Empathy for Negative Emotions1
| Voxel | ALE | x | y | z | Area | Label |
|---|---|---|---|---|---|---|
| 837 | 0.040 | −46 | 8 | 2 | L anterior insula | |
| 0.030 | −42 | 18 | −10 | 47 | L inferior frontal gyrus | |
| 0.028 | −40 | 20 | 2 | L anterior insula | ||
| 0.019 | −34 | 28 | 14 | L anterior insula | ||
| 650 | 0.028 | 4 | −18 | −8 | R red nucleus | |
| 0.023 | 0 | −26 | −12 | L red nucleus | ||
| 0.021 | −12 | −16 | −12 | L substantia nigra | ||
| 0.017 | 6 | −10 | 2 | R thalamus | ||
| 505 | 0.034 | 44 | 16 | 4 | R anterior insula | |
| 0.022 | 36 | 22 | −8 | 47 | R inferior frontal gyrus | |
| 406 | 0.025 | −2 | 10 | 50 | 6 | L MPFC |
| 0.021 | 6 | 8 | 50 | 6 | R MPFC | |
| 0.018 | 0 | −10 | 56 | 6 | L MPFC | |
| 165 | 0.018 | −20 | 8 | 10 | L putamen | |
| 0.017 | −18 | 4 | 0 | L putamen | ||
| 149 | 0.023 | 2 | 10 | 0 | R caudate nucleus | |
| 117 | 0.028 | −4 | 22 | −10 | 32 | L ACC/RCZa |
| 83 | 0.022 | 20 | 8 | 6 | R putamen |
PFDR < 0.05, k > 30, voxel size = 2 × 2 × 2 mm. ACC, anterior cingulate cortex; RCZa, anterior rostral cingulate zone; MPFC, medial prefrontal cortex; L, left; R, right. RCZa, RCZp, and CCZ are defined as by Fan et al. (2008).
TABLE 5.
Brain Regions Involved in Empathy for Positive Emotions1
| Voxel | ALE | x | y | z | Area | Label |
|---|---|---|---|---|---|---|
| 128 | 0.014 | −36 | 12 | −4 | L anterior insula | |
| 114 | 0.015 | 50 | −46 | 18 | 13 | R superior temporal gyrus |
| 0.011 | 54 | −34 | 20 | 13 | R superior temporal gyrus | |
| 60 | 0.015 | −38 | 14 | −26 | 38 | L superior temporal gyrus |
| 60 | 0.015 | 6 | 48 | 36 | 6 | R MPFC |
| 0.015 | 8 | 48 | 36 | 8 | R MPFC | |
| 60 | 0.014 | 50 | 2 | 40 | 6 | R mid frontal gyrus |
| 0.014 | 50 | 2 | 42 | 6 | R mid frontal gyrus | |
| 50 | 0.013 | 54 | 6 | 10 | 6 | R mid frontal gyrus |
| 46 | 0.010 | −60 | −32 | 40 | 44 | L precentral gyrus |
| 0.010 | −58 | −26 | 40 | 40 | L inferior parietal lobule | |
| 0.009 | −60 | −40 | 38 | 1 | L SI | |
| 43 | 0.012 | 34 | 28 | −2 | 40 | L inferior parietal lobule |
| 41 | 0.011 | 46 | −24 | −2 | 47 | L inferior frontal gyrus |
PFDR < 0.05, k > 30, voxel size = 2 × 2 × 2 mm. SI, primary somatosensory cortex; MPFC, medial prefrontal cortex; L, left; R, right.
Several aspects of these findings deserve attention. First, AIC, but not ACC, is consistently involved in the three categories of empathetic emotions examined, suggesting a unique role of AIC in processing affective visual stimuli regardless of valence (see x = −38 and 38 slices across Fig. 1A–D). Second, right AIC activation is absent in empathy for positive emotions and present only in empathy of pain and negative emotions, whereas left AIC is activated by all valence categories. This finding is consistent with the notion that right AIC encodes “energy-consuming” negative feelings (Craig, 2011). There are at least two possible explanations regarding the function of the left AIC: 1) it directly processes both positive and negative feelings and 2) it specifically encodes “energy-nourishing” positive feelings (Craig, 2011), yet there might be asymmetrical information flow between left and right AIC such that negative feelings are processed in the right AIC first and then sent to the left AIC, a downstream region of the right AIC. Further investigations using lesion and causal modeling of neuroimaging data are needed to answer this question. Third, although we were not able to compare gender differences in the current meta-analysis, males and females have been shown to use different strategies in emotion paradigms (Baron-Cohen and Wheelwright, 2004; Singer et al., 2006; Schulte-Ruther et al., 2008). A previous meta-analysis reported that males exhibit great right AIC activation to negative stimuli (Stevens and Hamann, 2012). Finer-grained analysis on gender differences in emotional awareness can offer insights into emotional awareness in relation to perceiver characteristics and implications for disorders such as autism (Baron-Cohen and Wheelwright, 2004).
Functional dissociation between AIC and ACC
In line with our meta-analysis on empathy studies, it has been commonly demonstrated that AIC and ACC are coactivated in various experimental paradigms (Critchley, 2004; Dosenbach et al., 2006; Craig, 2009; Medford and Critchley, 2010; Fan et al., 2011) as well as in resting states (Seeley et al., 2007; Britz et al., 2010; Cauda et al., 2011; Fan et al., 2012). As pointed out by Craig, “it is understandably mystifying that a region of the ventrolateral prefrontal cortex (the anterior insular cortex (AIC)) and a region of the medial prefrontal cortex (the anterior cingulate cortex (ACC)) are co-active in so many behaviors. . . .” (Craig, 2009). Indeed, ACC also has been considered a significant structure in emotional awareness (Lane et al., 1998; Lieberman, 2007). Therefore, experimental evidence showing functional dissociation between AIC and ACC is crucial in delineating the relationship between these two structures.
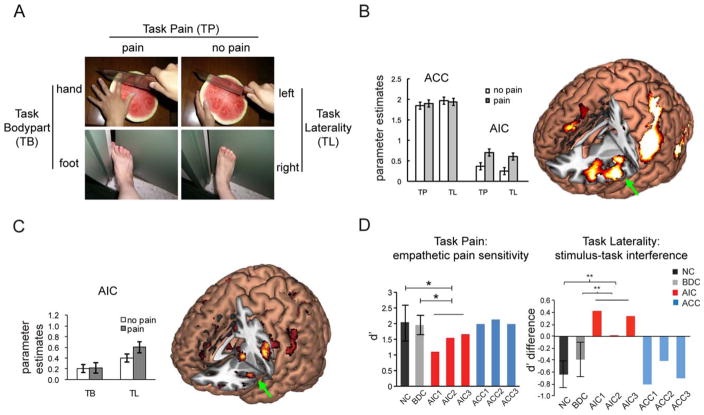
We recently showed a functional dissociation between AIC and ACC using an empathetic pain paradigm (Fig. 2A; modified from Gu et al., 2010, 2013). Participants were asked to judge whether the person in the pictorial stimulus is suffering from pain (“explicit” task pain; TP) or the laterality of limb (“implicit” task laterality; TL). A key manipulation in this design is equating the cognitive load among experimental conditions. The results suggest that, after controlling for cognitive load, AIC, but not ACC, showed increased activation for painful compared with neutral pictures (Fig. 2B, modified from Gu et al., 2010). This finding points out the importance of AIC, in dissociation from ACC, in emotional awareness. In line with this evidence, several other studies have shown functional dissociation between AIC and ACC in other domains (Sridharan et al., 2008; Eckert et al., 2009). Using Granger causal analysis, Sridharan and colleagues (2008) showed that the right AIC has a causal influence on ACC and plays a critical role in switching between the central executive network and the default mode network. The right AIC, but not the ACC, is also coupled with brain regions involved in task performance across different domains (Eckert et al., 2009).
Figure 2.
Empathetic pain as a test case to study the AIC–awareness relationship. A: Visual stimuli and task conditions used in empathetic pain paradigms (modified from Gu et al., 2010, 2013). B: After controlling for cognitive load, only AIC, and not ACC, showed increased activation for empathetic pain (modified from Gu et al., 2010). C: Cognition–emotion interaction effect in AIC (modified from Gu et al., 2013). D: AIC lesions, but not ACC lesions, are associated with diminished sensitivity to others’ pain in the explicit empathy task (left, indexed by smaller d′ based on signal detection theory) and lack of task–stimulus interference in the implicit empathy task (right, indexed by d′painful − d′nonpainful; for details see Gu et al., 2012). AIC, anterior insular cortex; ACC, anterior cingulate cortex; NC, neurologically intact controls; BDC, brain-damaged controls; TP, task painl; TL, task laterality; TB, task body part. * P < 0.05, **P < 0.01.
We consider this evidence complementary to, rather than conflicting with, the finding of joint involvement of AIC and ACC. ACC does not seem to be a critical region in generating awareness, yet it might receive input from AIC and convey the “feeling” information to other brain networks that serve voluntary control functions (Posner and Rothbart, 1998; Craig, 2009; Medford and Critchley, 2010; Valentini, 2010). This notion is supported by the functional connectivity between ACC and lateral prefrontal, primary, and supplementary motor areas during conflict processing (Fan et al., 2008). In other words, we borrow from the classical view that ACC serves as a limbic motor region and the AIC as a limbic sensory structure (Craig, 2009; Medford and Critchley, 2010).
AIC integrates top-down and bottom-up information
Functional integration concerns the convergence (superadditive effect) of multiple mental operations. To demonstrate the involvement of any brain region in functional integration, at least two axioms need to be met: 1) corepresentation of each of the mental processes in that brain region and 2) an interaction between operations in the same region (Calvert, 2001; Gu et al., 2013). This definition is analogical to the idea of multisensory integration (MSI); in the presence of multiple sensory inputs, the firing rate of an MSI cell exceeds the sum of its responses to each input if processed separately (Roelfsema et al., 1997; Calvert, 2001). Investigating the integration of top-down and bottom-up information is crucial because it directly speaks to the general organizational principles of the brain (Dayan et al., 1995; Friston, 2002, 2012; Corbetta et al., 2008) and consciousness (Dehaene et al., 2006; Tononi and Koch, 2008; Dehaene and Changeux, 2011; Edlund et al., 2011). Top-down processes are often driven by task demand and largely voluntary; bottom-up processes, on the other hand, are mostly stimulus-driven and subject to voluntary control. In the framework of predictive coding and hierarchical inference, top-down, higher-level cortical activities try to predict or “explain away” the bottom-up sensory information conveyed by lower-level brain regions (Friston, 2002, 2010). The integration of bottom-up input and top-down recurrent information is considered essential for consciousness to occur (Tononi and Koch, 2008) and is likely to be subserved by long-distance projection neurons (Dehaene et al., 2006; Dehaene and Changeux, 2011).
We propose that AIC serves as a key node in such information integration process, based on findings of superadditive effects of task demand and stimulus valence in AIC (Fig. 2C, modified from Gu et al., 2013; for review see Sterzer and Kleinschmidt, 2010). Although much research has focused on the modulatory effect of one mental process on another (Ochsner and Gross, 2005; Blair et al., 2007), fMRI evidence supporting functional integration is limited. One previous study suggests that dorsolateral PFC is involved in cognition–emotion integration (Gray et al., 2002); however, cognitive and emotional information were spatially and temporally segregated in the design, so one can speak only to the existence of distinct yet partially shared processing modules. In contrast, by simultaneously manipulating and top-down demand (e.g., laterality judgment TL and body-part judgment TB) and stimulus valence (e.g., pain, no pain), one can test a synergistic effect and introduce competition for neuroanatomical resources, an approach commonly used in sensory integration studies (Calvert, 2001). Behavioral data showed increased response time and error rates for painful compared with nonpainful stimuli under the difficult task TL relative to the easy task TB, indicating an interaction between stimulus valence (i.e., pain) and top-down influence (i.e., task demand). In parallel to behavioral findings, the activation of AIC and its related regions and networks such as somatosensory cortices showed corepresentation of stimulus valence and top-down effects and a superadditive interaction effect between cognitive load and stimulus valence, suggesting that information integration took place in these brain regions. Corepresentation of the cognitive evaluation and the stimulus valence of pain in AIC have been shown in previous studies (Salomons et al., 2004; Wager et al., 2004; Gu and Han, 2007a,b). Additionally, integration of stimulus predictability and subjective preference in right AIC is also reported for a juice delivery task (Berns et al., 2001).
The significance of these findings can be summarized as follows. First, The joint involvement of AIC and SI in integrating top-down and bottom-up information suggests that a possible “somatic marker” signal (Damasio, 1996) is activated when the processing of affective visual stimuli is guided by certain top-down requirements. Such signal might be subsequently conveyed to control regions such as ACC and prefrontal cortex for appropriate behavioral output. Second, information integration in AIC is supported by its anatomical (Mesulam and Mufson, 1982b; Saper, 2002) and intrinsic functional (Cauda et al., 2011; Deen et al., 2011; Touroutoglou et al., 2012; Chang et al., 2013) connectivity with a large scale network of sensorimotor, affective, and cognitive control regions. Third, the left AIC showed a more robust interaction effect (surviving in both ROI and whole-brain analyses) than the right AIC. We speculate that the left AIC might have advantageous access to structures involved in sensorimotor and cognitive control regions, which makes the integration easier compared with the right AIC. Fourth, such synergy did not occur in ACC, which further suggests that AIC and ACC are functionally dissociable and singles out the significance of AIC in subjective awareness. These findings provide important, although not necessarily conclusive evidence, to help uncover the nature of high-level information integration and, potentially, awareness.
AIC is necessary for emotional awareness
Finally, we demonstrate the necessity of AIC activity in emotional awareness by presenting evidence from neuropsychology studies. Although the insula has received increasing attention in the neuroimaging field, studies on the effects of focal insular lesions are still limited (for review see Jones et al., 2010). For instance, focal lesions in this region impair disgust perception (Calder et al., 2000). We examined both explicit and implicit empathetic pain perception in three patients with focal AIC lesions, in comparison with patients with focal ACC lesions and neurologically intact controls (Fig. 2D, modified from Gu et al., 2012). In the explicit task (Fig. 2D, left), AIC patients, but not ACC patients, displayed a striking impairment in sensitivity (measured by d′) to others’ pain when explicitly asked to evaluate pain, suggesting that AIC is necessary for explicit empathetic pain processing. In the implicit task of limb laterality judgment (Fig. 2D, right), control subjects showed a normal stimulus–task interference effect indexed by negative d′ difference between painful and nonpainful stimuli (i.e., pain worsened judgment of laterality). However, AIC patients lacked such interference between stimulus valence and laterality judgment, indicating that the integration of stimulus-driven and top-down information was impaired in patients with AIC lesions and that AIC is necessary for bottom-up and top-down integration. Taken together, these findings demonstrate the necessity for AIC, but not ACC, in emotional awareness. Although AIC and ACC are usually considered as one system with AIC as the input sensory region and ACC as the output control region (Seeley et al., 2007; Craig, 2009; Jones et al., 2010), the finding that ACC is usually activated by but not necessary for empathetic pain perception suggests the existence of multiple control regions other than ACC and that AIC is the only critical input region.
Three recent case studies specifically examined the effects of bilateral insular lesions on subjective awareness and reported intact interoceptive awareness (Khalsa et al., 2009) and self-awareness (Philippi et al., 2012; Damasio et al., 2013). The authors concluded that the insula is not necessary for awareness. Although we agree with the authors that emotional awareness is less likely to be subserved by one neural substrate such as the insula and is more likely to be processed by distributed networks including low-level brainstem and thalamic nuclei, we next discuss possible sources of discrepancies in the findings among studies. First, although the insular lesions in the two patients “Roger” (Khalsa et al., 2009; Philippi et al., 2012) and “M” (Damasio et al., 2013) are bilateral, there are still residual tissues in the insula that might convey some interoceptive information (Craig, 2011). Second, the “core” self-awareness assessed by Damasio and colleagues (Philippi et al., 2012; Damasio et al., 2013) is based on self-recognition tasks, which are subserved by distributed neural networks also involving the frontal–temporal network (Keenan et al., 2000) and cortical midline structures (Zhu et al., 2007; Han and Northoff, 2009). Therefore, Roger and M might also be very likely to utilize their intact frontal regions in self-recognition tasks. Third, patient Roger did exhibit deficits in certain aspects of interoceptive awareness, as reported in supplemental materials by Khalsa and colleagues (2009). For instance, he was unable to detect heart rate change at low doses of isoproterenol infusion, his response lag was significantly longer than controls, and his interoceptive awareness was greatly worsened after anesthetic application (Khalsa et al., 2009). These findings are consistent with our findings on unilateral AIC lesions in that interoceptive and emotional awareness is impaired but not completely abolished in these patients.
SUMMARY: A MODEL OF AIC AND EMOTIONAL AWARENESS
Based on the evidence described above, we propose that AIC plays a critical and necessary role in emotional awareness. Based on the hierarchical active inference scheme (Dayan et al., 1995; Friston, 2010) and several previous models on the insula (Craig, 2009; Singer et al., 2009; Seth et al., 2011) and awareness (Tononi and Koch, 2008), we consider a dual-process model (Fig. 3) in which AIC serves two major functions: 1) integrating bottom-up interoceptive prediction error signals with top-down predictions from high-level cortical areas and 2) providing descending interoceptive predictions to visceral systems that provide a point of reference for autonomic reflexes.
Figure 3.
Hypothetical model of insula and awareness. In this hierarchical scheme, each lower-level structure receives descending predictions from and sends ascending prediction errors to higher-level regions. Anterior insular cortex (AIC) serves two major processes in this model (center): 1) integrating bottom-up interoceptive prediction errors with top-down predictions from high-order brain regions such as the anterior cingulate cortex (ACC) and prefrontal cortex (PFC), analogous to the role of sensory cortices (e.g., visual and auditory areas) in exteroceptive processing (left) and 2) sending descending predictions to the autonomic system via smooth muscles to provide a point of reference for autonomic reflexes, similar to the role of motor cortex in generating proprioceptive output via striated muscles (right).
In the first (bottom-up) process, AIC functions as an interface between interoceptive input and top-down predictions from high-order cortical regions (i.e., PFC and ACC). This process is analogous to the role of sensory cortices such as visual and auditory areas in integrating bottom-up exteroceptive input with top-down signals (Fig. 3, left). This integration produces a signal representing the organism’s current awareness state. We consider this signal to represent the current state of the organism that, on the one hand, is consistent with top-down predictions from higher-level representations of goals, actions, and attention (e.g., from ACC) and, on the other hand, best predicts future awareness states. This integration or synthesis of forward and backward signals is consistent with formulations of hierarchical inference in the brain and neuronal implementations based on predictive coding (see, e.g., Seth et al., 2011).
This notion is supported by empirical evidence of an integrative role of AIC, reviewed in previous sections, and is consistent with generative models of the brain and consciousness that suggest that the integration of bottom-up and top-down signals is important for subjective awareness (Friston, 2002, 2010; Tononi and Koch, 2008). According to a predictive coding account of the brain (Dayan et al., 1995; Friston, 2002, 2010), bottom-up stimulus-driven projections convey prediction errors, and top-down pathways convey active predictions. Therefore, functional integration in AIC also suggests a predictive coding role of AIC (Singer et al., 2009; Seth et al., 2011). One study specifically showed that AIC encodes both prediction of pain and pain prediction error in the same task (Seymour et al., 2004), which directly supports the functional integration and predictive encoding account of AIC.
Recent developments in computational neuroscience support a role for AIC in encoding predictions and updating these predictions on the basis of bottom-up interoceptive prediction error. In line with the conceptual framework proposed by Singer and colleagues (2009), a recent empirical study shows that subjects use Bayesian rules to update their feelings and that (feeling) variance in prediction errors scale parametrically with AIC activation (Xiang et al., 2013). Another study showed that AIC is activated by not only prediction of risk but also the prediction error of risk (Preuschoff et al., 2008), which relates to unexpected uncertainty and state transition (Yu and Dayan, 2005). Sudden changes in reward contingencies and inquisitive policies also activate AIC (Li et al., 2006).
The second (top-down) process corresponds to the provision of descending predictions to visceral systems (i.e., via smooth muscles) that provide a point of reference for autonomic reflexes and sympathetic/parasympathetic outflow and for generating future awareness states. In other words, the AIC both responds to and controls the internal milieu or literally “gut feelings.” The AIC is perfectly placed anatomically to do this; it is equipped with the anatomical and functional foundation to perform the very important task of inducing transitions in physiological states. As reviewed above, neurons in AIC innervate the viscera directly and indirectly, for example, through projections to the hypothalamic area via the amygdala. In short, AIC is able to cause changes in the physiological states of the body, in addition to perceiving changes from the body.
To illustrate the simplicity and potential power of this model, consider the analogous role of the AIC in controlling autonomic reflexes via smooth muscles and the motor cortex in controlling proprioceptive reflexes via striated muscles (Fig. 3, right). Recent predictive coding formulations of motor control consider descending corticospinal signals from motor cortex to provide predictions or set-points for classical reflex arcs in the spinal cord (Adams et al., 2013). In this view, descending predictions control behavior by enslaving peripheral reflexes. Our proposal here is exactly the same; however, the descending predictions are not of proprioceptive states but of interoceptive states, and the reflexes become autonomic in nature. Put simply, one might think of the insular cortex as a ventral extension of the sensorimotor strip that is concerned not with proprioception (and exteroception) but with interoception (Craig, 2002, 2009, 2011). This perspective has been developed by a number of authors (Allman et al., 2005; Seeley et al., 2006; Butti and Hof, 2010; Evrard et al., 2012) and nicely accommodates several observations reviewed above.
This model resolves the conceptual dialectic between the James-Lange theory and the Cannon-Bard formulations, in the sense that they are both right: bodily sensations both cause and are caused by central representations. This is a necessary consequence of hierarchical Bayesian inference and the recurrent exchange of neuronal signals implicit in predictive coding. The model also explains the findings suggesting that the insula serves a dual visceromotor and viscerosensory function.
By analogy with the motor cortex, our model explains why the insular cortex possesses viscerotopic maps. Furthermore, like the motor cortex, the AIC is agranular. This is a remarkable exception to the laminar structure of the neocortex, which is shared only by the motor cortex, the ACC, and the AIC. This suggests a privileged role in the generation of descending predictions to peripheral systems.
In predictive coding schemes, it is generally thought that top-down predictions originate in infragranular pyramidal cells. For example, in the motor cortex, descending predictions originate from large pyramidal cells (e.g., Betz cells) in deep cortical layers. It is tempting to speculate that VENs of the AIC (which are located in layer V) play this role, as suggested by several investigators (Allman et al., 2005; Seeley et al., 2006; Butti and Hof, 2010; Evrard et al., 2012).
This perspective on the AIC as an integral part of hierarchical predictive coding in the brain explains the involvement of AIC across low-level autonomic and sensorimotor (Craig et al., 2000; Sterzer and Kleinschmidt, 2010; Fan et al., 2012) to high-level cognitive and social (Montague and Lohrenz, 2007; King-Casas et al., 2008; Bossaerts, 2010; Kirk et al., 2011) domains. This proposal does not preclude the participation of other brain regions in emotional awareness. On the contrary, AIC as well as other subregions of the insular cortex work closely coupled with other brain regions and networks (Cauda et al., 2011; Deen et al., 2011; Peltz et al., 2011) to translate different modalities of information effectively into subjective awareness. A posterior-to-anterior gradient of processing complexity exists within the insular cortex, with AIC representing the most complex and abstract end of this axis (Craig, 2009, 2010). AIC, in this sense, could be where the “sentient self” resides.
In summary, the proposed model extends previous models of AIC (Craig, 2009; Singer et al., 2009; Seth et al., 2011), although many details in the proposed AIC model remain unknown. For instance, what happens at the neuronal and molecular levels during the actual information integration process? How does information flow among AIC, ACC, and many other closely related structures? How do deficits in these processes manifest in disease? Finer-grained quantitative investigations and combination of neuroimaging, lesion, stimulation, biochemical methods, and theoretical neurobiology are needed to answer these questions, to advance our understanding of functions of the insular cortex and human emotional awareness.
Acknowledgments
Grant sponsor: National Institute of Health; Grant number: R21 MH083164 and R01 MH094305 (to J.F.); Grant sponsor: James S. McDonnell Foundation; Grant number: 22002078 (to P.R.H.); Grant sponsor: The Wellcome Trust (to K.J.F).
We thank Dr. P. Read Montague and the Human Neuroimaging Laboratory for support and Dr. Xun Liu and Ji Young Kim, David Fan, and Gabrielle Frenkel for help with the meta-analysis. Dr. Montague provided funding to X.G. through a Wellcome Trust Principal Award. The contents of the present article are solely the responsibility of the authors and do not necessarily represent the official views of funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ROLE OF AUTHORS
Drafting of the manuscript: XG. Meta-analysis: XG, JF. Design of the study: XG, JF, PRH. Critical revision of the manuscript for important intellectual content: PRH, KJF, JF.
LITERATURE CITED
- Adams RA, Shipp S, Friston KJ. Predictions not commands: active inference in the motor system. Brain Struct Funct. 2013;218:611–643. doi: 10.1007/s00429-012-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Akitsuki Y, Decety J. Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage. 2009;47:722–734. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- Bach DR, Dolan RJ. Knowing how much you don’t know: a neural organization of uncertainty estimates. Nat Rev Neurosci. 2012;13:572–586. doi: 10.1038/nrn3289. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–2120. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am J Physiol. 1928;84:490–515. [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bauernfeind AL, de Sousa AA, Avasthi T, Dobson SD, Raghanti MA, Lewandowski AH, Zilles K, Semendeferi K, Allman JM, Craig AD, Hof PR, Sherwood CC. A volumetric comparison of the insular cortex and its subregions in primates. J Hum Evol. 2013;64:263–279. doi: 10.1016/j.jhevol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem DJ. Self-perception: an alternative interpretation of cognitive dissonance phenomena. Psychol Rev. 1967;74:183–200. doi: 10.1037/h0024835. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Schaller K, Clusmann H. The seminal contributions of Johann-Christian Reil to anatomy, physiology, and psychiatry. Neurosurgery. 2007;61:1091–1096. doi: 10.1227/01.neu.0000303205.15489.23. discussion 1096. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–1525. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Struct Funct. 2010;214:645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage. 2010;52:1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- Butti C, Hof PR. The insular cortex: a comparative perspective. Brain Struct Funct. 2010;214:477–493. doi: 10.1007/s00429-010-0264-y. [DOI] [PubMed] [Google Scholar]
- Butti C, Sherwood CC, Hakeem AY, Allman JM, Hof PR. Total number and volume of von Economo neurons in the cerebral cortex of cetaceans. J Comp Neurol. 2009;515:243–259. doi: 10.1002/cne.22055. [DOI] [PubMed] [Google Scholar]
- Butti C, Santos M, Uppal N, Hof PR. von Economo neurons: clinical and evolutionary perspectives. Cortex. 2013;49:312–326. doi: 10.1016/j.cortex.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The wisdom of the body. New York: W.W. Norton and Co; 1932. p. 312. [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Soc Neurosci. 2006;1:364–384. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham M, Pedroni AF, Antley A, Slater M, Jancke L. Virtual milgram: empathic concern or personal distress? Evidence from functional MRI and dispositional measures. Front Hum Neurosci. 2009;3:29. doi: 10.3389/neuro.09.029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Paul R, Zawacki TM, Moser DJ, Sweet L, Wilkinson H. Emotional and personality changes following cingulotomy. Emotion. 2001;1:38–50. doi: 10.1037/1528-3542.1.1.38. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini M, Galati G, Romani GL, Aglioti SM. Empathic neural reactivity to noxious stimuli delivered to body parts and non-corporeal objects. Eur J Neurosci. 2008;28:1222–1230. doi: 10.1111/j.1460-9568.2008.06406.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Damasio A, Damasio H, Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cereb Cortex. 2013;23:833–846. doi: 10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Dayan P, Hinton GE, Neal RM, Zemel RS. The Helmholtz machine. Neural Comput. 1995;7:889–904. doi: 10.1162/neco.1995.7.5.889. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Echols S, Correll J. The blame game: the effect of responsibility and social stigma on empathy for pain. J Cogn Neurosci. 2010;22:985–997. doi: 10.1162/jocn.2009.21266. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI, Schneider F, Habel U. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. Affect is a form of cognition: a neurobiological analysis. Cogn Emot. 2007;21:1184–1211. doi: 10.1080/02699930701437931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Ferri F, Salone A, Perrucci MG, D’Amico L, Ferro FM, Romani GL, Gallese V. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J Cogn Neurosci. 2011;23:1808–1822. doi: 10.1162/jocn.2010.21551. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund JA, Chaumont N, Hintze A, Koch C, Tononi G, Adami C. Integrated information increases with fitness in the evolution of animats. PLoS Comput Biol. 2011;7:e1002236. doi: 10.1371/journal.pcbi.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC, Forro T, Logothetis NK. von Economo neurons in the anterior insula of the macaque monkey. Neuron. 2012;74:482–489. doi: 10.1016/j.neuron.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Logothetis NK, Craig AD. Modular architectonic organization of the insula in the macaque monkey. J Comp Neurol. 2013 doi: 10.1002/cne.23436. in press. [DOI] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Fan J, Gu X, Liu X, Guise KG, Park Y, Martin L, de Marchena A, Tang CY, Minzenberg MJ, Hof PR. Involvement of the anterior cingulate and frontoinsular cortices in rapid processing of salient facial emotional information. Neuroimage. 2011;54:2539–2546. doi: 10.1016/j.neuroimage.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, Hof PR. Spontaneous brain activity relates to autonomic arousal. J Neurosci. 2012;32:11176–11186. doi: 10.1523/JNEUROSCI.1172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A. Anatomy of the insula-functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- Friston K. Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu Rev Neurosci. 2002;25:221–250. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Gallay DS, Gallay MN, Jeanmonod D, Rouiller EM, Morel A. The insula of Reil revisited: multiarchitectonic organization in macaque monkeys. Cereb Cortex. 2012;22:175–190. doi: 10.1093/cercor/bhr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Beacher FD, Minati L, Nagai Y, Kemp AH, Harrison NA, Critchley HD. Emotional appraisal is influenced by cardiac afferent information. Emotion. 2012;12:180–191. doi: 10.1037/a0025083. [DOI] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007a;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Gu X, Han S. Neural substrates underlying evaluation of pain in actions depicted in words. Behav Brain Res. 2007b;181:218–223. doi: 10.1016/j.bbr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30:3739–3744. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, Fan J. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition–emotion integration in the anterior insular cortex. Cereb Cortex. 2013;23:20–27. doi: 10.1093/cercor/bhr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeem AY, Sherwood CC, Bonar CJ, Butti C, Hof PR, Allman JM. von Economo neurons in the elephant brain. Anat Rec. 2009;292:242–248. doi: 10.1002/ar.20829. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G. Understanding the self: a cultural neuroscience approach. Prog Brain Res. 2009;178:203–212. doi: 10.1016/S0079-6123(09)17814-7. [DOI] [PubMed] [Google Scholar]
- Han S, Gu X, Mao L, Ge J, Wang G, Ma Y. Neural substrates of self-referential processing in Chinese Buddhists. Soc Cogn Affect Neurosci. 2009;5:332–339. doi: 10.1093/scan/nsp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. J Neurosci. 2010;30:12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Macey PM, Macey KE, Frysinger RC, Woo MA, Harper RK, Alger JR, Yan-Go FL, Harper RM. Brain responses associated with the Valsalva maneuver revealed by functional magnetic resonance imaging. J Neurophysiol. 2002;88:3477–3486. doi: 10.1152/jn.00107.2002. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, Lange KW, Ceballos-Baumann AO. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U. Brief report: cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J Autism Dev Disord. 2004;34:229–235. doi: 10.1023/b:jadd.0000022613.41399.14. [DOI] [PubMed] [Google Scholar]
- Hof PR, Van der Gucht E. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae) Anat Rec. 2007;290:1–31. doi: 10.1002/ar.20407. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Mentalizing about emotion and its relationship to empathy. Soc Cogn Affect Neurosci. 2008;3:204–217. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proc Natl Acad Sci U S A. 2009;106:8021–8026. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind os-IX. 1884:188–205. [Google Scholar]
- Jones CL, Ward J, Critchley HD. The neuropsychological impact of insular cortex lesions. J Neurol Neurosurg Psychiatry. 2010;81:611–618. doi: 10.1136/jnnp.2009.193672. [DOI] [PubMed] [Google Scholar]
- Joukamaa M, Saarijarvi S, Muuriaisniemi ML, Salokangas RK. Alexithymia in a normal elderly population. Compr Psychiatry. 1996;37:144–147. doi: 10.1016/s0010-440x(96)90576-3. [DOI] [PubMed] [Google Scholar]
- Kaufman JA, Paul LK, Manaye KF, Granstedt AE, Hof PR, Hakeem AY, Allman JM. Selective reduction of von Economo neuron number in agenesis of the corpus callosum. Acta Neuropathol. 2008;116:479–489. doi: 10.1007/s00401-008-0434-7. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Wheeler MA, Gallup GG, Jr, Pascual-Leone A. Self-recognition and the right prefrontal cortex. Trends Cogn Sci. 2000;4:338–344. doi: 10.1016/s1364-6613(00)01521-7. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12:1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Sidhu M, Gaus SE, Huang EJ, Hof PR, Miller BL, DeArmond SJ, Seeley WW. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb Cortex. 2012;22:251–259. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kim SE, Kim JJ, Jeong B, Park CH, Son AR, Song JE, Ki SW. Compassionate attitude towards others’ suffering activates the mesolimbic neural system. Neuropsychologia. 2009;47:2073–2081. doi: 10.1016/j.neuropsychologia.2009.03.017. [DOI] [PubMed] [Google Scholar]
- King AB, Menon RS, Hachinski V, Cechetto DF. Human forebrain activation by visceral stimuli. J Comp Neurol. 1999;413:572–582. [PubMed] [Google Scholar]
- King JA, Blair RJ, Mitchell DG, Dolan RJ, Burgess N. Doing the right thing: a common neural circuit for appropriate violent or compassionate behavior. Neuroimage. 2006;30:1069–1076. doi: 10.1016/j.neuroimage.2005.10.011. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Downar J, Montague PR. Interoception drives increased rational decision-making in meditators playing the ultimatum game. Front Neurosci. 2011;5:49. doi: 10.3389/fnins.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkonen P, Karvonen JT, Veijola J, Laksy K, Jokelainen J, Jarvelin MR, Joukamaa M. Prevalence and sociodemographic correlates of alexithymia in a population sample of young adults. Compr Psychiatry. 2001;42:471–476. doi: 10.1053/comp.2001.27892. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Mohammadi B, Donamayor N, Samii A, Munte TF. Emotional and cognitive aspects of empathy and their relation to social cognition—an fMRI-study. Brain Res. 2010;1311:110–120. doi: 10.1016/j.brainres.2009.11.043. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Sjostrom T, Chen YP, Wang YH, Huang CY. Intuition and deliberation: two systems for strategizing in the brain. Science. 2009;324:519–522. doi: 10.1126/science.1165598. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JD. Self-attribution of emotion: the effects of expressive behavior on the quality of emotional experience. J Pers Soc Psychol. 1974;29:475–486. doi: 10.1037/h0036125. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J. Is the extrastriate body area (EBA) sensitive to the perception of pain in others? Cereb Cortex. 2008;18:2369–2373. doi: 10.1093/cercor/bhn006. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007a;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007b;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. J Cogn Neurosci. 2010;22:362–376. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- Lane RD, Schwartz GE. Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. Am J Psychiatry. 1987;144:133–143. doi: 10.1176/ajp.144.2.133. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lange C. Om sindsbevaegelser: et psyko-fysiologisk studie. Kjbenhavn: Jacob Lunds; 1885. Reprinted in: Lange CG, James W, editors. The emotions. Haupt IA, trans. Baltimore: Williams & Wilkins. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Li J, McClure SM, King-Casas B, Montague PR. Policy adjustment in a dynamic economic game. PLoS ONE. 2006;1:e103. doi: 10.1371/journal.pone.0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lindgren L, Westling G, Brulin C, Lehtipalo S, Andersson M, Nyberg L. Pleasant human touch is represented in pregenual anterior cingulate cortex. Neuroimage. 2012;59:3427–3432. doi: 10.1016/j.neuroimage.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. Neuroimage. 2010;51:1468–1475. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982b;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Montague PR, Lohrenz T. To detect and correct: norm violations and their enforcement. Neuron. 2007;56:14–18. doi: 10.1016/j.neuron.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007;37:642–651. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cogn Affect Behav Neurosci. 2004;4:270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Morrison I, Peelen MV, Downing PE. The sight of others’ pain modulates motor processing in human cingulate cortex. Cereb Cortex. 2007;17:2214–2222. doi: 10.1093/cercor/bhl129. [DOI] [PubMed] [Google Scholar]
- Naidich TP, Kang E, Fatterpekar GM, Delman BN, Gultekin SH, Wolfe D, Ortiz O, Yousry I, Weismann M, Yousry TA. The insula: anatomic study and MR imaging display at 1.5 T. Am J Neuroradiol. 2004;25:222–232. [PMC free article] [PubMed] [Google Scholar]
- Newman-Norlund RD, Ganesh S, van Schie HT, De Bruijn ER, Bekkering H. Self-identification and empathy modulate error-related brain activity during the observation of penalty shots between friend and foe. Soc Cogn Affect Neurosci. 2009;4:10–22. doi: 10.1093/scan/nsn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Vogt BA, Morrison JH, Hof PR. Spindle neurons of the human anterior cingulate cortex. J Comp Neurol. 1995;355:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci U S A. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Parkkola R, Hietanen JK. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage. 2008;43:571–580. doi: 10.1016/j.neuroimage.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, Glover GH, Mackey SC. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3:144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A, Soares JJ. “Unconscious anxiety”: phobic responses to masked stimuli. J Abnorm Psychol. 1994;103:231–240. doi: 10.1037//0021-843x.103.2.231. [DOI] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwijk S, Lindquist KA, Anderson E, Dautoff R, Moriguchi Y, Barrett LF. States of mind: emotions, body feelings, and thoughts share distributed neural networks. Neuroimage. 2012;62:2110–2128. doi: 10.1016/j.neuroimage.2012.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J, Derbyshire SW. Pain sensation evoked by observing injury in others. Pain. 2010;148:268–274. doi: 10.1016/j.pain.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Peltz E, Seifert F, DeCol R, Dorfler A, Schwab S, Maihofner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage. 2011;54:1324–1335. doi: 10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin Neurobiol. 2005;15:188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Feinstein JS, Khalsa SS, Damasio A, Tranel D, Landini G, Williford K, Rudrauf D. Preserved self-awareness following extensive bilateral brain damage to the insula, anterior cingulate, and medial prefrontal cortices. PLoS ONE. 2012;7:e38413. doi: 10.1371/journal.pone.0038413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Picard F. State of belief, subjective certainty and bliss as a product of cortical dysfunction. Cortex. 2013 doi: 10.1016/j.cortex.2013.01.006. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Fustos J, Critchley HD. On the generalised embodiment of pain: how interoceptive sensitivity modulates cutaneous pain perception. Pain. 2012;153:1680–1686. doi: 10.1016/j.pain.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radna RJ, MacLean PD. Vagal elicitation of respiratory-type and other unit responses in striopallidum of squirrel monkeys. Brain Res. 1981;213:29–44. doi: 10.1016/0006-8993(81)91246-4. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Engel AK, Konig P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–161. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another’s face. Cereb Cortex. 2007;17:230–237. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45:810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, Heinsen H, Schmitz C, Hof PR. von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res. 2011;1380:206–217. doi: 10.1016/j.brainres.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19:1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42:393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Seeley WW. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct. 2010;214:465–475. doi: 10.1007/s00429-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, Dearmond SJ. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Merkle FT, Gaus SE, Craig AD, Allman JM, Hof PR, Economo CV. Distinctive neurons of the anterior cingulate and frontoinsular cortex: a historical perspective. Cereb Cortex. 2012;22:245–250. doi: 10.1093/cercor/bhr005. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Simeon D, Giesbrecht T, Knutelska M, Smith RJ, Smith LM. Alexithymia, absorption, and cognitive failures in depersonalization disorder: a comparison to posttraumatic stress disorder and healthy volunteers. J Nerv Ment Dis. 2009;197:492–498. doi: 10.1097/NMD.0b013e3181aaef6b. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct Funct. 2010;214:611–622. doi: 10.1007/s00429-010-0252-2. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Levenson RW. Alexithymia in neurodegenerative disease. Neurocase. 2011;17:242–250. doi: 10.1080/13554794.2010.532503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ. Recent developments in alexithymia theory and research. Can J Psychiatry. 2000;45:134–142. doi: 10.1177/070674370004500203. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA. The 20-Item Toronto Alexithymia Scale: IV. Reliability and factorial validity in different languages and cultures. J Psychosom Res. 2003;55:277. doi: 10.1016/s0022-3999(02)00601-3. [DOI] [PubMed] [Google Scholar]
- Thompson E, Varela FJ. Radical embodiment: neural dynamics and consciousness. Trends Cogn Sci. 2001;5:418–425. doi: 10.1016/s1364-6613(00)01750-2. [DOI] [PubMed] [Google Scholar]
- Tononi G, Koch C. The neural correlates of consciousness: an update. Ann N Y Acad Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushida T, Ikemoto T, Tanaka S, Shinozaki J, Taniguchi S, Murata Y, McLaughlin M, Arai YC, Tamura Y. Virtual needle pain stimuli activates cortical representation of emotions in normal volunteers. Neurosci Lett. 2008;439:7–12. doi: 10.1016/j.neulet.2008.04.085. [DOI] [PubMed] [Google Scholar]
- Valentini E. The role of anterior insula and anterior cingulate in empathy for pain. J Neurophysiol. 2010;104:584–586. doi: 10.1152/jn.00487.2010. [DOI] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc Neurosci. 2007;2:179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- von Economo C. Eine neue Art Spezialzellen des Lobus cinguli und Lobus insulae. Z Ges Neurol Psychiat. 1926;100:706–712. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Xiang T, Lohrenz T, Montague PR. Computational substrates of norms and their violations during social exchange. J Neurosci. 2013;33:1099–1108. doi: 10.1523/JNEUROSCI.1642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. J Neurosci. 2009;29:8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kawamura Y. Cortical responses to electrical and gustatory stimuli in the rabbit. Brain Res. 1975;94:447–463. doi: 10.1016/0006-8993(75)90228-0. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2:276–291. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc Natl Acad Sci U S A. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. Neuroimage. 2007;34:1310–1316. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]