Abstract
Background
Patients with newly diagnosed HIV may be part of social networks with elevated prevalence of undiagnosed HIV infection. Social network recruitment by persons with newly diagnosed HIV may efficiently identify undiagnosed cases of HIV infection. We assessed social network recruitment as a strategy for identifying undiagnosed cases of HIV infection.
Methods
In an STI clinic in Lilongwe, Malawi, three groups of 45 “seeds” were enrolled: STI patients with newly diagnosed HIV, STI patients who were HIV-uninfected, and community controls. Seeds were asked to recruit up to 5 social “contacts” (sexual or non-sexual). Mean number of contacts recruited per group was calculated. HIV prevalence ratios and number of contacts needed to test to identify one new case of HIV were compared between groups using generalized estimating equations with exchangeable correlation matrices.
Results
Mean number of contacts recruited was 1.3 for HIV-infected clinic seeds, 1.8 for HIV-uninfected clinic seeds and 2.3 for community seeds. Contacts of HIV-infected clinic seeds had a higher HIV prevalence (PR: 3.2, 95% CI: 1.3, 7.8) than contacts of community seeds, but contacts of HIV-uninfected clinic seeds did not (PR: 1.1, 95% CI: 0.4, 3.3). Results were similar when restricted to non-sexual contacts. To identify one new case of HIV it was necessary to test 8 contacts of HIV-infected clinic seeds, 10 contacts of HIV-uninfected clinic seeds, and 18 contacts of community seeds.
Conclusions
Social contact recruitment by newly diagnosed STI patients efficiently led to new HIV diagnoses. Research to replicate findings and guide implementation is needed.
Keywords: HIV, social network, sexually transmitted infection, Malawi, HIV counseling and testing, syndromic management
Background
Timely HIV diagnosis is a necessary step for accessing HIV care and treatment and an important step for reducing HIV transmission1–3. In spite of recent scale-up of HIV counseling and testing in sub-Saharan Africa, many adults still have not been tested for HIV3. In Malawi in 2009, 11% of the adult population was infected with HIV, but only one third of HIV-infected adults knew they were HIV-infected4. The remaining two thirds either never tested or received an HIV-negative result at the time of their last test4. Because persons unaware of HIV infection contribute disproportionately to HIV transmission4–6, strategies are needed to identify these persons.
Several strategies are available for increasing HIV testing and counseling (HTC). Stand-alone voluntary counseling and testing addresses a need for client-driven HTC, but misses those who do not seek services. Opt-out HTC reaches most care-seekers in many clinical settings7, but misses populations that do not routinely present for care. Community-based strategies, such as door-to-door HTC, have been effective and reasonably efficient at reaching first-time testers8–13. However, such efforts typically have been implemented once in settings where most adults have never been tested. In settings where most adults have been tested, the efficiency of door-to-door testing is diminished, as many prevalent cases have already been detected14,15. In Malawi, where 11% of adults are HIV-infected, and a large share know their HIV status, it would be necessary to test many adults to identify each new case of HIV.
In light of recent scale-up of HCT, new strategies are needed to identify hard-to-reach undiagnosed cases of HIV infection. Asking high risk patients with sexually transmitted infections (STIs) and newly diagnosed HIV to recruit social contacts is one possible strategy. Success of such a strategy would hinge on three premises: 1) Feasibility: STI patients can successfully recruit members of their social networks; 2) Effectiveness: STI patients have social networks with high HIV prevalence; and 3) Efficiency: Few contacts require screening to identify one new case of HIV. Demonstration projects of social contact recruitment in the United States have led to improved case-finding, with HIV-infected persons and high-risk HIV-uninfected persons more likely to recruit other HIV-infected persons16–18. Similarly, respondent driven sampling has effectively found undiagnosed cases of STIs and HIV in concentrated epidemics19, and been piloted in generalized epidemics.20–22 However, social contact recruitment based on STI and HIV status has never been formally assessed as a strategy to identify undiagnosed cases of HIV in a generalized HIV epidemic. This strategy is promising in light of “differential affiliation,” persons affiliating with social groups who have comparable HIV risk.23
In an STI clinic in Lilongwe, Malawi, we evaluated a social contact recruitment program. We assessed 1) whether newly diagnosed HIV-infected and HIV-uninfected STI patients were able to recruit social network members for HIV screening (feasibility), 2) the distribution of HIV among contacts who presented (effectiveness), and 3) number of contacts recruited to identify one new case of HIV (efficiency). For assessments of feasibility, effectiveness, and efficiency we compared contacts of HIV-infected and HIV-uninfected STI patients to contacts of community controls.
Methods
This study was conducted at the sexually transmitted infection (STI) clinic at Kamuzu Central Hospital in Lilongwe, Malawi from November 2010-February 2012. This clinic serves persons with symptomatic STIs and their partners. During this period, patients were routinely assessed for STIs using Malawi’s syndromic management algorithm. Using this algorithm, patients reporting STI symptoms received clinical exams. In clinical exams, patients were examined for genital ulcer disease, urethral/vaginal discharge, genital warts, and bubo. Females were screened for lower abdominal pain and males for balanitis. During this period all patients were offered HTC using parallel HIV-1 antibody rapid tests: Alere Determine™ HIV-1/2 Rapid Test and Trinity Biotech Uni-Gold™ HIV Rapid Test. Patients with two positive HIV antibody tests were classified as having established HIV-infection. Patients with at least one HIV-negative antibody test result were offered HIV RNA PCR screening with Abbott Real Time HIV-1 Assay through CHAVI 001, a concomitant study in this setting. Patients with positive PCR results were classified with acute HIV infection (AHI), and those with negative PCR results were classified as HIV-uninfected.
Seed Participant Procedures
Three groups of 45 “seeds” were enrolled: newly diagnosed HIV-infected STI clinic patients with STI syndromes, HIV-uninfected STI clinic patients with STI syndromes, and community controls.
To recruit clinic seeds, up to four patients were randomly selected from the STI clinic roster each day and screened for participation. Patients were eligible for seed participation if they were 18–45 years, residing within the Lilongwe City catchment area, diagnosed with an STI syndrome in the last two weeks, and not referred by a sexual contact. Patients who previously received an HIV-positive test result were excluded. All patients recently diagnosed with AHI were invited to participate as HIV-infected seeds, regardless of random selection from the clinic roster or meeting other eligibility criteria.
Community control seeds were recruited from the STI clinic catchment area using frequency matching based on clinic seeds’ ages, genders, and areas. Forty-five areas (the primary Lilongwe geographic units) were selected from the areas where the 90 clinic seeds resided. Within each of the 45 areas, geographic coordinates were selected randomly in SAS 9.2 to identify the location for community recruitment. A trained community team of educators, counselors and nurses with GPS devices was given the coordinates and asked to recruit one person within a specified age range and gender at each set of coordinates. The community team followed a structured set of procedures to identify which residences and residents to approach. Community members at these residences were eligible if they met the specified age and gender criteria, were willing to test for HIV, and had not tested HIV-positive previously. Once an eligible person was identified, the study was described and the person was invited to participate. For those community seeds who agreed to participate, all study procedures were conducted at a private location in the community. Prior to community recruitment, community workers sensitized community leaders to facilitate participation.
Study procedures were similar for community and clinic seeds. All seeds had one initial visit and were encouraged to come to the clinic one month later for one follow-up visit. A travel reimbursement of approximately $5 was provided for each study visit. At the initial visit, participants were consented by trained study staff and asked to respond to an interviewer-administered questionnaire. The questionnaire assessed demographics, socioeconomic status, HIV testing history, sexual behaviors, and characteristics of five social contacts. Clinic seeds had HIV and STI information transferred from clinic records to study forms at their initial visit. Community seeds received HIV testing and counseling in the community at their initial visit and were assessed for STIs using the clinic’s syndromic management algorithm at follow-up. At follow-up, all seeds answered questions about their participation in the program and were given $2 for each successfully referred contact.
The social contact recruitment program was described to seeds at the initial visit. It was described as a “health promotion program,” rather than an STI or HIV program to avoid stigma. Study staff explained that the program included HIV testing, STI screening, and other health services, including blood pressure screening and a health discussion. Seeds were asked to recruit up to five contacts and provided with five vouchers linked to the seed’s study identification number. Seeds were encouraged to refer social contacts who they thought would benefit from the health promotion program. Seeds were permitted to define whom they considered a social contact, and were not restricted from recruiting sexual partners or family members.
Social Contact Participant Procedures
Contacts were eligible if they were 18–45 years of age and residing in the Lilongwe City catchment area. When contacts presented, they were consented; interviewed about their demographic characteristics, sexual behavior and HIV testing history; assessed for STIs using the same syndromic management procedures; and assessed for HIV using the same antibody test protocol as seeds. Contacts were not systematically assessed for AHI 24 and were not excluded if they already knew their HIV status. Contacts were also offered blood pressure screening and a health promotion discussion on cardiovascular disease, diabetes, clean water and hygiene, family planning, STIs, or malaria. They received $5 for transport reimbursement.
Data Analyses
Descriptive statistics were calculated for seeds (Table 1) and contacts (Table 3) using means and proportions. Proportions of seeds with each characteristic were compared between groups using Chi-squared tests.
Table 1. Characteristics of Seed Participants by Seed Group.
Demographic, behavioral, and diagnostic characteristics of seeds are described and compared by group
| Clinic Based-Seeds | Community Seeds | ||||||
|---|---|---|---|---|---|---|---|
| HIV- uninfected (N=45) |
HIV- infected (N=45) |
(N=45) | Pearson Chi2 |
||||
| N | % | N | % | N | % | p-value | |
| Gender | |||||||
| Male | 16 | (36%) | 28 | (62%) | 17 | (38%) | |
| Female | 29 | (64%) | 17 | (38%) | 28 | (62%) | 0.02 |
| Age | |||||||
| 18–25 | 19 | (42%) | 15 | (33%) | 18 | (40%) | |
| 26–35 | 20 | (44%) | 24 | (53%) | 22 | (49%) | |
| 36–45 | 6 | (13%) | 6 | (13%) | 5 | (11%) | 0.9 |
| Marital status | |||||||
| Never married | 9 | (20%) | 14 | (31%) | 12 | (27%) | |
| Married | 30 | (67%) | 25 | (56%) | 28 | (62%) | |
| Divorced/separated/widowed | 6 | (13%) | 6 | (13%) | 5 | (11%) | 0.8 |
| Education | |||||||
| ≤primary | 19 | (42%) | 19 | (42%) | 15 | (33%) | |
| some secondary | 16 | (36%) | 19 | (42%) | 15 | (33%) | |
| ≥secondary | 10 | (22%) | 7 | (16%) | 15 | (33%) | 0.4 |
| Ran out of food in the last 3 months? | |||||||
| Yes | 31 | (69%) | 38 | (84%) | 28 | (62%) | |
| No | 14 | (31%) | 7 | (16%) | 17 | (38%) | 0.06 |
| Condom use over last 5 acts | |||||||
| 0 | 23 | (74%) | 18 | (55%) | 26 | (87%) | |
| 1–4 | 5 | (16%) | 9 | (27%) | 4 | (13%) | |
| 5 | 3 | (10%) | 6 | (18%) | 0 | (0%) | 0.05 |
| Sex partners in last 3 months | |||||||
| 0 | 4 | (9%) | 2 | (4%) | 11 | (24%) | |
| 1 | 35 | (78%) | 30 | (67%) | 32 | (71%) | |
| ≥2 | 6 | (13%) | 13 | (29%) | 2 | (4%) | 0.002 |
| Exchange sex for money in last 3 months | |||||||
| Yes | 3 | (7%) | 12 | (27%) | 1 | (2%) | |
| No | 42 | (93%) | 33 | (73%) | 44 | (98%) | 0.0007 |
| Previously tested for HIV | |||||||
| Never | 2 | (4%) | 18 | (40%) | 7 | (16%) | |
| Once | 10 | (22%) | 18 | (40%) | 9 | (20%) | |
| ≥Twice | 33 | (73%) | 9 | (20%) | 29 | (64%) | <0.0001 |
| HIV Status | |||||||
| HIV-negative | 45 | (100%) | 0 | (0%) | 44 | (98%) | |
| HIV-positive (established) | 0 | (0%) | 33 | (73%) | 1 | (2%) | |
| HIV-positive (acute) | 0 | (0%) | 12 | (27%) | NA | <0.0001 | |
| Any STI | |||||||
| Yes | 45 | (100%) | 37 | (82%) | 0 | (0%) | |
| No | 0 | (0%) | 8 | (18%) | 28 | (100%) | <0.0001 |
| Genital Ulcer Disease | |||||||
| Yes | 10 | (24%) | 16 | (37%) | 0 | (0%) | |
| No | 32 | (76%) | 27 | (63%) | 28 | (100%) | 0.001 |
| Urethral Discharge | |||||||
| Yes | 10 | (24%) | 15 | (35%) | 0 | (0%) | |
| No | 32 | (76%) | 28 | (65%) | 28 | (100%) | 0.002 |
| Vaginal Discharge2 | |||||||
| Yes | 18 | (64%) | 7 | (44%) | 0 | (0%) | 0.0002 |
| No | 10 | (36%) | 9 | (56%) | 16 | (100%) | |
| Lower Abdominal Pain2 | |||||||
| Yes | 14 | (48%) | 2 | (13%) | 0 | (0%) | |
| No | 15 | (52%) | 14 | (88%) | 16 | (100%) | 0.0007 |
For community seeds, STI information is only available for those who came to the clinic for a follow-up visit.
Information on vaginal discharge and lower abdominal pain is only presented for female seeds.
Table 3. Characteristics of Contacts by Seed Group.
Demographic, behavioral, and diagnostic characteristics of contacts are described and compared by seed group.
| Clinic Based-Seeds | Community Seeds | |||||
|---|---|---|---|---|---|---|
| HIV-uninfected | HIV-infected | |||||
| N | (%) | N | (%) | N | (%) | |
| Gender | ||||||
| Male | 34 | (45%) | 32 | (56%) | 40 | (42%) |
| Female | 42 | (55%) | 25 | (44%) | 55 | (58%) |
| Age | ||||||
| 18–25 | 36 | (47%) | 28 | (49%) | 39 | (41%) |
| 26–35 | 31 | (41%) | 25 | (44%) | 45 | (47%) |
| 36–45 | 9 | (12%) | 4 | (7%) | 11 | (12%) |
| Marital Status | ||||||
| Never married | 10 | (13%) | 20 | (35%) | 27 | (28%) |
| Married | 51 | (67%) | 28 | (49%) | 56 | (59%) |
| Divorced/separated/widowed | 15 | (20%) | 9 | (16%) | 12 | (13%) |
| Education | ||||||
| ≤primary | 29 | (41%) | 18 | (32%) | 30 | (33%) |
| some secondary | 26 | (37%) | 25 | (44%) | 41 | (45%) |
| ≥secondary | 16 | (23%) | 14 | (25%) | 21 | (23%) |
| Ran out of food in last 3 months | ||||||
| Yes | 66 | (87%) | 52 | (91%) | 76 | (80%) |
| No | 10 | (13%) | 5 | (9%) | 19 | (20%) |
| Condom use over last 5 acts | ||||||
| 0 | 36 | (77%) | 18 | (60%) | 50 | (83%) |
| 1–4 | 10 | (21%) | 10 | (33%) | 6 | (10%) |
| 5 | 1 | (2%) | 2 | (7%) | 4 | (7%) |
| Sex partners <3 months | ||||||
| 0 | 15 | (21%) | 14 | (25%) | 20 | (22%) |
| 1 | 51 | (72%) | 35 | (61%) | 66 | (73%) |
| ≥2 | 5 | (7%) | 8 | (14%) | 5 | (5%) |
| Exchange sex for money <3 months | ||||||
| Yes | 10 | (13%) | 11 | (19%) | 22 | (23%) |
| No | 66 | (87%) | 46 | (81%) | 73 | (77%) |
| Previously tested for HIV | ||||||
| Never | 11 | (15%) | 20 | (36%) | 17 | (18%) |
| Once | 21 | (30%) | 17 | (31%) | 23 | (25%) |
| At least twice | 39 | (55%) | 18 | (33%) | 53 | (57%) |
| HIV Status | ||||||
| HIV-negative | 64 | (89%) | 37 | (69%) | 83 | (89%) |
| Known positive | 1 | (1%) | 10 | (19%) | 4 | (4%) |
| New positive | 7 | (10%) | 7 | (13%) | 6 | (6%) |
| Any STI | ||||||
| Yes | 21 | (29%) | 10 | (19%) | 8 | (9%) |
| No | 52 | (71%) | 44 | (81%) | 84 | (91%) |
| GUD | ||||||
| Yes | 1 | (1%) | 2 | (4%) | 1 | (1%) |
| No | 71 | (99%) | 52 | (96%) | 91 | (99%) |
| Urethral Discharge | ||||||
| Yes | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| No | 72 | (100%) | 54 | (100%) | 92 | (100%) |
| Vaginal discharge1 | ||||||
| Yes | 18 | (45%) | 7 | (30%) | 5 | (9%) |
| No | 22 | (55%) | 16 | (70%) | 50 | (91%) |
| Lower Abdominal Pain1 | ||||||
| Yes | 4 | (10%) | 2 | (9%) | 2 | (4%) |
| No | 35 | (90%) | 21 | (91%) | 53 | (96%) |
Inforamtion on vaginal discharge and lower abdominal pain is only presented for female contacts.
To assess feasibility, the proportion of seeds who successfully recruited at least one contact was compared between groups using a Chi-squared test. The mean number of contacts recruited per seed was compared using analysis of variance.
To assess effectiveness, the prevalence of previous HIV testing, sexual behaviors, STIs, and HIV were compared between groups. Prevalence ratios (PR) and 95% confidence intervals (CI) were calculated using generalized estimating equations with a binomial distribution, log link, and exchangeable correlation matrix to account for clustering by seed. We also explored whether the prevalence of these behaviors and infections was different between the contacts of patients with established and acute HIV-infection.
To assess efficiency, we calculated the proportion of contacts newly tested for HIV and compared proportions between groups using generalized estimating equations with a binomial distribution, log link, and an exchangeable correlation matrix. The number of contacts needed to test to identify one new case of HIV or any STI syndrome was also calculated using generalized estimating equations with a log link, Poisson distribution, and exchangeable correlation matrix.
Ethical Approval
Permission for collecting these data was granted by the Malawi National Health Science Research Committee and the School of Medicine Institutional Review Board at the University of North Carolina, Chapel Hill. All seeds and contacts provided written consent to participate. Information about seeds was not shared with contacts and vice versa.
Results
Seed participant characteristics
Of 245 randomly selected clinic participants, 118 were eligible. The most common reasons for non-eligibility were known HIV-positive status (N=57, 45%), not meeting age or catchment area requirements (N=13, 10%), being the sex partner of an STI patient (N=15, 12%), or not having an STI (N=12, 9%). Of the 118 eligible clinic participants, 76% consented (N=90). In the community, 108 locations were visited to recruit 45 community seeds. Some coordinates did not lead to residences (N=22), some led to residences with nobody home (N=10), and some led to residences where no one met eligibility criteria (N=25). Of the 48 residences with an eligible person present, 93% were willing to participate. As specified by the protocol, the seed population included 45 newly diagnosed HIV-infected patients, 45 HIV-uninfected patients, and 45 community controls. Twelve HIV-infected seeds had AHI.
Among all seeds, 45% were male (Table 1), the mean age was 27.6 years, and most (61%) were married. Most (71%) had not used a condom in any of their last five sex acts. In the last three months, 12% exchanged sex for money and 16% had two or more partners, although proportions were higher among clinic-based seeds. Almost all seeds (80%) had been tested for HIV at least once, although this was lowest (60%) among HIV-infected seeds. Among HIV-infected clinic seeds, 27% had recently been diagnosed with AHI. Among community seeds, one (2%) had HIV. All clinic seeds had an STI except for eight of the seeds with AHI.
Feasibility
Overall, the 135 seeds recruited 244 contacts (36% of the maximum number possible). The proportion recruiting at least one contact was somewhat higher among community seeds (69%) than among HIV-infected clinic seeds (47%) or HIV-uninfected clinic seeds (53%) (p=0.09) (Table 2). However, among seeds recruiting at least one contact, the mean number of contacts was the same between the three groups: HIV-infected seeds, 2.9; HIV-uninfected seeds, 3.4; and community seeds, 3.3 (p=0.5).
Table 2. Contact Recruitment by Seed Group.
Table 2 reports several indicators of feasibility by seed group.
| Clinic Based-Seeds | Community Seeds | ||||||
|---|---|---|---|---|---|---|---|
| HIV-uninfected | HIV-infected | p-value | |||||
| Seeds recruiting 0–5 contacts (N, %) | |||||||
| 0 | 21 | (47%) | 24 | (53%) | 14 | (31%) | |
| 1 | 3 | (7%) | 5 | (11%) | 7 | (16%) | |
| 2 | 6 | (13%) | 7 | (16%) | 4 | (9%) | |
| 3 | 2 | (4%) | 1 | (2%) | 4 | (9%) | |
| 4 | 5 | (11%) | 2 | (4%) | 4 | (9%) | |
| 5 | 8 | (18%) | 6 | (13%) | 12 | (27%) | 0.4 |
| Contacts recruited (mean, SD) | 1.8 | (2.0) | 1.3 | (1.8) | 2.3 | (2.1) | 0.07 |
| Seeds recruiting ≥1 contact (N, %) | 24 | (53%) | 21 | (47%) | 31 | (69%) | 0.09 |
| Contacts recruited among seeds with ≥1 contact (mean, SD) | 3.4 | (1.5) | 2.9 | (1.6) | 3.3 | (1.6) | 0.5 |
SD=Standard deviation
Among HIV-infected seeds, 39% of those with established HIV infection and 67% of those with AHI recruited at least one contact. The mean number of contacts recruited per seed was higher among those with AHI (mean=2.0) than established HIV infection (mean=1.1). This difference may have been due to additional counseling provided through CHAVI 001.
Social contact characteristics
Of the 244 contacts recruited, 228 (93%) participated. Of those who did not, most were ineligible due to being >45 years. Of participating contacts, 62% were friends or neighbors of the seed, 18% were family members (primarily siblings and cousins), 11% were sexual contacts (primarily spouses), and 8% had another relationship. Most had known the recruiting seed for ≥1 year (79%), reported knowing the seed very well (87%), saw the seed several times each week (93%), interacted with the seed primarily at a home (81%), and described conversation as their primary activity together (89%).
Among contacts, 46% were male (Table 3), the mean age was 27.5 years, and most (59%) were married. Most (78%) reported at least on HIV test before the study. Most (76%) had not used a condom during any of the last five sex acts. In the last three months, 19% exchanged sex for money and 8% had ≥2 sex partners.
Effectiveness
Contacts of the HIV-infected clinic seeds were more likely to be HIV-infected (31%) than contacts of community seeds (11%). HIV prevalence was 3.2 times higher (95% CI: 1.3, 7.8) among contacts of HIV-infected clinic seeds than among contacts of community seeds. Contacts of the HIV-uninfected clinic seeds were not more likely to be HIV-infected (10% established HIV infection, 1% AHI) than contacts of community seeds (prevalence ratio: 1.1, 95% CI: 0.4, 3.3). When analyses were restricted to non-sexual contacts, these prevalence ratio estimates were similar: 3.0 and 1.4, respectively. When analyses were adjusted for seed sexual behavior (condom use and number of partners), prevalence ratio estimates were also similar: 3.4 and 1.0, respectively.
The contacts of the HIV-infected and HIV-uninfected clinic seeds were more likely to have an STI syndrome (29% and 19%, respectively) than the contacts of the community seeds (9%). STI syndrome prevalence was 2.0 times higher (95% CI: 0.8, 5.3) among contacts of HIV-infected clinic seeds and 3.2 times higher (95% CI: 1.4, 7.2) among contacts of HIV-uninfected clinic seeds compared to contacts of community seeds. When analyses were restricted to non-sexual contacts prevalence ratio estimates were similar: 1.9 and 3.3, respectively. When analyses were adjusted for seed sexual behavior, prevalence ratio estimates were also similar: 2.1 and 3.2, respectively.
Contacts of seeds with established HIV infection and AHI were compared. The prevalence of HIV was nearly the same among contacts of seeds with established HIV infection (32%) and contacts of seeds with AHI (30%). Most contacts were not assessed for AHI. The prevalence of an STI was higher (24%) among the contacts of clinic seeds with established HIV infection than among contacts of clinic seeds with AHI (10%).
Efficiency
Of the 180 HIV-uninfected contacts, few (19%) were tested for HIV for the first time through the study. Of 35 contacts with HIV infection, seven (20%) were being tested for HIV the first time through the study, 13 (37%) had been tested previously and sero-converted afterwards, and 15 (43%) already knew they were HIV-infected. Of 20 contacts who learned their HIV-positive status through the study, seven were recruited by HIV-infected seeds, seven by HIV-uninfected seeds, and six by community seeds.
To identify one new case of HIV, 8.1 contacts of HIV-infected clinic seeds, 9.7 contacts of HIV-uninfected clinic seeds, and 17.5 contacts of community seeds were screened. To identify one new case of an STI, 5.5 contacts of HIV-infected clinic seeds, 3.5 contacts of HIV-uninfected clinic seeds, and 11.4 contacts of community seeds were screened. To identify one new case of an STI or HIV, 3.7 contacts of HIV-infected clinic seeds, 2.8 contacts of HIV-uninfected clinic seeds, and 7.3 contacts of community seeds were screened.
Discussion
Asking STI patients to recruit their social contacts was a feasible, effective, and efficient way of diagnosing new HIV cases in a generalized HIV epidemic. Half of the clinic seeds in our study were able to successfully recruit at least one contact, and contacts of HIV-infected clinic seeds had a higher HIV prevalence than contacts of community seeds. To identify one new case of HIV infection only 8–10 contacts of clinic seeds needed to be tested for HIV, much better efficiency than random testing in the population.
High risk persons tend to associate with other persons who engage in similar high risk activities. However, this relationship typically has been assessed in concentrated HIV epidemics20, 24, 25, with fewer assessments in generalized epidemics.21 In contrast, we used social contact recruitment in a generalized epidemic among persons with biological evidence of risk—a newly diagnosed case of HIV and/or an STI. By using a well-designed community-based comparison group, we were able to demonstrate effectiveness. Even in the context of a generalized HIV epidemic, STI and HIV risk was not evenly distributed, but rather, clustered in social networks.
Understanding the reasons for social contact recruitment effectiveness is important. One possible explanation is that members of the same social networks have similar risk behaviors. Formal exploration of this possibility is being assessed in a separate analysis. However, informal comparisons of sexual behavior between seeds and corresponding contacts suggest this explanation alone does not account for these results. An alternative explanation for the observed HIV disease clustering is that the social network itself is a risk factor. Contacts of HIV-infected seeds may be part of sexual networks with a higher HIV prevalence. In other words, the network population may be a more salient exposure than the behaviors within that network, an observation that has been made in concentrated epidemics25,26 and other generalized27 HIV epidemic settings. Sexual relationships between seeds and contacts are not the primary reason for the observed clustering, even though a high prevalence of HIV-concordance has been observed among couples in this setting28. When analyses were restricted to only non-sexual contacts elevated HIV prevalence persisted.
Social contact recruitment by patients with AHI may also be a promising way of effectively finding the “leading edge” of the HIV epidemic. In this study, we were only able to explore this possibility through enrollment of a few persons with AHI. On average, these patients were willing to recruit two social contacts and the HIV prevalence among their contacts was high (30%). However, we were not able to explore whether their contacts had AHI. Exploring AHI in social contacts of AHI patients is a key next step, as these persons may be exposed to networks with elevated HIV incidence.
Social contact recruitment was feasible in all groups, but more feasible for community-based seeds. Clinic based seeds recruited fewer social contacts. Lower recruitment may have been due to stigma or fear of contacts learning their STI or HIV results, factors under exploration in an analysis of acceptability. In spite of clinic-based seeds recruiting fewer contacts, half of the seeds were successfully able to recruit at least one contact.
Lower feasibility coupled with greater effectiveness led to greater efficiency of social contact recruitment by clinic-based seeds. The total number of newly diagnosed contacts was approximately the same in all three groups. However, the number of contacts needed to test to identify one new case of HIV was considerably lower among contacts of clinic-based seeds. For routine implementation, screening fewer high-risk contacts is more efficient than more low-risk contacts. Efficiency may be improved further by targeting those seeds most likely to recruit undiagnosed HIV-infected persons. Such targeting could reduce the number of additional persons presenting to a busy clinical setting, while simultaneously reaching those with greatest need. Demographic, behavioral, and relationship characteristics associated with recruitment of high risk contacts is a direction to explore.
Several operational considerations deserve further research. First, in addition to receiving $5 per research visit, all seeds received a $2 incentive for each contact who presented to the clinic. This amount was considered motivational, but not coercive by local staff and community advisors. However, because this amount did not vary, we could not assess whether a larger incentive would have improved contact recruitment. Additionally, all seeds were exposed to the same messages regarding contact recruitment. They were encouraged to bring friends who would benefit from the health promotion program. But other messages, such as encouraging recruitment of high risk contacts, may be more effective. Future studies could randomize whether different incentive amounts and messages are associated with different degrees of feasibility, effectiveness, and efficiency.
Replication in other clinical settings is warranted. STI clinics serve patients with greater biological and behavioral risk for HIV and these patients were part of social networks with elevated undiagnosed HIV infection. Whether newly diagnosed HIV-infected patients in other settings would also be part of higher risk networks is unknown. However, recruitment by only HIV-infected seeds may result in inadvertent disclosure of seed HIV status. Assessment in other settings, with attention to inadvertent disclosure, is an important direction for future research.
Our findings reflect a novel strategy for addressing a pressing public health need: identifying undiagnosed, hard-to-reach cases of HIV infection. We demonstrated that asking STI patients to recruit their social contacts was a feasible, effective, and efficient way of identifying this population. These observations support social contact recruitment extending the reach of the health care screening system. Such an approach could become a powerful way of identifying HIV in hard-to-reach populations earlier.
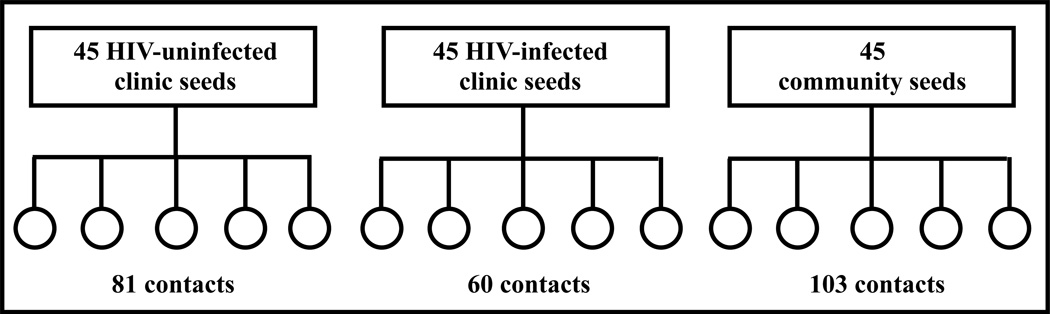
Figure 1. Study Schematic.
Figure 1 illustrates the study design. There were 45 participants (depicted by rectangles) in each seed group. Each seed could recruit up to five contacts (depicted by circles). The total number of contacts who presented by seed group is reported.
Table 4. Comparisons of Clinic Contacts to Community contacts for Prevalence of Infections and Behaviors.
Contacts of HIV-infected and HIV-uninfected seeds are compared to contacts of community seeds for the prevalence of several infections and outcomes.
| Clinic Based-Seeds | Community Seeds | ||||
|---|---|---|---|---|---|
| HIV-uninfected | HIV-infected | ||||
| PR | (95% CI) | PR | (95% CI) | Reference | |
| STI syndrome (any versus none) | 3.2 | (1.4, 7.2) | 2.0 | (0.8, 5.3) | 1. |
| HIV status (positive versus negative) | 1.1 | (0.4, 3.3) | 3.2 | (1.3, 7.8) | 1. |
| Previous HIV test (yes versus no) | 1.2 | (0.7, 2.1) | 1.3 | (0.7, 2.3) | 1. |
| New positive (new positive versus other) | 1.8 | (0.5, 6.4) | 2.3 | (0.7, 8.0) | 1. |
| Condom use in last 5 acts (never versus ever) | 0.9 | (0.8, 1.1 ) | 0.7 | (0.5, 1.0) | 1. |
| Sex partners in last 3 months (>2 versus 0 or 1) | 1.5 | (0.4, 6.4) | 2.5 | (0.7, 9.2) | 1. |
| Sex for money in last 3 months (yes versus no) | 0.6 | (0.3, 1.3) | 0.8 | (0.4, 1.7) | 1. |
PR=prevalence ratio; CI=confidence interval
Acknowledgements
We would like to thank the clinic and community staff and participants for their contributions.
Conflicts of Interest and Sources of Funding:
This research was funded by a 2010 developmental grant from the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program (P30 AI50410), and by an MP3 study (5-R01 AI083059-03). NER was supported by NIH HIV/STD training grant (T32 AI007001-34) and UNC Hopkins Morehouse Tulane Fogarty Global Health Fellows Program (R25 TW009340). SER is funded by F30 MH098731-01 and T32 GM008719. The authors are grateful for contributions by clinic and community staff and study participants.
Footnotes
Previous Reporting
- Rosenberg NE, Pettifor AE, Kamanga G, Bonongwe N, Mapanje C, Hoffman I, Martinson F, Miller WC. “Social Networks of STI Patients have Higher STI Prevalence than Social Networks of Community Controls.” STI & AIDS World Congress, 2013.
- Rosenberg NE, Bonongwe N, Pettifor A, Kamanga G, Mapanje C, Hoffman I, Martinson F, Miller WC. “Acceptability of a Social Contact Recruitment Program for Identifying HIV and STIs in Lilongwe, Malawi” Consortium of Universities for Global Health Annual Meeting, March 2013.
- Rosenberg NE, Kamanga G, Bonongwe N, Pettifor A, Mapanje C, Nkhata L, Rutstein SE, Hoffman I, Martinson F Miller WC. “Social Network Recruitment by STI Patients is a Promising Strategy for Identifying STIs and HIV in Resource-Limited Settings” XIX International AIDS Conference, July 2012.
Author contributions
GK, WCM, NER, and AP conceptualized the study under the guidance of IF and FM. NER, SR, AP and GK developed data collection tools. MW developed the study database. GK, NB, and CM oversaw study implementation. NER conducted all analyses under the guidance of WCM. NER drafted the initial manuscript. All authors provided substantive edits to the manuscript and approved the final draft.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 3.WHO, UNAIDS, UNICEF. HIV Testing and Counseling. Towards Universal Access: Scaling up Priority HIV/AIDS Interventions in the Health Sector, Progress Report 2010
- 4.Macro International. Malawi Demographic and Health Survey 2010. Zomba, Malawi and Calverton, Maryland, USA: 2011. [Google Scholar]
- 5.Marks G, Crepaz N, Senterfitt JW, et al. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 6.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011 Jul 16;378(9787):256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg NE, Pettifor AE, Bruyn GD, et al. HIV Testing and Counseling Leads to Immediate Consistent Condom Use Among South African Stable HIV-Discordant Couples. J Acquir Immune Defic Syndr. 2013 Feb 1;62(2):226–233. doi: 10.1097/QAI.0b013e31827971ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roura M, Watson-Jones D, Kahawita TM, et al. Provider-initiated testing and counselling programmes in sub-Saharan Africa: a systematic review of their operational implementation. AIDS. 2013 Feb 20;27(4):617–626. doi: 10.1097/QAD.0b013e32835b7048. [DOI] [PubMed] [Google Scholar]
- 9.Were W, Mermin J, Bunnell R, et al. Home-based model for HIV voluntary counselling and testing. Lancet. 2003 May 3;361(9368):1569. doi: 10.1016/S0140-6736(03)13212-6. [DOI] [PubMed] [Google Scholar]
- 10.Menzies N, Abang B, Wanyenze R, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009 Jan 28;23(3):395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 11.Dalal W, Feikin DR, Amolloh M, et al. Home-Based HIV Testing and Counseling in Rural and Urban Kenyan Communities. J Acquir Immune Defic Syndr. 2013 Feb 1;62(2):e47–e54. doi: 10.1097/QAI.0b013e318276bea0. [DOI] [PubMed] [Google Scholar]
- 12.Tumwesigye E, Wana G, Kasasa S, et al. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care STDs. 2010 Nov;24(11):735–741. doi: 10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- 13.Were WA, Mermin JH, Wamai N, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr. 2006 Sep;43(1):91–95. doi: 10.1097/01.qai.0000225021.81384.28. [DOI] [PubMed] [Google Scholar]
- 14.Negin J, Wariero J, Mutuo P, et al. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Tropical Med Int Health. 2009 Aug;14(8):849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 15.Armbruster B, Helleringer S, Kalilani-Phiri L, et al. Exploring the relative costs of contact tracing for increasing HIV case finding in sub-Saharan countries. J Acquir Immune Defic Syndr. 2011 Oct 1;58(2):e29–e36. doi: 10.1097/QAI.0b013e31822a9fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helleringer S, Mkandawire J, Reniers G, et al. Should Home-Based HIV Testing and Counseling Services be Offered Periodically in Programs of ARV Treatment as Prevention? A Case Study in Likoma (Malawi) AIDS Behav. 2012 Nov 20; doi: 10.1007/s10461-012-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimbrough LW, Fisher HE, Jones KT, et al. Accessing social networks with high rates of undiagnosed HIV infection: The social networks demonstration project. Am J Public Health. 2009 Jun;99(6):1093–1099. doi: 10.2105/AJPH.2008.139329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoy SI, Shiu K, Martz TE, et al. Improving the Efficiency of HIV Testing With Peer Recruitment, Financial Incentives, and the Involvement of Persons Living with HIV Infection. J Acquir Immune Defic Syndr. 2013 Feb 11; doi: 10.1097/QAI.0b013e31828a7629. [DOI] [PubMed] [Google Scholar]
- 19.Golden MR, Gift TL, Brewer DD, et al. Peer referral for HIV case-finding among men who have sex with men. AIDS. 2006 Oct 3;20(15):1961–1968. doi: 10.1097/01.aids.0000247118.74208.6a. [DOI] [PubMed] [Google Scholar]
- 20.Malekinejad M, Johnston LG, Kendall C, et al. Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav. 2008 Jul;12(4 Suppl):S105–S130. doi: 10.1007/s10461-008-9421-1. [DOI] [PubMed] [Google Scholar]
- 21.Townsend L, Zembe Y, Mathews C, et al. Estimating HIV prevalence and HIV-related risk behaviors among heterosexual women who have multiple sex partners using respondent-driven sampling in a high risk community in South Africa. J Acquir Immune Defic Syndr. 2012 Dec 18; doi: 10.1097/QAI.0b013e3182816990. [DOI] [PubMed] [Google Scholar]
- 22.Ssali S, Wagner G, Tumwine C, et al. HIV Clients as Agents for Prevention: A Social Network Solution. AIDS Res Treat. 2012;2012:815823. doi: 10.1155/2012/815823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latkin CA, Knowlton AR, Sherman S. Routes of drug administration, differential affiliation, and lifestyle stability among cocaine and opiate users: implications to HIV prevention. J. Subst. Abuse. 2001;13(1–2):89–102. doi: 10.1016/s0899-3289(01)00070-0. [DOI] [PubMed] [Google Scholar]
- 24.Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. J Acquir Immune Defic Syndr. 2007 Oct 18;21(16):2237–2242. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis AM, Murillo W, de Maria Hernandez F, et al. Social network-based recruitment successfully reveals HIV-1 transmission networks among high-risk individuals in El Salvador. J Acquir Immune Defic Syndr. 2013 May 1;63(1):135–141. doi: 10.1097/QAI.0b013e318288b246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latkin C, Donnell D, Liu TY, et al. The dynamic relationship between social norms and behaviors: the results of an HIV prevention network intervention for injection drug users. Addiction. 2013 May;108(5):934–943. doi: 10.1111/add.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK USA: a meta-analysis. Lancet. 2012 Jul 28;380(9839):341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 28.Helleringer S, Kohler HP. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007 Nov 12;21(17):2323–2332. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- 29.Brown LB, Miller WC, Kamanga G, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011 Apr 15;56(5):437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]