Abstract
In all of the mammalian species studied to date, the short-wavelength-sensitive (S) cones and the S-cone bipolar cells that receive their input are very similar, but the retinal ganglion cells that receive synapses from the S-cone bipolar cells appear to be quite different. Here, we review the literature on mammalian retinal ganglion cells that respond selectively to stimulation of S-cones and respond with opposite polarity to longer wavelength stimuli. There are at least three basic mechanisms to generate these color-opponent responses, including: (1) opponency is generated in the outer plexiform layer by horizontal cells and is conveyed to the ganglion cells via S-cone bipolar cells, (2) inputs from bipolar cells with different cone inputs and opposite response polarity converge directly on the ganglion cells, and (3) inputs from S-cone bipolar cells are inverted by S-cone amacrine cells. These are not mutually exclusive; some mammalian ganglion cells that respond selectively to S-cone stimulation seem to utilize at least two of them. Based on these findings, we suggest that the small bistratified ganglion cells described in primates are not the ancestral type, as proposed previously. Instead, the known types of ganglion cells in this pathway evolved from monostratified ancestral types and became bistratified in some mammalian lineages.
Keywords: Color vision, Blue, Evolution, Primate, Neuronal types
Introduction
Nearly all mammals have retinal ganglion cells that respond selectively to the stimulation of short wavelength-sensitive (S) cones. These retinal ganglion cells can be subdivided into ON types that respond with an increase in firing rate to increments in the intensity of S-cone stimulation and OFF types that respond to the same stimuli with decreases in firing rate. The bipolar cells that provide their excitatory input can also be divided into two types. Typically, those with hyperpolarizing, or OFF, responses to light terminate in the outer half of the inner plexiform layer (IPL), sublamina a, and those with depolarizing, or ON responses to light, terminate in the inner half, sublamina b (Dowling, 2012).
Three different physiological mechanisms have been proposed to account for color opponency in retinal ganglion cells that respond selectively to S-cone stimulation. These include: (1) horizontal cell feedback onto cones, (2) direct antagonistic input to ganglion cell dendrites from ON and OFF bipolar cells with input from different types of cones, and (3) chromatically selective inhibition of bipolar cell axons and ganglion cell dendrites from amacrine cells. Each has been observed in mammalian retinas, and the most thoroughly studied retinas, those of lagomorphs (rabbits) and primates, appear to utilize all three mechanisms. Horizontal cells impose a chromatic surround onto S-cones, and this may be regarded as the canonical mechanism of color opponency (Packer et al., 2010; Crook et al., 2011). The summation of ON and OFF inputs from cone bipolar cells with inputs from different cone types can also produce color opponency. A major benefit of this arrangement is that the receptive field surrounds of the two bipolar cells tend to cancel one another, leading to a receptive field that is purely color-opponent, without spatial opponency (Rodieck, 1991; Dacey, 2000; Sher & DeVries, 2012).
Amacrine cells have been found to produce color-opponent ganglion cells by two different mechanisms. In ground squirrels, inputs from M-ON bipolar cells and an S-ON amacrine cell converge to generate S-OFF/M-ON color opponent ganglion cells (Chen & Li, 2012; Sher & DeVries, 2012). In the mouse, on the other hand, spiking GABAergic amacrine cells exploit regional differences in cone opsin expression to effect color opponency (Chang et al., 2013). S-ON amacrine cells that invert the responses of S-ON bipolar cells are also likely to be found in primate retinas because two types of S-OFF ganglion cells costratify with S-ON bipolar cells (Dacey & Packer, 2003; Dacey et al., 2005).
Most mammals are dichromats and have color-opponent ganglion cells that respond with opposite polarity to stimulation of S-cones and medium wavelength-sensitive (M) cones (Puller & Haverkamp, 2011). Color-opponent ganglion cells of only one marsupial mammal, the wallaby, have been studied to date. Placental mammals whose color-opponent ganglion cells have been studied include: cats, mice, guinea pigs, ground squirrels, rabbits, and several species of primates. In some cases, the morphology and synaptic connections of the ganglion cells have been studied, but other types of are known only from recordings of their light responses. In this review, we will describe the light responses of S-cone opponent ganglion cells in each species and then describe the neural circuits that generate them.
The retinal ganglion cells that respond selectively to S-cone stimulation appear to be morphologically different in each of the mammals studied to date (Famiglietti, 2008). In this review, we will describe the morphology of the S-cone opponent ganglion cells in each species, and, using the results from other mammalian retinas, we will also propose an evolutionary scenario to account for the diversity of S-cone opponent ganglion cells in primates.
Wallaby
Using a whole, isolated wallaby (Macropus eugenii) retina preparation in vitro, responses were recorded from color-opponent ganglion cells using patch electrodes. All had S-OFF and M-ON responses to full-field stimuli, and there was no apparent spatial opponency. Because the S-cone components had latencies 15 ms longer than the M-cone component, the authors proposed that the S-OFF response originated from the inversion of S-ON bipolar cell responses by intervening amacrine cells, the third mechanism (Hemmi et al., 2002). The morphology of the color-opponent cells in wallabies was not described, however, and the OFF response in the ganglion cell might have originated from S-OFF bipolar cells, as in lagomorphs, rodents, and some primates (see below).
Cat
The first recordings of S-cone selective ganglion cells in cat (Felis domesticus) retina were made using extracellular electrodes (Cleland & Levick, 1974). These cells were extremely rare, comprising less than 1% of a very large sample, and all had tonic, ON responses to stimulation of S-cones. Of the six cells that were identified, five had OFF responses to longer wavelengths and axons with relatively fast conduction velocities, comparable to those of brisk sustained, or X, cells. The receptive field was organized differently than in typical X cells, however. ON responses to S-cone stimulation and OFF responses to longer wavelengths were both detectable in the receptive field centers. In some cells, the area of S-cone sensitivity was larger than that of longer wavelengths, but the opposite was true in others. The sixth ganglion cell had a larger S-ON receptive field, no OFF responses, and an axon with much slower conduction velocity. Another study of cat retina confirmed that ganglion cells driven by S-cones lacked spatial opponency (Ringo & Wolbarsht, 1986). However, two later studies described cat ganglion cells sensitive to S-cone stimulation with center-surround receptive field organization (Guenther & Zrenner, 1993; Rowe & Cox, 1993).
The ganglion cells in cat retina that respond to short wavelengths have not been identified morphologically. All that is known about the presumed S-ON ganglion cell is that it was not the alpha or beta morphological type (Cohen & Sterling, 1990a). A GABAergic neuron somewhere in the pathway providing input to the S-ON cells was clearly important in regulating their sensitivity. The GABAa antagonist, bicuculline, blocked the transient loss of sensitivity to short wavelength stimuli after the offset of a yellow adapting background in recordings from the optic nerve (Schuurmans & Zrenner, 1981).
The neural circuit that provides input to the S-ON ganglion cell has been studied by three-dimensional reconstruction from serial ultrathin sections. Type b5 bipolar cells were identified as the S-ON type by their inputs from a sparse population of cones and their axon terminals in the lower part of sublamina b (Cohen & Sterling, 1990b). The b5 bipolar cell axon had a distinctive pattern of synaptic connections in the IPL. It directed about two-thirds of its output to ganglion cells, more than any other type of ON bipolar cell, and it did not make gap junctions with AII amacrine cells or with bipolar cells. The axon terminals received a relatively small number of inputs from amacrine cells, and only a few of these amacrine cells received input from the same bipolar cell. This type of bipolar cell has also been described using the Golgi method and called wfb (Famiglietti, 1981).
Mouse
Typical color opponent ganglion cells in mouse (Mus musculus) retina were first recorded using extracellular electrodes (Ekesten & Gouras, 2005). About 2% of retinal ganglion cells had S-ON and M-OFF responses to full-field stimuli, and all had sustained, or tonic, responses to light. The S-ON ganglion cells have not been identified morphologically, but S-ON responses are known to originate from type 9 bipolar cells, which receive inputs from S-cones (Haverkamp et al., 2005) and M-OFF responses from type 1 bipolar cells (Breuninger et al., 2011).
In addition, Ekesten and Gouras found that at least one cell with transient, or phasic, responses also had M-ON and S-OFF responses (see their Fig. 2b). A recent study of color opponent ganglion cells in mice suggests a possible explanation (Chang et al., 2013). In the dorsal retina, M cones express only M-opsin, but in the ventral retina, M-cones co-express S and M opsin. Alpha-like cells located near the boundary between dorsal and ventral retina have both S-OFF/M-ON responses and S-ON/M-OFF responses generated by amacrine cells, the third mechanism. The color opponency in these cells depends on inhibitory inputs from spiking GABAergic amacrine cells because it is blocked by the sodium channel blocker, tetrodotoxin, or by GABA antagonists.
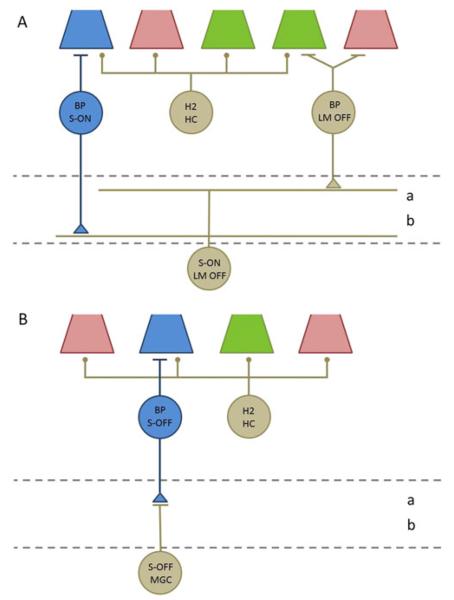
Fig. 2.
Two types of ganglion cells from Old World anthropoid retinas that respond selectively to stimulation of S-cones. In addition, primates have two types of monostratified S-OFF ganglion cells whose inputs are thought to be similar to those illustrated in Fig. 1B. (A). Small bistratified cells have branches in both sublamina a and sublamina b of the IPL (dashed lines). Their S-ON responses are generated by excitatory synapses (circles) from S-ON bipolar cells (BP). Their OFF responses to L- and M-cone stimulation have two sources, excitatory synapses from diffuse bipolar cells with input from L and M cones, and inhibitory synapses from H2 horizontal cells (H2) onto cones. The synaptic inputs to the large bistratified cells have not been studied but are thought to be similar. (B) Some OFF midget ganglion cells (MGC) in central retina have S-OFF responses because their excitatory input comes from a single OFF midget bipolar cell, which receives input from a single S-cone. Their ON responses to L- and M-cone stimulation are thought to originate from H2 horizontal cells.
Guinea pig
Using an in vitro eyecup preparation from guinea pigs (Cavia porcellus), light responses were recorded from retinal ganglion cells with loose patch electrodes, and then the cells were filled with Lucifer yellow using sharp intracellular electrodes. A small proportion of the ganglion cells, fewer than 1%, had color-opponent responses. In the superior retina, where the majority of cones express M-opsin and the others express S-opsin, both S-ON/M-OFF and S-OFF/M-ON cells were found in approximately equal numbers. Neither type showed spatial opponency; the ON and OFF responses could be elicited throughout the receptive fields. Both types were monostratified; that is, their distal dendrites all branched in a single stratum of the IPL, the innermost part of sublamina b in this case. The authors proposed that the S-ON cells received direct excitatory input from S-ON bipolar cells and that the S-OFF cells received input from S-ON bipolar cells indirectly via amacrine cells (Yin et al., 2009). Thus, the S-ON responses are apparently generated by the first of the three mechanisms, and the S-OFF responses are generated by the third.
Ground squirrel
Color opponent ganglion cells were more abundant in the ground squirrel retina (Spermophilis (Citellus) mexicanus), comprising 24% of a large sample recorded with extracellular electrodes in the optic nerve (Michael, 1968). Based on their responses to full-field monochromatic stimuli, roughly half were S-ON/M-OFF, and half were S-OFF/M-ON. The receptive fields of these cells were also mapped with spots and annuli. In the majority of color-opponent cells, the ON and OFF responses to spots that stimulate S-cones and M-cones were found throughout the receptive fields, and annuli had no effect. Other color opponent cells had similar receptive field centers but surrounds that were sensitive only to S-cones. A few color-opponent ganglion cells showed more pronounced spatial antagonism; their receptive field centers were sensitive only to M-cone stimulation and their surrounds only to S-cone stimulation.
In a more recent study using a different species (Spermophilis (Ictidomys) tridecemlineatus), isolated ground squirrel retinas in vitro were stimulated with chromatic white noise, and extracellular recordings of ganglion cells were made using a multielectrode array. Both S-ON/M-OFF and S-OFF/M-ON responses with roughly coextensive excitatory and inhibitory receptive fields were observed, like the first types described previously. To block responses mediated by ON bipolar cells without changing their membrane potentials, the retina was treated with a combination of the group III metabo-tropic glutamate receptor agonist, DL-2-amino-4-phosphonobutyric acid (APB) and an antagonist of that receptor, LY341495. Light responses of both the S-ON and the S-OFF types were blocked; APB applied alone produced similar results. The S-OFF, but not the M-ON, responses were blocked by strychnine, an antagonist of the inhibitory transmitter glycine. Thus, the S-OFF responses must have been generated by glycinergic S-ON amacrine cells (Sher & DeVries, 2012). Using intracellular recording and Neurobiotin injection, amacrine cells with S-ON responses to light were identified. They contained immunoreactive glycine transporter, a marker for glycinergic amacrine cells; their dendrites costratified with the axon terminals of S-ON bipolar cells, and their responses to S-cone stimulation were also blocked by APB (Chen & Li, 2012). Taken together, these two papers provided the clearest demonstration to date of amacrine cell-mediated S-cone opponent responses, the third mechanism.
The presynaptic bipolar cells have also been identified. B1 bipolar cells were first described in a closely related species (Spermophilis beechyi) using retinas labeled using the Golgi method (West, 1976). They were proposed as S-cone selective bipolar cells based on the contacts of their dendrites with sparse regularly arranged cone pedicles, and they were predicted to have ON-type light responses based on the termination of their invaginating dendrites and their axons in the innermost stratum of sublamina b (Linberg et al., 1996). This was confirmed using physiological methods, and the cells were renamed bb (Li & DeVries, 2006). The S-ON bipolar cells of Spermophilis (Ictidomys) tridecemlineatus were also studied using double light microscopic immunolabeling and electron microscopic immunolabeling (Puller et al., 2011). M-OFF bipolar cells have also been identified in physiological experiments (Li & DeVries, 2006). Both types have been predicted to contact the bistratified G13 ganglion cell type, based on its apparent homology with small bistratified ganglion cells in primate retinas (Linberg et al., 1996; Li & DeVries, 2006). The evidence for S-OFF bipolar cells in ground squirrel retinas is less compelling because their light responses have not been recorded. Using the Golgi method, they were tentatively identified as the b7 type (Linberg et al., 1996), and in a later study, they were labeled by intracellular injection and renamed X cells (Light et al., 2012).
Rabbit
Studies with extracellular electrodes
The first description of color opponent ganglion cells in rabbit (Oryctolagus cuniculus) retina used extracellular electrodes in the visual streak and stimulating electrodes in the optic chiasm to measure axonal conduction velocity. The color-opponent cells all had ON responses to S-cone stimulation and OFF responses to M-cone stimulation. The light responses were relatively sustained, and the receptive fields were concentrically organized, with S-ON centers and M-OFF surrounds. Based on these findings and the relatively fast conduction velocity of their axons, they were classified as a subtype of X cell (Caldwell & Daw, 1978). A second type of color-opponent cell with an S-OFF center, an M-ON surround, and a slower axonal conduction velocity was reported in another study using similar techniques (Vaney et al., 1981).
Three types of ganglion cells that respond selectively to S-cone stimulation were identified morphologically in a recent study using loose patch electrodes to record their light responses and to fill the cells with Neurobiotin via electroporation (Mills & Tian, 2012). The first type was monostratified, with all of its dendrites in the inner part of sublamina b. It had S-ON/M-OFF responses, and because both components of the response were blocked by APB, they must be mediated by S-ON bipolar cells via the first mechanism. Further evidence that the responses originated in the outer plexiform layer (OPL) comes from the finding that the M-OFF component is also reduced by 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), a buffer that blocks horizontal cell feedback onto cones.
The second type was also monostratified and had dendrites branching in the same stratum of the IPL as the first type, but it had S-OFF responses. The S-OFF component also originated in S-ON bipolar cells because it was blocked by APB. It was also blocked by strychnine, and taken together, these findings indicated that the S-cone signals were inverted by glycinergic amacrine cells in the pathway providing input to the monostratified S-OFF ganglion cells. Because the M-ON signals persisted under these conditions, they must be mediated by excitatory input from M-ON bipolar cells. Thus, these cells use the third mechanism for generating color opponecy, as described previously in ground squirrels (Sher & DeVries, 2012).
The third type was bistratified, with dendrites branching in sublamina a and also in sublamina b. These cells had OFF responses to S-cone stimulation, but unlike the monostratified type, its OFF responses persisted in APB or strychnine. Therefore, they must be mediated by an excitatory input from an S-OFF bipolar cell in sublamina a, rather than by inversion of a signal from S-ON bipolar cells. The M-ON responses were likely to be mediated by horizontal cells because they persisted in APB but were reduced by HEPES. Thus, the color opponent responses in these cells were generated by the first mechanism. These findings are summarized in Fig. 1.
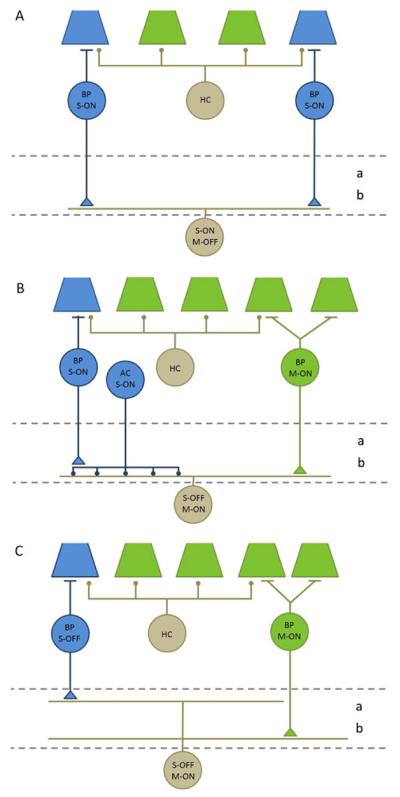
Fig. 1.
Three types of ganglion cells from rabbit retina respond selectively to stimulation of S-cones. (A) Monostratified S-ON ganglion cells ramify exclusively in sublamina b of the IPL (bounded by dashed lines). They receive excitatory input (triangles) from ON-bipolar cells (BP) that contact S-cones. OFF responses to M-cone stimulation are generated by inhibitory synapses (circles) from horizontal cells (HC) to cones and are conveyed to the IPL via the axons of the S-ON bipolar cells. (B) Monostratified S-OFF ganglion cells also ramify in sublamina b, and their responses to S-cone stimulation also originate from S-ON bipolar cells, but they are reversed in polarity by inhibitory amacrine cells. The S-ON bipolar cells make excitatory synapses onto S-ON amacrine cells which, in turn, make inhibitory synapses onto the S-OFF amacrine cells. M-ON responses are generated by excitatory synapses from M-ON bipolar cells. (C) Bistratified S-OFF ganglion cells have branches in both sublamina a and sublamina b. They receive excitatory inputs from S-OFF bipolar cells in sublamina a and from M-OFF bipolar cells in sublamina b.
Anatomical studies
Candidates for S-cone bipolar cells were first identified using the Golgi method. Both had large dendritic fields and contacted only a small percentage of the cones. Type wa was expected to have OFF responses because its axons ramified in sublamina a, and type wb was expected to have ON responses because its axons ramified in sublamina b (Famiglietti, 1981, 2008). Type wa bipolar cells were also labeled by intracellular injection with Neurobiotin. Defining 0% as the lower border of the inner nuclear layer (INL) and 100% as the upper border of the ganglion cell layer (GCL), the wa axons ramified between 5% and 15% depth in the IPL. These bipolar cells received input exclusively from S-cones labeled with antibody to S-opsin (Liu & Chiao, 2007). Type wb cells were also labeled by uptake of biocytin injected into the vitreous humor and visualized with avidin-fluorescein. Their dendrites contacted 2–7 S-cones identified with antibody to S-opsin (MacNeil & Gaul, 2008).
Candidates for S cone-selective ganglion cells have been proposed based on anatomical studies. The G3 ganglion cell had a relatively sparse dendritic arbor in sublamina b and a denser dendritic arbor in sublamina a, and it was proposed that it had S-ON responses to light based on its similarity to small bistratified cells of primates (Rockhill et al., 2002). The same bistratified ganglion cell, also called type III.2a, was proposed to have OFF responses to S-cone stimulation based on the costratification of its dendrites with the axon terminals of wa bipolar cells (Famiglietti, 2008). The morphology of G3 ganglion cells was later described in detail (Hoshi & Mills, 2009). Recordings from these cells showed them to be OFF-center cells with a preference for vertically oriented stimuli (Venkataramani & Taylor, 2010). Hoshi and Mills tested many G3 ganglion cells with S-cone isolating stimuli and found no evidence for chromatic opponency (unpublished observations).
A more promising candidate for an S-OFF ganglion cell was described using the Golgi method, a bistratified ganglion cell called BS3, which ramified at 6–14% depth in sublamina a and 80–88% depth in sublamina b (Famiglietti, 2009). This cell co-stratified with S-OFF cone bipolar cells and also resembled the second, bistratified S-OFF ganglion cell type (Mills & Tian, 2012). The monostratified IIb2 type was proposed as an S-ON ganglion cell based on close contacts of wb bipolar cell axon terminals with the dendrites of this ganglion cell (Famiglietti, 2008). This was confirmed in a physiological study; the monostratifed S-ON cell, the first type described above, was similar in morphology and stratification to the IIb2 type (Mills & Tian, 2012). No obvious candidate for the monostratified S-OFF type has been described previously. Because these are morphologically very similar to the S-ON type, the two types may have been grouped together in earlier anatomical studies.
Primates
Studies with extracellular electrodes
With the exception of the nocturnal species lacking S-cones, all primates that have been studied to date have retinal ganglion cells with ON responses to S-cone stimulation and OFF responses to stimuli of longer wavelengths. These were first described in extracellular recordings from the spider monkey (Ateles sp.) optic nerve (Hubel & Wiesel, 1960). In macaque (Macaca mulatta) retina, they had tonic responses to light, and compared with the tonic ganglion cells stimulated by long wavelength-sensitive (L) cones and M-cones, the ganglion cells excited by S-cones had larger receptive field centers (Gouras, 1968). A later study reported that 7% of the cells in the macaque (Macaca sp.) retina had S-ON/LM-OFF responses. Cells with S-OFF/LM-ON responses were far less numerous, comprising only 2% of the total (de Monasterio & Gouras, 1975). The same percentages were found in a third study of central macaque ganglion cells using similar techniques (de Monasterio et al., 1975). In many of the ganglion cells in these two studies, the area from which responses to L- and M-cones could be elicited was larger or smaller than the area from which responses to S-cones could be elicited; these cells were described as having concentric surrounds. The same was true in another study of central macaque retinal ganglion cells, and based on their receptive field organization and axonal conduction velocities, they were classified as X cells (de Monasterio, 1978 b).
In other macaque ganglion cells, the responses to S cones and the responses of opposite polarity to L and M cones were coextensive, as in the type II cells described previously in macaque lateral geniculate nucleus (de Monasterio & Gouras, 1975; de Monasterio, 1978a). In a later study of macaque retina, all of the S-ON cells were described as having the type II receptive field organization, and they comprised 6% of the total. Fewer than 1% of the ganglion cells in that sample were the S-OFF type (Zrenner & Gouras, 1981). Unlike other primate retinal ganglion cells, S-ON cells were able to detect a boundary between an yellow and a white stimulus (Gouras & Eggers, 1982), and, unlike color opponent cells sensitive to L and M cones, S-ON cells had a consistent spectral sensitivity and neutral point, the wavelength at which excitation switched to inhibition (Zrenner, 1983). However, macaque S-ON cells were very similar to color opponent cells sensitive to L- and M-cones in their temporal response properties (Yeh et al., 1995).
Small bistratifi ed ganglion cells
The major type of S-ON ganglion cell in primates was identified morphologically as the small bistratified type using intracellular recording and Neurobiotin injection (Dacey & Lee, 1994). These had two sets of dendrites, one in the outermost stratum of sublamina a and the other in the innermost stratum of sublamina b. Because their dendritic field diameters and spatial density as a function of eccentricity were known (Dacey, 1993), it was possible to identify small bistratified cells in many of the electrophysiological studies that followed. Using a multielectrode array and stimulating with chromatic white noise, both small bistratified cells and the S-cones that provided their excitatory input could be identified (Chichilnisky & Baylor, 1999). Each S-cone provided the major input to one small bistratified cell and smaller inputs to the other neighboring cells of the same type; these authors also showed that the inputs from S-cones sum linearly. Very similar ganglion cells were found in capuchin monkey (Cebus apella) retinas (Silveira et al., 1999). With temporally modulated stimuli based on movies of natural scenes, small bistratified cells responded well to increases in S-cone stimulation but less so to luminance changes. Most of the information transmitted to the brain by these cells would be at low temporal frequencies (van Hateren et al., 2002).
Small bistratified cells in the peripheral macaque retina had responses that were more transient than those recorded from the central retina, but otherwise they were very similar. The receptive field for S-ON responses was slightly smaller than that for the LM-OFF responses, and the inputs from all three cone types had the same latency (Solomon et al., 2005). In a later study, small bistratified ganglion cells in central macaque retina were recorded with either loose patch or intracellular electrodes, and many of the cells were identified by injecting Neurobiotin. Rod input to small bistratified cells with the same sign as the input from S-cones was observed, and the S-ON and LM-OFF components of the receptive field were coextensive (Crook et al., 2009).
In four recent studies, large-scale multielectrode arrays were used to record the activity of all the small bistratified cells in a patch of peripheral macaque retina. The small bistratified cells formed a regular mosaic, and the receptive fields had a concentric organization, in which the diameter of the LM-OFF component was about 50% larger than that of the S-ON component. The slow kinetics of the LM-OFF response suggested that it originated from horizontal cells (Field et al., 2007). Rod input to small bistratified cells was confirmed and found to originate from the primary rod pathway, rod bipolar cells, and AII amacrine cells, for stimuli in the low scotopic range (Field et al., 2009). Under constant photopic stimulation, the firing of small bistratified cells was correlated with that of other cells of the same type and with other types of ON cells. There were no fast correlations between neighboring small bistratified cells, however (Greschner et al., 2011). This was surprising because Neurobiotin injected intracellularly into small bistratified ganglion cells in human and macaque retinas spread to a subset of amacrine cells and to neighboring small bistratified cells, presumably via gap junctions (Dacey, 1993). Small bistratified cells were similar to other common types of macaque retinal ganglion cells in their responses to focal electrical stimulation (Jepson et al., 2013).
In parallel, there have been a number of studies of the synaptic inputs to small bistratified cells. Light microscopic immunolabeling was used to identify the bipolar cells presynaptic to small bistratified ganglion cells that had been injected with Neurobiotin in peripheral marmoset (Callithrix jacchus) retina. Inputs from DB3 diffuse bipolar cells with the dendrites were found in sublamina a and from S-ON bipolar cells with the dendrites in sublamina b (Ghosh et al., 1997). Reconstructing the small bistratified cells from serial ultrathin sections of central macaque retina, these findings were confirmed and DB2 diffuse bipolar cells were also found to be presynaptic. Each bistratified cell received most of its input from a single S-ON bipolar cell and smaller numbers of inputs from one or two other S-ON bipolar cells. Approximately 45% of the input synapses onto small bistratified cells in central macaque retina were from amacrine cells (Calkins et al., 1998). Intracellularly injected small bistratified cells from peripheral marmoset retina had a much higher percentage of amacrine cell input, and large dense-cored vesicles were found in some of the presynaptic amacrine cells (Ghosh et al., 1999). Using a combination of retrograde labeling and light microscopic immunolabeling, a high percentage of the bipolar cell input to small bistratified cells was observed in central marmoset retina, as in central macaque retina (Percival et al., 2009). In all of these studies, there were more bipolar cell synapses onto dendrites in sublamina b than dendrites in sublamina a, and this was thought to compensate for the lower spatial density of S-cones. Small bistratified cells are the only type of ganglion cell proven to generate color opponent responses by the second mechanism, that is, direct inputs from bipolar cells driven by two different cone types and having opposite response polarities.
There has been some controversy about the contributions of S-ON bipolar cell receptive field surrounds, mediated by H2 horizontal cells, to the light responses of small bistratified ganglion cells. Initially, two components were identified in the LM-OFF responses of small bistratified cells using intracellular electrodes. One component must have originated from the receptive field surrounds of S-ON bipolar cells because it was blocked by APB. The second component, mediated by OFF-diffuse bipolar cells, persisted after APB treatment (Dacey, 2000). The stimuli and recording conditions used in a study using a microelectrode array must have favored the first component. The LM-OFF component was completely blocked by APB and therefore appeared to originate in the OPL. Under these conditions, the receptive field surround was larger than the receptive field center (Field et al., 2007). The conditions used in a later study apparently favored the second component. The S-ON component of the response was blocked by APB, but the entire LM-OFF component persisted in the presence of APB. Under these conditions, the two components of the receptive field were approximately coextensive (Crook et al., 2009). These authors suggested that the difference between their results and the ones reported earlier arose because their recordings were from the central retina and the earlier ones from the peripheral retina, an interpretation consistent with the differences between central and peripheral retina in the proportion of the bipolar cell input. They also pointed out that the stimulus intensities and the adaptation states of the retina were different in the two studies. Thus, it appears that small bistratified cells use either horizontal cells and apposed inputs from cone-selective bipolar cells to generate color opponent responses, depending on the eccentricity, the stimulus, and the adaptation state.
It is clear that amacrine cells do not generate color opponent responses in small bistratified ganglion cells because opponency is unaffected by antagonists of GABA and glycine, the primary amacrine cell neurotransmitters. However, amacrine cells are clearly important in the pathways that provide input to small bistratified ganglion cells in primates because they provide direct inhibitory input (Calkins et al., 1998; Ghosh & Grunert, 1999). In experiments using high-contrast chromatic stimuli and intracellular electrodes, the GABA antagonist picrotoxin had two subtle effects on small bistratified cells. The amplitudes of the responses to S-cone stimulation and to L- and M-cone stimulation were both increased, and the responses to stimulation of L- and M-cones became slower and more prolonged (Crook et al., 2009). The effects of amacrine cells on the light responses of small bistratified ganglion cells were more apparent in subsequent voltage-clamp studies with patch electrodes. S-cone-isolating stimuli did not generate detectable inhibition, but L+M-isolating stimuli produced both feed-forward and crossover inhibition. The feed-forward inhibition was blocked by a GABA antagonist and the crossover inhibition by a glycine antagonist; these findings suggest that at least two amacrine cell types are involved (Dacey et al., 2011). The absence of inhibition with S-cone stimulation is consistent with the finding that the amacrine cells postsynaptic to S-cone bipolar cells rarely make feed-forward synapses onto the postsynaptic ganglion cells (Marshak et al., 1990). The amacrine cells that mediate these interactions have not been identified, but one candidate is an amacrine cell that contains the neuropeptide secretoneurin (Marshak, 1997). These amacrine cells have dendrites that costratify with axons of S-ON bipolar cells and the dendrites of small bistratified ganglion cells. They receive a large proportion of their input from bipolar cells and direct a large proportion of their output to ganglion cells and to OFF bipolar cells, a pattern of synaptic connections that is consistent with the physiological results.
Other types of S-cone opponent ganglion cells
A second type of macaque ganglion cell with S-ON/LM-OFF responses and type II receptive field organization, the large bistratified cell, was described using a combination of retrograde labeling, intracellular recording and Neurobiotin injection, and also using the Golgi method. Its dendrites were found in the same two strata of the IPL as those of the small bistratified cells, but their dendritic and receptive field diameters were much larger (Dacey, 1993; Peterson & Dacey, 2000; Dacey et al., 2003). Large bistratified cells have also been described using intracellular Neurobiotin injection in macaque retina, using a combination of retrograde labeling and photofilling in central marmoset retina and using transient transfection with GFP in peripheral marmoset retina (Yamada et al., 2005; Moritoh et al., 2013; Percival et al., 2013). Because large bistratified cells have relatively small perikarya and a low spatial density, it is uncertain whether any were sampled in the earlier studies of primate retina using extracellular electrodes. Although it is possible, based on their stratification pattern, that large bistratified cells use the second mechanism to generate color opponency, this is uncertain because their synaptic inputs have not been described.
Two types of ganglion cells with S-OFF/LM-ON responses have been identified using similar techniques, large sparse and giant sparse (melanopsin-containing) cells. Their receptive fields also lacked spatial opponency, and they had relatively low spatial densities (Dacey et al., 2003; Dacey et al., 2005; Percival et al., 2013). Both types had dendrites ramifying in the innermost part of sublamina b, and both received excitatory input from DB6 ON-diffuse bipolar cells (Jusuf et al., 2007; Grunert et al., 2011; Percival et al., 2011). Bistratified, color-opponent bipolar cells have been proposed as a possible source for these responses (Kolb et al., 1997). However, there is psychophysical evidence suggesting that the S-OFF responses arise from amacrine cells, via the third mechanism. Responses to increments in the intensity of S-cone isolating stimuli were significantly faster than those to decrements in the same stimulus, possibly reflecting an additional synapse in the S-OFF pathway (Shinomori & Werner, 2012). The presynaptic amacrine cells have not been identified, but they are expected to have S-ON responses, like those described in ground squirrel retina (Chen & Li, 2012). One candidate is the amacrine cell that is labeled when Neurobiotin is injected into small bistratified ganglion cells. It has varicose dendrites ramifying in the same strata of the IPL as those of the small bistratified ganglion cell (Dacey, 1993). Gap junctions with small bistratified ganglion cells would be expected to generate S-ON responses in these amacrine cells.
Another potential source of S-OFF responses is a subset of central OFF midget ganglion cells that receive input from midget bipolar cells with input from a single S-cone; these would generate opponent responses via the first mechanism. These were identified in an electron microscopic study of central macaque retina (Klug et al., 2003). These cells have not been identified by intracellular recording and tracer injection, but in peripheral macaque retina, receptive field centers of OFF midget ganglion cells received input from S-cones, but ON midget ganglion cells did not (Field et al., 2010). Psychophysical evidence supporting the existence of this pathway is discussed by Kiyuharo et al. (this issue). Thus, the various types of primate retinal ganglion cells utilize all three of the mechanisms to generate S-cone opponent responses. These findings are summarized in Fig. 2.
Discussion
Color opponency in S-cone pathways
There are three basic mechanisms to generate color opponent responses in ganglion cells that respond selectively to stimulation of S-cones. The mechanism listed first in the introduction of this review is the simplest; the opponency is generated by horizontal cells in the OPL and conveyed to the ganglion cell by excitatory synapses from S-cone bipolar cells. In rabbits, guinea pigs and, possibly, other species, there are monostratified, S-ON ganglion cells whose color-opponent responses are generated this way via inputs from S-ON bipolar cells. In rabbits, color-opponency is also generated similarly in one type of S-OFF ganglion cell via excitatory inputs from S-OFF bipolar cells. In the central retinas of Old World monkeys and, presumably, humans, this mechanism is expected to generate color-opponent responses in a subset of OFF midget ganglion cells that receive input from a single S-cone via an OFF midget bipolar cell. Under some conditions, small bistratified cells of primates also have opponent responses generated partially or entirely by horizontal cells.
The mechanism listed second, in which excitatory inputs from S-ON bipolar cells are combined with excitatory inputs from OFF bipolar cells driven by other cone types, appears to be found only in primates. This accounts for the opponency in small bistratified ganglion cells, particularly those closest to the center of the retina. The mechanism listed third and described most recently, S-OFF responses in ganglion cells generated by inhibitory inputs from S-ON amacrine cells, may be the oldest phylogenetically. Ganglion cells like these have been found in marsupials and three orders of placental mammals: rodents, lagomorphs, and primates.
The small bistratified cells of primates utilize two different mechanisms to generate color-opponent responses, and their receptive field organization varies accordingly. Because small bistratified cells have a much higher spatial density than other types of S-cone selective ganglion cells, they were likely the source of vast the majority of the early extracellular recordings. In retrospect, the cells with concentric surrounds and the cells with coextensive surrounds were probably small bistratified cells under different conditions. The same may also be true for ganglion cells that respond selectively to S-cone stimulation in other mammals.
Evolution of S-cone-selective ganglion cells
Five types of ganglion cells respond selectively to S-cone stimulation in have-been-identified Old World anthropoids (cercopithecoids, apes, and humans). An evolutionary scenario to account for this diversity is illustrated in Fig. 3. We propose that there are two distinct processes underlying the evolution of these retinal ganglion cells. The first process is duplication of a neuronal type followed by diversification, which we propose occurred at three different times. This would explain the appearance of two cell types that are very similar in their morphology and stratification patterns, but different in one or two functionally significant properties. Duplication and diversification like this appear to have occurred during the evolution of brisk-transient ganglion cells, as well. In primates, for example, these include smooth bistratified cells, which are similar in many respects to parasol cells but have much larger dendritic field diameters (Petrusca et al., 2007; Crook et al., 2008). A similar process of duplication and diversification has been proposed to account for the similarities between some types of bipolar cells in ground squirrel retina (Light et al., 2012).
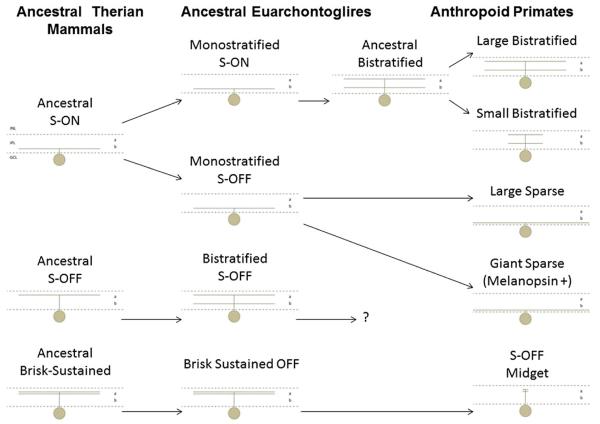
Fig. 3.
An evolutionary tree was constructed to account for the diversity of primate retinal ganglion cells that respond selectively to stimulation of short wavelength-sensitive (S) cones. The positions of the INL and the GCL are indicated in the upper left panel, and the conventions are the same in the other panels. The boundaries of the IPL are indicated with dashed lines, and the outer (a) and inner (b) sublaminae are labeled. The hypothetical monostratified S-ON, S-OFF, and OFF brisk-sustained ganglion cells of the Therian common ancestor of the marsupial and placental mammals are indicated on the left. In the Euarchontoglires (center), which gave rise to the rodents, rabbits, and primates among other mammals, the ancestral S-ON cell was duplicated to become monostratified S-ON and monostratified S-OFF cells like those of modern guinea pigs and rabbits. The ancestral S-OFF cell became a bistratified S-OFF cell like that of modern rabbits. The question mark indicates that its homolog in primates, if any, is unknown. Early in the primate lineage (right), the monostratified S-ON cell became bistratified, and then it was duplicated, giving rise to the small and large bistratified types. The monostratified S-OFF type was duplicated, giving rise to the large sparse and giant sparse types. The OFF brisk-sustained type was relatively unchanged during mammalian evolution; it simply became smaller in primates, where it is called a midget ganglion cell. In Old World anthropoids, a subset of central OFF midget ganglion cells receives input from a single S-cone.
The second process is transformation of a monostratified ganglion cell type into a bistratified type; we propose that this happened twice during the evolution of mammalian ganglion cells that respond selectively to S-cone stimulation. Developing retinal ganglion cells have broadly stratified dendritic arbors that become more narrowly stratified later. A small change in the developmental program might generate two sets of narrowly stratified dendrites rather than one. Because the early dendritic arbors are found around the center of the IPL, it seems quite plausible that one set of dendrites would ramify in sublamina a and the other in sublamina b in the adult (Tian, 2008).
On the left side of Fig. 3 are three types of ganglion cells that we propose were found in a hypothetical therian mammal, the ancestor of the placental and marsupial mammals. In the supraordinal group Euarchontoglires, including rodents, lagomorphs, and primates, we propose that the ancestral, monostratifed S-ON ganglion cell was duplicated, giving rise to monostratified S-ON and S-OFF types, similar to those found in modern guinea pigs and rabbits. In both species, these are very similar in their morphology and stratification patterns. The monostratified S-ON type receives excitatory input from the S-ON bipolar cell, but a single change in a cell-to-cell recognition pathway might prevent this synapse from developing. The monostratified S-OFF type would, instead, receive inhibitory input from an S-ON amacrine cell branching in the same stratum of the IPL. As a result, the S-OFF cell would evolve with no new circuitry elements required. Support for this idea comes from physiological experiments with monostratified S-OFF cells in rabbit retina. When the strychnine is applied, a residual ON response is unmasked (Mills, unpublished observations). This finding suggests that the monostratified S-OFF cell may have retained some inputs from the S-ON cell bipolar cell, but strong upregulation of the glycinergic inhibition suppressed the ON responses.
We propose that an ancestral, monostratified S-OFF ganglion cell that receives excitatory input from an S-OFF bipolar cell in sublamina a became a bistratified S-OFF cell like that observed in modern rabbits. If there is a homologous type of S-OFF ganglion cell in primates, it has not yet been described. Because S-OFF bipolar cells have been tentatively identified in a study of human retinas using the Golgi method (Kolb et al., 1992), this is certainly plausible. In addition, the ancestral, monostratified S-ON type became bistratified early in the primate lineage, and later it was duplicated to generate the large bistratified cell and small bistratified cell of modern primates. The extra set of dendrites would provide access to bipolar cells and monostratified amacrine cells with opposite response polarities, and this might be advantageous for color opponency. The strata of the IPL contain different combinations of bipolar cells and amacrine cells, imparting distinct characteristics to each one (Werblin, 2011). We also propose that the monostratified S-OFF cell was duplicated in the primate lineage to become the large sparse cell and the giant sparse cell of primates, which are morphologically very similar. The hypothetical, ancestral OFF-brisk sustained ganglion cell was apparently relatively unchanged during evolution, simply becoming smaller in primates and, in Old World anthropoids, receiving excitatory input from S-OFF midget bipolar cells.
These hypotheses are speculative, but they are potentially testable in experiments comparing color-opponent ganglion cell types both within and between species. This would require a much larger sample of color-opponent ganglion cells from mammals other than primates. At present, only a few color opponent ganglion cells have been identified in nonprimate mammals using intracellular recording and tracer injection, probably because of the low spatial density of these ganglion cells and the necessity to select ganglion cells for recording at random (Yin et al., 2009; Mills & Tian, 2012; Chang et al., 2013). These sampling problems could be alleviated by using transgenic markers to selectively label populations of color-opponent ganglion cells, either by chance or by identification of genes specific to those populations. While many genetic markers used currently result in labeling of ganglion cell types that are not obviously related, genetic analyses using ganglion cell transcriptomes may soon lead to identification of transcripts that would identify types that are related either by functional or evolutionary commonalities. Such genetic analyses would also provide a basis for testing these ideas, and this is already feasible in mouse retina (Siegert et al., 2012).
Genetic manipulations are currently restricted to mouse retina, but mouse ganglion cells can also be labeled by viral transfection (Hong et al., 2011). This method is applicable to other species, as well; viral vectors have been used to label macaque retinal ganglion cells (Koilkonda et al., 2009) and ground squirrel bipolar cells (Light et al., 2012). Transient transfection of marmoset retinal ganglion cells in vitro with GFP has been used to label S-cone opponent ganglion cells in marmoset retina, and this method is also applicable in other species (Moritoh et al., 2013). Studies of blue-yellow opponent ganglion cells using multielectrode arrays have already yielded valuable results and are growing in popularity (Field et al., 2007; Sher & DeVries, 2012). When combined with techniques that label a subset of retinal ganglion cells, multielectrode array studies have the potential to provide correlative morphology (EJ Chichilnisky, personal communication). The yield of blue-yellow opponent ganglion cells in anatomical studies could be increased by combining S-cone isolating stimulation with activity-dependent labeling techniques (Marc et al., 2005).
Conclusions
Our understanding of S-cone pathways has until very recently been informed solely by the difficult technique of blind extracellular recording, whose drawbacks include selection bias, low encounter rates, and inability to characterize the cells morphologically. The result has likely been underestimation of the diversity of S-cone pathways and the opponent mechanisms that comprise them. Homologies between species have necessarily been difficult to identify; the S-ON ganglion cell in dichromatic mammals does not resemble the small bistratified S-ON ganglion cell in primates, for example. Recent work in primates, guinea pigs, ground squirrels, and rabbits suggests that there are at least two and as many as five types of retinal ganglion cells that respond selectively to stimulation of S-cones in each species. We have compared the older results with newer data collected using patch electrode recordings from whole cells and multielectrode arrays and suggested an evolutionary scenario to account for the diversity of primate retinal ganglion cells that respond selectively to S-cone stimulation.
Acknowledgments
This work was supported by grants EY06472, EY010121, and EY10608 from the National Eye Institute. We are grateful to John Concha for preparing the illustrations and to E.J. Chichilnisky, Peter Gouras, Hideo Hoshi, E. Christopher Kirk, and Lin-Min Tiang for valuable discussions and for sharing unpublished results.
References
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. The Journal of Neuroscience. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW. New properties of rabbit retinal ganglion cells. The Journal of Physiology. 1978;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina. The Journal of Neuroscience. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Breuninger T, Euler T. Chromatic coding from cone-type unselective circuits in the mouse retina. Neuron. 2013;77:559–571. doi: 10.1016/j.neuron.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Chen S, Li W. A color-coding amacrine cell may provide a blue-off signal in a mammalian retina. Nature Neuroscience. 2012;15:954–956. doi: 10.1038/nn.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky EJ, Baylor DA. Receptive-field microstructure of blue-yellow ganglion cells in primate retina. Nature Neuroscience. 1999;2:889–893. doi: 10.1038/13189. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Levick WR. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. The Journal of Physiology. 1974;240:457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Convergence and divergence of cones onto bipolar cells in the central area of cat retina. Philosophical Transactions of the Royal Society of London. 1990a;330:323–328. doi: 10.1098/rstb.1990.0202. Series B, Biological Sciences. [DOI] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Demonstration of cell types among cone bipolar neurons of cat retina. Philosophical Transactions of the Royal Society of London. 1990b;330:305–321. doi: 10.1098/rstb.1990.0201. Series B, Biological Sciences. [DOI] [PubMed] [Google Scholar]
- Crook JD, Davenport CM, Peterson BB, Packer OS, Detwiler PB, Dacey DM. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. The Journal of Neuroscience. 2009;29:8372–8387. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates “red-green” color opponency in midget ganglion cells of the primate retina. The Journal of Neuroscience. 2011;31:1762–1772. doi: 10.1523/JNEUROSCI.4385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Peterson BB, Packer OS, Robinson FR, Gamlin PD, Troy JB, Dacey DM. The smooth monostratified ganglion cell: Evidence for spatial diversity in the Y-cell pathway to the lateral geniculate nucleus and superior colliculus in the macaque monkey. The Journal of Neuroscience. 2008;28:12654–12671. doi: 10.1523/JNEUROSCI.2986-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Visual Neuroscience. 1993;10:1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annual Review of Neuroscience. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The `blue-on' opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Current Opinion in Neurobiology. 2003;13:421–427. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: In vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Crook JD, Manookin MB, Packer OS. Association for Research in Vision and Ophthalmology Annual Meeting. Fort Lauderdale, FL: 2011. Absence of synaptic inhibition associated with S-cone on excitatory input to the small bistratified, blue-yellow opponent ganglion cell of the macaque monkey retina. [Google Scholar]
- de Monasterio FM. Center and surround mechanisms of opponent-color X and Y ganglion cells of retina of macaques. Journal of Neurophysiology. 1978a;41:1418–1434. doi: 10.1152/jn.1978.41.6.1418. [DOI] [PubMed] [Google Scholar]
- de Monasterio FM. Properties of concentrically organized X and Y ganglion cells of macaque retina. Journal of Neurophysiology. 1978b;41:1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]
- de Monasterio FM, Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. The Journal of Physiology. 1975;251:167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Monasterio FM, Gouras P, Tolhurst DJ. Trichromatic colour opponency in ganglion cells of the rhesus monkey retina. The Journal of Physiology. 1975;251:197–216. doi: 10.1113/jphysiol.1975.sp011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The Retina: An Approachable Part of the Brain. Harvard University Press; Cambridge, MA: 2012. [Google Scholar]
- Ekesten B, Gouras P. Cone and rod inputs to murine retinal ganglion cells: evidence of cone opsin specific channels. Visual Neuroscience. 2005;22:893–903. doi: 10.1017/S0952523805226172. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Wide-field cone bipolar cells and the blue-ON pathway to color-coded ganglion cells in rabbit retina. Visual Neuroscience. 2008;25:53–66. doi: 10.1017/S0952523808080061. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Bistratified ganglion cells of rabbit retina: Neural architecture for contrast-independent visual responses. Visual Neuroscience. 2009;26:195–213. doi: 10.1017/S0952523808080929. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV., Jr. Functional architecture of cone bipolar cells in mammalian retina. Vision Research. 1981;21:1559–1563. doi: 10.1016/0042-6989(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Field GD, Gauthier JL, Sher A, Greschner M, Machado TA, Jepson LH, Shlens J, Gunning DE, Mathieson K, Dabrowski W, Paninski L, Litke AM, Chichilnisky EJ. Functional connectivity in the retina at the resolution of photoreceptors. Nature. 2010;467:673–677. doi: 10.1038/nature09424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Greschner M, Gauthier JL, Rangel C, Shlens J, Sher A, Marshak DW, Litke AM, Chichilnisky EJ. High-sensitivity rod photoreceptor input to the blue-yellow color opponent pathway in macaque retina. Nature Neuroscience. 2009;12:1159–1164. doi: 10.1038/nn.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Sher A, Gauthier JL, Greschner M, Shlens J, Litke AM, Chichilnisky EJ. Spatial properties and functional organization of small bistratified ganglion cells in primate retina. The Journal of Neuroscience. 2007;27:13261–13272. doi: 10.1523/JNEUROSCI.3437-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh F, Bruun A, Ehinger B. Graft-host connections in long-term full-thickness embryonic rabbit retinal transplants. Investigative Ophthalmology & Visual Science. 1999;40:126–132. [PubMed] [Google Scholar]
- Ghosh KK, Martin PR, Grunert U. Morphological analysis of the blue cone pathway in the retina of a New World monkey, the marmoset Callithrix jacchus. The Journal of Comparative Neurology. 1997;379:211–225. [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. The Journal of Physiology. 1968;199:533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Eggers H. Ganglion cells mediating the signals of blue sensitive cones in primate retina detect white-yellow borders independently of brightness. Vision Research. 1982;22:675–679. doi: 10.1016/0042-6989(82)90103-1. [DOI] [PubMed] [Google Scholar]
- Greschner M, Shlens J, Bakolitsa C, Field GD, Gauthier JL, Jepson LH, Sher A, Litke AM, Chichilnisky EJ. Correlated firing among major ganglion cell types in primate retina. The Journal of Physiology. 2011;589:75–86. doi: 10.1113/jphysiol.2010.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunert U, Jusuf PR, Lee SC, Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Visual Neuroscience. 2011;28:39–50. doi: 10.1017/S095252381000026X. [DOI] [PubMed] [Google Scholar]
- Guenther E, Zrenner E. The spectral sensitivity of dark- and light-adapted cat retinal ganglion cells. The Journal of Neuroscience. 1993;13:1543–1550. doi: 10.1523/JNEUROSCI.13-04-01543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. The Journal of Neuroscience. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi JM, James A, Taylor WR. Color opponent retinal ganglion cells in the tammar wallaby retina. Journal of Vision. 2002;2:608–617. doi: 10.1167/2.9.3. [DOI] [PubMed] [Google Scholar]
- Hong YK, Kim IJ, Sanes JR. Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. The Journal of Comparative Neurology. 2011;519:1691–1711. doi: 10.1002/cne.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, Mills SL. Components and properties of the G3 ganglion cell circuit in the rabbit retina. The Journal of Comparative Neurology. 2009;513:69–82. doi: 10.1002/cne.21941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of optic nerve fibres in the spider monkey. The Journal of Physiology. 1960;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson LH, Hottowy P, Mathieson K, Gunning DE, Dabrowski W, Litke AM, Chichilnisky EJ. Focal electrical stimulation of major ganglion cell types in the primate retina for the design of visual prostheses. The Journal of Neuroscience. 2013;33:7194–7205. doi: 10.1523/JNEUROSCI.4967-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. The European Journal of Neuroscience. 2007;26:2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- Klug K, Herr S, Ngo IT, Sterling P, Schein S. Macaque retina contains an S-cone OFF midget pathway. The Journal of Neuroscience. 2003;23:9881–9887. doi: 10.1523/JNEUROSCI.23-30-09881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koilkonda RD, Hauswirth WW, Guy J. Efficient expression of self-complementary AAV in ganglion cells of the ex vivo primate retina. Molecular Vision. 2009;15:2796–2802. [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Goede P, Roberts S, McDermott R, Gouras P. Uniqueness of the S-cone pedicle in the human retina and consequences for color processing. The Journal of Comparative Neurology. 1992;386:443–460. doi: 10.1002/(sici)1096-9861(19970929)386:3<443::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kolb H, Linberg KA, Fisher SK. Neurons of the human retina: a Golgi study. The Journal of Comparative Neurology. 1992;318:147–187. doi: 10.1002/cne.903180204. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nature Neuroscience. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Light AC, Zhu Y, Shi J, Saszik S, Lindstrom S, Davidson L, Li X, Chiodo VA, Hauswirth WW, Li W, DeVries SH. Organizational motifs for ground squirrel cone bipolar cells. The Journal of Comparative Neurology. 2012;520:2864–2887. doi: 10.1002/cne.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, Suemune S, Fisher SK. Retinal neurons of the California ground squirrel, Spermophilus beecheyi: A Golgi study. The Journal of Comparative Neurology. 1996;365:173–216. doi: 10.1002/(SICI)1096-9861(19960205)365:2<173::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Liu PC, Chiao CC. Morphologic identification of the OFF-type blue cone bipolar cell in the rabbit retina. Investigative Ophthalmology & Visual Science. 2007;48:3388–3395. doi: 10.1167/iovs.06-1531. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Gaul PA. Biocytin wide-field bipolar cells in rabbit retina selectively contact blue cones. The Journal of Comparative Neurology. 2008;506:6–15. doi: 10.1002/cne.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Kalloniatis M, Jones BW. Excitation mapping with the organic cation AGB2+ Vision Research. 2005;45:3454–3468. doi: 10.1016/j.visres.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Marshak D. Secretoneurin-IR amacrine cells of the macaque retina. Investigative Ophthalmology & Visual Science. 1997;38:S50. [Google Scholar]
- Marshak DW, Aldrich LB, Del Valle J, Yamada T. Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. The Journal of Neuroscience. 1990;10:3045–3055. doi: 10.1523/JNEUROSCI.10-09-03045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael CR. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. 3. Opponent color units. Journal of Neurophysiology. 1968;31:268–282. doi: 10.1152/jn.1968.31.2.268. [DOI] [PubMed] [Google Scholar]
- Mills SL, Tian L-M. Association for Research in Vision and Ophthalmology Annual Meeting. Ft. Lauderdale, FL: 2012. The morphology and physiology of blue/ green ganglion cells in the rabbit retina. [Google Scholar]
- Moritoh S, Komatsu Y, Yamamori T, Koizumi A. Diversity of retinal ganglion cells identified by transient GFP transfection in organotypic tissue culture of adult marmoset monkey retina. PLoS One. 2013;8:e54667. doi: 10.1371/journal.pone.0054667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer OS, Verweij J, Li PH, Schnapf JL, Dacey DM. Blue-yellow opponency in primate S cone photoreceptors. The Journal of Neuroscience. 2010;30:568–572. doi: 10.1523/JNEUROSCI.4738-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival KA, Jusuf PR, Martin PR, Grunert U. Synaptic inputs onto small bistratified (blue-ON/yellow-OFF) ganglion cells in marmoset retina. The Journal of Comparative Neurology. 2009;517:655–669. doi: 10.1002/cne.22183. [DOI] [PubMed] [Google Scholar]
- Percival KA, Martin PR, Grunert U. Synaptic inputs to two types of koniocellular pathway ganglion cells in marmoset retina. The Journal of Comparative Neurology. 2011;519:2135–2153. doi: 10.1002/cne.22586. [DOI] [PubMed] [Google Scholar]
- Percival KA, Martin PR, Grunert U. Organisation of koniocellular-projecting ganglion cells and diffuse bipolar cells in the primate fovea. The European Journal of Neuroscience. 2013;37:1072–1089. doi: 10.1111/ejn.12117. [DOI] [PubMed] [Google Scholar]
- Peterson BB, Dacey DM. Morphology of wide-field bistratified and diffuse human retinal ganglion cells. Visual Neuroscience. 2000;17:567–578. doi: 10.1017/s0952523800174073. [DOI] [PubMed] [Google Scholar]
- Petrusca D, Grivich MI, Sher A, Field GD, Gauthier JL, Greschner M, Shlens J, Chichilnisky EJ, Litke AM. Identification and characterization of a Y-like primate retinal ganglion cell type. The Journal of Neuroscience. 2007;27:11019–11027. doi: 10.1523/JNEUROSCI.2836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puller C, Haverkamp S. Bipolar cell pathways for color vision in non-primate dichromats. Visual Neuroscience. 2011;28:51–60. doi: 10.1017/S0952523810000271. [DOI] [PubMed] [Google Scholar]
- Puller C, Ondreka K, Haverkamp S. Bipolar cells of the ground squirrel retina. The Journal of Comparative Neurology. 2011;519:759–774. doi: 10.1002/cne.22546. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Wolbarsht ML. Spectral coding in cat retinal ganglion cell receptive fields. Journal of Neurophysiology. 1986;55:320–330. doi: 10.1152/jn.1986.55.2.320. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. The Journal of Neuroscience. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. Which Cells Code for Color? In: Valberg A, Lee BB, editors. From Pigments to Perception. Plenum Press; New York: 1991. pp. 83–93. [Google Scholar]
- Rowe MH, Cox JF. Spatial receptive-field structure of cat retinal W cells. Visual Neuroscience. 1993;10:765–779. doi: 10.1017/s0952523800005459. [DOI] [PubMed] [Google Scholar]
- Schuurmans RP, Zrenner E. Responses of the blue sensitive cone system from the visual cortex and the arterially perfused eye in cat and monkey. Vision Research. 1981;21:1611–1615. doi: 10.1016/0042-6989(81)90043-2. [DOI] [PubMed] [Google Scholar]
- Sher A, DeVries SH. A non-canonical pathway for mammalian blue-green color vision. Nature Neuroscience. 2012;15:952–953. doi: 10.1038/nn.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomori K, Werner JS. Aging of human short-wave cone pathways. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13422–13427. doi: 10.1073/pnas.1119770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Cabuy E, Scherf BG, Kohler H, Panda S, Le YZ, Fehling HJ, Gaidatzis D, Stadler MB, Roska B. Transcriptional code and disease map for adult retinal cell types. Nature Neuroscience. 2012;15:487–495. S481–482. doi: 10.1038/nn.3032. [DOI] [PubMed] [Google Scholar]
- Silveira LC, Lee BB, Yamada ES, Kremers J, Hunt DM, Martin PR, Gomes FL. Ganglion cells of a short-wavelength-sensitive cone pathway in New World monkeys: Morphology and physiology. Visual Neuroscience. 1999;16:333–343. doi: 10.1017/s0952523899162138. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Lee BB, White AJ, Ruttiger L, Martin PR. Chromatic organization of ganglion cell receptive fields in the peripheral retina. The Journal of Neuroscience. 2005;25:4527–4539. doi: 10.1523/JNEUROSCI.3921-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N. Synaptic activity, visual experience and the maturation of retinal synaptic circuitry. The Journal of Physiology. 2008;586:4347–4355. doi: 10.1113/jphysiol.2008.159202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hateren JH, Ruttiger L, Sun H, Lee BB. Processing of natural temporal stimuli by macaque retinal ganglion cells. The Journal of Neuroscience. 2002;22:9945–9960. doi: 10.1523/JNEUROSCI.22-22-09945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Levick WR, Thibos LN. Rabbit retinal ganglion cells. Receptive field classification and axonal conduction properties. Experimental Brain Research. 1981;44:27–33. doi: 10.1007/BF00238746. [DOI] [PubMed] [Google Scholar]
- Venkataramani S, Taylor WR. Orientation selectivity in rabbit retinal ganglion cells is mediated by presynaptic inhibition. The Journal of Neuroscience. 2010;30:15664–15676. doi: 10.1523/JNEUROSCI.2081-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. The retinal hypercircuit: A repeating synaptic interactive motif underlying visual function. The Journal of Physiology. 2011;589:3691–3702. doi: 10.1113/jphysiol.2011.210617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RW. Light and electron microscopy of the ground squirrel retina: functional considerations. The Journal of Comparative Neurology. 1976;168:355–377. doi: 10.1002/cne.901680304. [DOI] [PubMed] [Google Scholar]
- Yamada ES, Bordt AS, Marshak DW. Wide-field ganglion cells in macaque retinas. Visual Neuroscience. 2005;22:383–393. doi: 10.1017/S095252380522401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh T, Lee BB, Kremers J. Temporal response of ganglion cells of the macaque retina to cone-specific modulation. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1995;12:456–464. doi: 10.1364/josaa.12.000456. [DOI] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Physiology and morphology of color-opponent ganglion cells in a retina expressing a dual gradient of S and M opsins. The Journal of Neuroscience. 2009;29:2706–2724. doi: 10.1523/JNEUROSCI.5471-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner E. Neurophysiological Aspects of Color Vision in Primates. Springer-Verlag; New York: 1983. [Google Scholar]
- Zrenner E, Gouras P. Characteristics of the blue sensitive cone mechanism in primate retinal ganglion cells. Vision Research. 1981;21:1605–1609. doi: 10.1016/0042-6989(81)90042-0. [DOI] [PubMed] [Google Scholar]