Abstract
Despite the recognized joint impact of climate and land cover change on facets of biodiversity and their associated functions, risk assessments have primarily evaluated impacts on species ranges and richness. Here we quantify the sensitivity of the functional structure of European avian assemblages to changes in both regional climate and land cover. We combine species range forecasts with functional trait information. We show that species sensitivity to environmental change is randomly distributed across the functional tree of the European avifauna and that functionally unique species are not disproportionately threatened by 2080. However, projected species range changes will modify the mean species richness and functional diversity of bird diets and feeding behaviours. This will unequally affect the spatial structure of functional diversity, leading to homogenization across Europe. Therefore, global changes may alter the functional structure of species assemblages in the future in ways that need to be accounted for in conservation planning.
Both climate and land cover change are major causes of the current unprecedented rates of global biodiversity loss that may, ultimately, deteriorate the structure of biota1, ecosystem stability2 and ecosystem service provisioning3. Indeed, the current and future response of species to climate and land use changes can substantially impact species assemblages and, therefore, alter phylogenetic and functional structures4. When evaluating how changes in land cover and regional climate might impinge on biodiversity, focus on facets of biological diversity that go beyond the commonly studied species richness or turnover is crucial4. Phylogenetic diversity (PD) in species assemblages is, for instance, important for explaining the role of species interactions and biogeographic histories in structuring communities5. Further, functional diversity (FD), reflecting the diversity of morphological, physiological and ecological traits within biological assemblages6 better depicts ecosystem functions and associated services than simple patterns of species richness and turnover7. Beyond aesthetic, patrimonial and philosophical arguments, the maintenance of functional diversity is a powerful argument to halt the so-called 6th extinction3. Loss of functions provided by particular species, if these are forced to relocate or to become locally extinct due to changes in land cover or climate, likely jeopardizes important regional ecosystem processes8. This underscores the importance of quantifying how functional uniqueness and diversity of species assemblages relates to the projected sensitivity of species to environmental changes.
Not all species are equally influenced by changes in climate or land cover. Generalist species are often perceived as being less sensitive to such changes than specialists that have traits adapted to a narrower range of conditions9. Indeed, a recent modelling study on Alpine plants indicates lower extinction risk for generalists compared to rare and threatened plant species10. Increase in forests, agriculture and urban areas at the expense of semi-natural grasslands, together with change in precipitation regimes and temperature increase, may influence the structure of avian assemblages11 and their associated functional diversity. Bird assemblages are interesting to study as they heavily depend on both vegetation structure and climate, and have been shown to have important ecological role on ecosystem functioning and associated services12. Through their mutualisms with plants, birds act as genetic linkers by pollenating flowers and transporting seeds, thereby helping to maintain plant diversity by supporting gene flow12, 13. Scavengers on carcasses help limit disease spread while predators on vertebrates and insects play important roles in the regulation of prey density12, 13, 14. As another example, cavity-drillers and nest-burrowers are recognized as ecosystem engineers that provide shelter to additional species13, 15, 16. Beside these direct functions, birds also provide important cultural services for nature enthusiasts and contribute to global nutrient dynamics13. Climate- or land cover-induced modifications in bird assemblages could have cascading negative effects in trophic chains, and strongly reduce the provision of some functions. For instance, a decline in top-predators could benefit prey species, with radiating effects on all lower-trophic levels17, 18. Therefore, biological simplification of agricultural lands or forests through land use intensification may decrease the provisioning of pest control and other ecosystem services by birds if their taxonomic and functional diversity decline15, 19.
Moreover, if global changes lead to more homogenous landscapes, then this naturally translates into more similar animal assemblages20. Functionally diverse assemblages likely show greater complementarity in resource use and thus provide enhanced ecosystem functioning21. Alternatively, assemblages with numerous similar species have a greater chance to provide more functional insurance against environmental changes (e.g. pesticides or diseases) than functionally diverse assemblages because redundancy buffers against loss of functions otherwise provided by single species22. Although these specific threats are difficult to account for or predict, it is nevertheless crucial to project the potential detrimental or beneficial effects on functional diversity by projected climate and land cover change at large spatial scales23.
Here we report impact analyses of changes in land cover and regional climate on the distribution of 402 European breeding bird species and the resulting effects on the functional diversity of bird assemblages. Functional diversity is represented here by behavioural traits during feeding to reflect how species acquire resources from their environment (feeding behaviour, feeding location and activity), and by body mass and diet traits to reflect the resource use requirements of species. We consider these as effect traits that determine the impact of a given organism on community structure and ecosystem functioning24, 25, although the distinction between effect and response traits (traits that stand for the response of organisms to environmental change) is not always straightforward for animals14. In order to project current and future suitable habitats for each species, we use consensus projections extracted from multiple species distribution models, several up-to-date high-resolution regional climate models, and land cover change scenarios, where the latter two originate from recently finished EU projects. First, we ask whether species sensitivity to climate and land cover change is randomly distributed across a functional tree of the European avifauna, depicted as a dendrogram based on inter-specific functional distances. Second, we test whether functionally unique species (species bearing singular combination of traits) are projected to experience more-severe changes in suitable climates and habitats than species bearing more common traits syndromes. Third, we ask whether changes in species habitat suitability influence the richness (i.e. the number of species bearing each function) and functional diversity of different guilds. To do so, we investigate species richness and functional diversity in diet, feeding behaviour and location, and activity and body mass over Europe. By investigating whether the functional diversity in feeding behaviour and location within each diet type (and similarly for the other trait types) responds to global change, we identify the functions that will likely increase or decrease in frequency and diversity. Finally, we test for spatial structure in expected change of functional diversity. To this end, we map current and future functional diversity of bird assemblages, and we investigate spatial changes in regional functional diversity across Europe. Under the assumptions that bird species will track their suitable climate and land cover, we showed that species bearing unique trait combinations were not more sensitive than other species, and that the trait diversity of some guilds was projected to change drastically (i.e. insectivores) while other guilds should not be strongly affected. Overall, the spatial distribution of trait diversity should change across Europe, leading to functional homogenization of its avifauna.
Results
Species sensitivity to climate and land cover changes
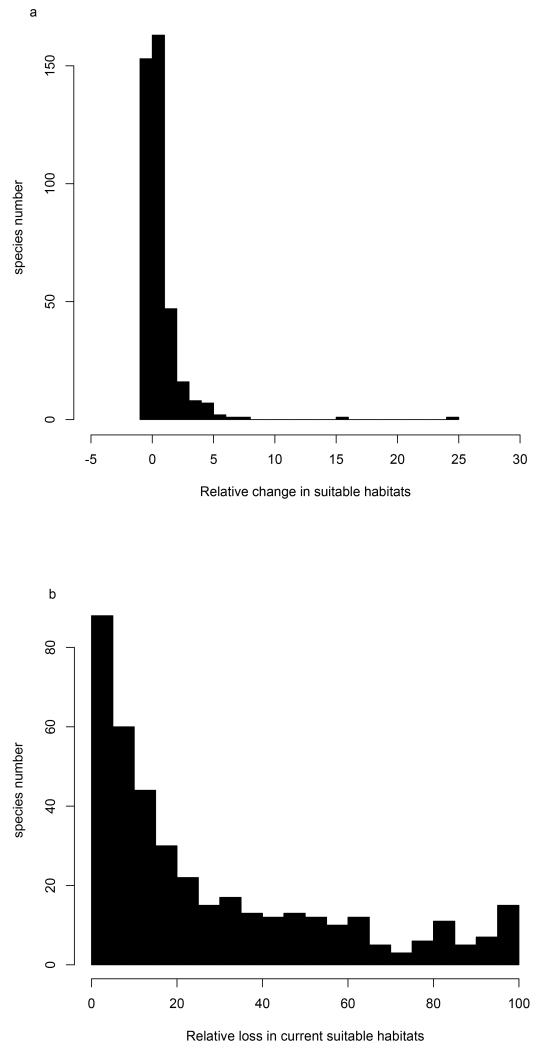
Species sensitivity to both climate and land use change are estimated as the change in the amount of suitable habitat assuming that all species fully disperse to newly suitable habitats and track their shifting niche without any response lag. Most species are predicted to shift their range North- and up-ward11, with a moderate increase in the amount of suitable habitat for most species under the A1B scenario (Fig. 1A, Supplementary Fig. 1 for the other regional climate and land cover scenarios). This implies that although several species are predicted to lose a substantial part of their current suitable habitat (Fig. 1B, Supplementary Fig. 1B), the majority is predicted to find larger extents of suitable habitat elsewhere in Europe under future conditions (Fig. 1A, Supplementary Fig. 1A).
Figure 1.
Distribution of changes in suitable habitats and loss in currently suitable habitats. Histograms representing the projected relative change in suitable habitats (a) and loss in currently suitable habitats (b) (in percentage) under the A1b emission scenarios by 2080, using the RCA30 regional climate model driven by the ECHAM5 global circulation model and ensembles of five species distribution models. The Y-axis represents the number of species for each class of projected change in suitable habitats. In (a), most of species are projected to experience between −2% and +5% of change in suitable habitats (with negative values standing for a loss in suitable habitat while positive values are a gain).
Species sensitivity distribution along the functional tree
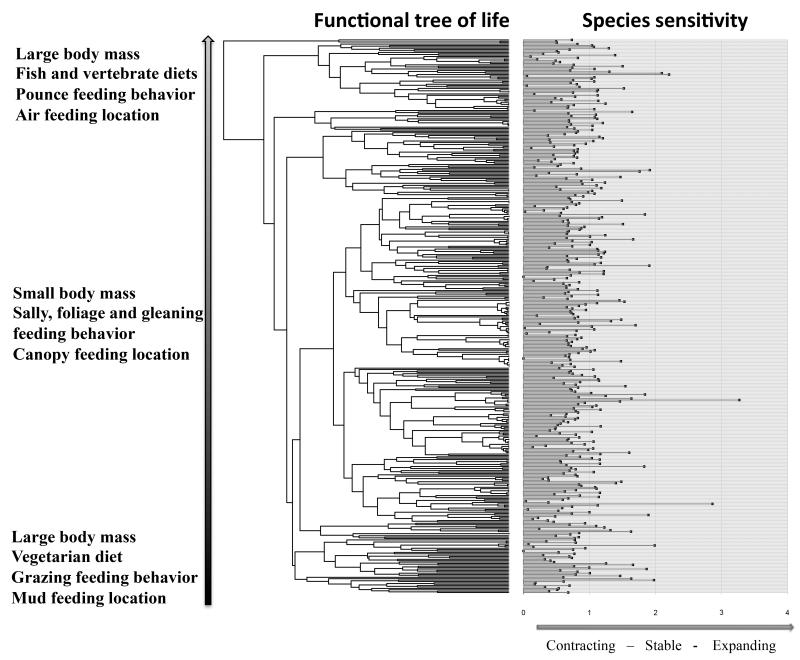
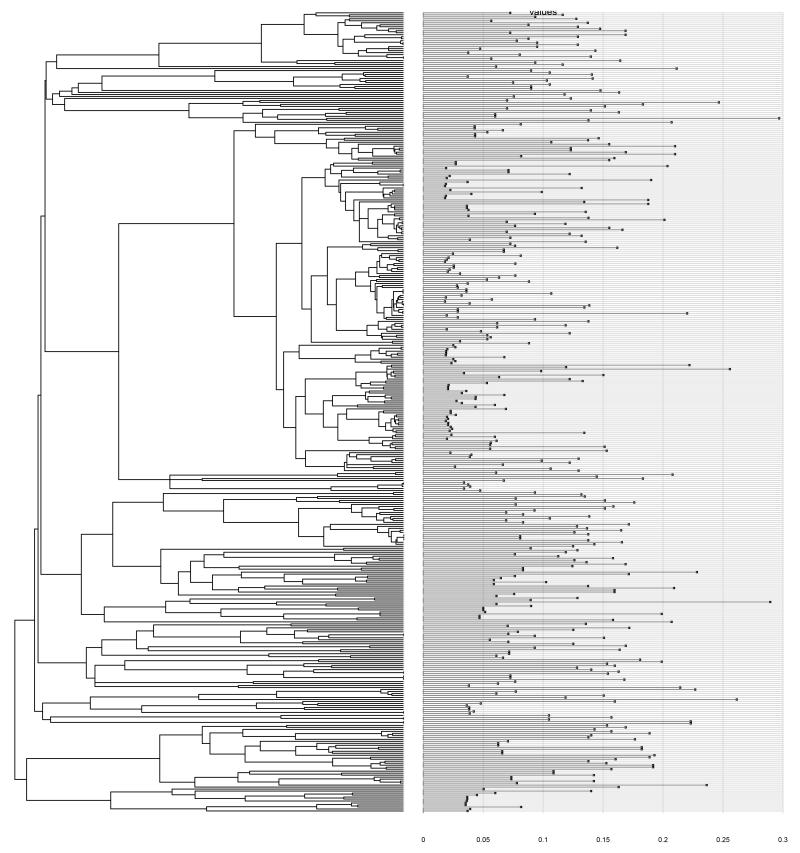
Among European bird species, we find only a weak, non-significant relationship between relative changes in the size of suitable habitat area following climate and land cover change and the position of species on the functional tree (Fig. 2, Supplementary Fig. 2 and Supplementary Table 1). This demonstrates that no group of functionally similar species is predicted particularly sensitive or insensitive to global change. This is surprising since large body mass and other life history traits usually predispose species to increased extinction risks26. Importantly, functionally unique species are unlikely more sensitive to environmental change than are functionally less unique species (Supplementary Table 2). The functional uniqueness of species is therefore not clustered on the phylogenetic tree of the European avifauna (Fig. 3).
Figure 2.
Link between the European functional tree of bird life and species sensitivity to climate change. Species sensitivity measured as change in suitable habitat and mapped onto the functional tree of the avifauna for one emission scenario (A1B) by 2080, using the RCA30 regional climate model driven by the ECHAM5 global circulation model and ensembles of five species distribution models. Species sensitivity was log transformed (log(CHS-1-min(CHS)) for this analysis.
Figure 3.
Functional uniqueness of the European avifauna mapped onto the phylogenetic tree 63. There was no significant phylogenetic signal of functional uniqueness (Pagel’s lambda likelihood ratio test p>0.0561). Functionally unique species were not more closely related to each other than if sampled randomly along the phylogeny.
Change in richness and diversity across functional groups
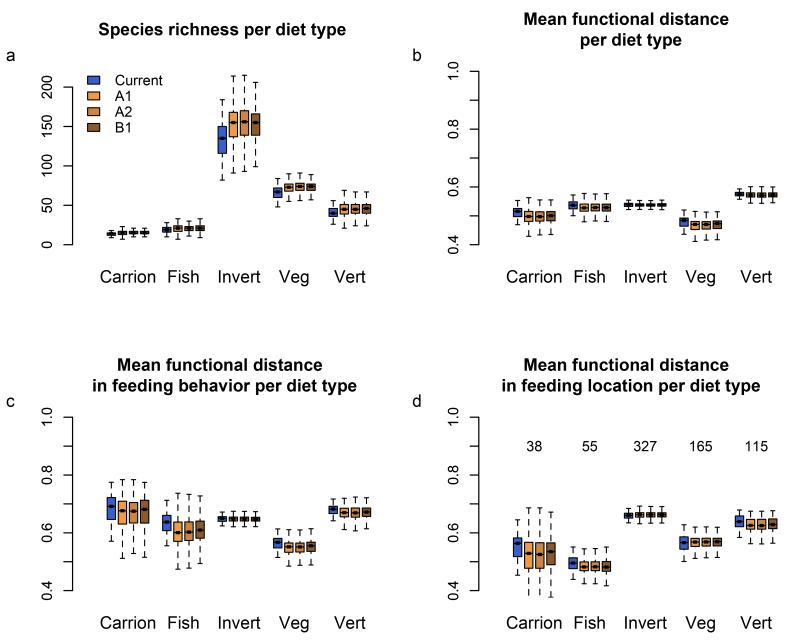
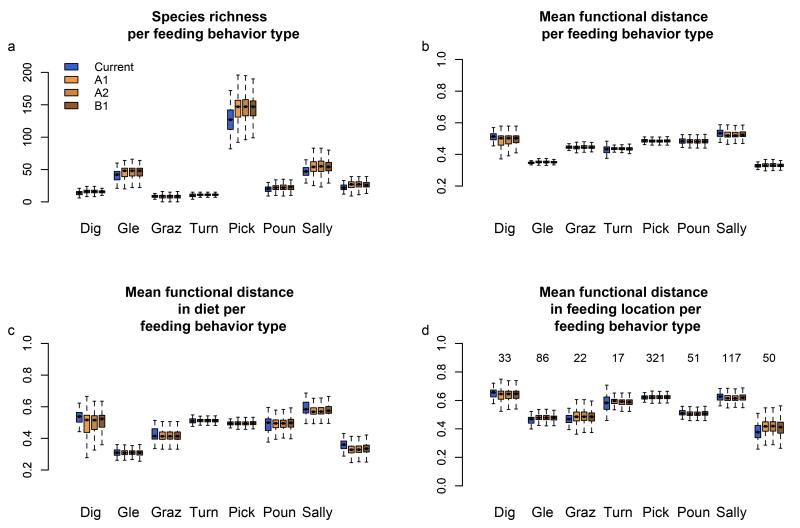
Interestingly, the projected species richness and functional diversity within each of the five groups of analysed traits (diet, feeding behaviour, feeding location, feeding activity, and body mass) show diverging patterns in response to environmental changes (Fig. 4 for diet, Fig. 5 for feeding behaviour and Supplementary Figs. 3, 4 and 5 for the other traits). Whereas mean and variance in body mass per pixel did not significantly change (Supplementary Fig. 5), there was a noticeable increase in the mean species richness of invertebrate diet and picking and pecking feeding behaviour with environmental change. Interestingly, this increase of species richness for these two specific diet and feeding behaviour groups is not followed by an increase in functional diversity (as measured by MFD), whether or not we consider all traits or single traits. In other words, the increase in species richness for the invertebrate diet will not result in a higher diversity in feeding behaviour or feeding locations. This is because all of these behaviours are already represented within each pixel. In summary, our results reveal an increase in redundancy for invertebrate diet and picking and pecking feeding behaviour. On the contrary, other diet groups are projected to experience an increase in species richness per pixel, while their functional diversity is projected to decline at the same time (Fig. 4) like, for instance, bird assemblages with a vertebrate diet. The diversity of feeding behaviours within the vertebrate diet group is projected to slightly decrease, resulting in a decrease of complementarity. In contrast, the fish diet group is projected to experience decreased functional diversity in feeding behaviours and locations, without an associated change in species richness.
Figure 4.
Species richness and functional diversity per diet type across Europe under current and three future climate and land cover scenarios. Each bar of the boxplot (sample size = 402 species) represents the median, first and third quartiles (defining the filled box) and minimum and maximum values (error bars excluding outliers) of the distributions of: species richness (a), MFD (mean pair-wise functional distance) considering all remaining traits except diet (b), MFD considering feeding behaviour only (c), and MFD considering feeding location only (d) mapped over Europe. The Y-axis represents the number of species (a) and the MFD values per functional group (b-c-d). Colour code is indicated in panel a. Species number per feeding behaviour is indicated in panel d. Only projections for climatic scenarios by 2080 and modelled under the RCA30 regional climate model are represented. The influence of regional climate models is represented in Supplementary Fig. 8.
Figure 5.
Species richness and functional diversity per feeding behaviour type across Europe under current and three future climate and land cover scenarios. Each bar of the boxplot (sample size = 402 species) represents the median, first and third quartiles (defining the filled box) and minimum and maximum values (error bars excluding outliers) of the distributions of: species richness (a), MFD (mean pair-wise functional distance) considering all remaining traits except feeding behaviour (b), MFD considering diet only (c), and MFD considering feeding location only (d) mapped over Europe. Y-axis represents the number of species (a) and the MFD values per functional group (b-c-d). Colour code is indicated in panel a. Species number per feeding behaviour is indicated in panel d. Only projections for climatic scenarios by 2080 and modelled under the RCA30 regional climate model are represented. Abbreviations for feeding behaviour type are: dig=digging, Gle=foliage gleaning, Graz=grazing, Turn=overturning, Pick= picking/pecking/stabbing, Poun=pouncing, Sally=sally. The influence of regional climate models is represented in Supplementary Fig. 8
Current and future trait diversity distribution
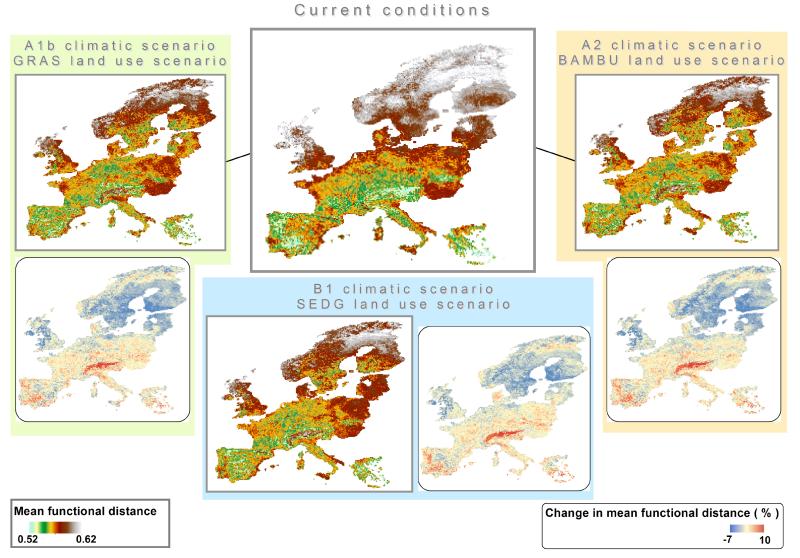
The spatial distribution of the overall functional diversity (calculated as MFD with all traits included) was calculated on a pixel basis among species that were projected to be present at each time period. Our analyses reveal that the projected functional diversity of the avifauna is not homogenously structured across Europe under current conditions, with northern regions and Atlantic coasts having the largest functional diversity and the European Alps and centre of Iberian Peninsula the lowest. However, despite these projections, European biogeographic regions are not equally affected (Fig. 6). Under current conditions, northern Europe and the northern UK currently exhibit markedly higher bird functional diversity compared to central Europe (e.g. southern Germany), the center of the Iberian Peninsula and the outer Alps (Fig. 6). Under projected global change, however, the marked difference between Northern and central Europe tends to be reduced. In particular, mountainous regions of central and southern Europe are projected to experience marked increase in functional diversity. For southern Scandinavia (i.e. nemoral and boreal regions) we predict reduction in functional diversity in many parts. In other words, the expected upward shift of suitable habitats for European birds in central European mountains may lead to a relative increase in functional diversity (assemblages being functionally less redundant). In contrast, for northern latitudes we predict assemblages to become functionally more redundant. The simulated differences between the various climate and land cover scenarios are relatively small and do not greatly alter spatial patterns (Fig. 6). In general, under the A1b climatic scenario and the associated GRAS land use scenario, the projected changes are the most marked, with stronger relative increase in functional diversity in the Alps and centre of the Iberian Peninsula, and stronger relative decrease in Northern UK and southern Scandinavia than under the A2 and B1 scenarios (Fig. 6).
Figure 6.
Mean pair-wise functional distance and its projected changes across Europe under current and future conditions. Large panels represent the per-pixel functional diversity of European avifauna. Small panels show the relative change in functional diversity between future and current conditions.
Discussion
The analysis of joint climate and land cover change impact on the functional diversity of an entire species group over large spatial scales is challenging. Our study addresses these challenges and presents a unique large-scale assessment of the potential impacts of combined climate and land cover changes on the functional diversity and richness of European avifaunal assemblages. Our study addresses important drawbacks of most existing global change risk assessments. In methodological terms, our study is one of the first to model the response of species to both regional climate and land use changes. For instance, Thuiller et al.27 quantified the influence of climate change on the phylogenetic diversity of European biota, but only focused on climate change as simulated from global (not regional) circulation models and ignored potential additional effects of projected land cover change. As suggested by Barbet-Massin28, we estimate the climatic and land cover requirements of species for the whole Western Palearctic region including Northern Africa. This allows us to account for species that may immigrate to Europe from North Africa, and ensure that the ecological requirements of the modelled species were fully captured. These estimates are consistent with recent analyses on the same group of species28 and slightly less alarming than previous studies29. The divergence from results of Huntley and colleagues29 likely originates from inclusion of the southern and eastern range limits of the modelled European bird species in North Africa and the Middle East28. In addition, we use the latest release of regional climate models and also include land cover variables that certainly buffer the direct effects of climate change. Finally, we have employed ensemble-forecasting methodologies by combining highly predictive species distribution models (Supplementary Fig. 6) to generate robust projections and, thus, use four different regional climate models and three socio-economic scenarios in order to incorporate into our projections all recognized sources of uncertainty.
In summary, we show that although the overall functional avian diversity of Europe is expected to only weakly change under projected climate and land cover change, some regions might experience increased functional complementarity (e.g. the European Alps), or simply an increase of species richness per guild (e.g. Boreal and Nemoral regions). Overall, this reshuffling should lead to a functional homogenisation of Europe, with most combinations of traits occurring being available everywhere in the landscape. This result complements the current opinion that the global avifauna is experiencing functional homogenization due to loss of specialist and proliferation of generalist species9. In our case, the causal factors are slightly different as this homogenization is due to a spatial re-structuring of assemblages and, notably, the arrival of species with new combinations of traits in specific regions (i.e. artic and alpine) increasing their functional complementarity. Thus, assemblages with projected increases in functional diversity may provide enhanced ecosystem functioning as a result of more efficient resource use, a beneficial effect that is projected to occur primarily in mountain areas. In any case, we show that species richness in a given guild is not predicted to dramatically drop meaning that no key functional groups (i.e. top predator) are predicted to go locally extinct, which could have had importance consequences on trophic cascade.
Interestingly, our results demonstrating species with unique combinations of traits are not disproportionally sensitive to climate and land cover change mirror a recent analysis carried out for 32 fish species in France30. This study evaluates the potential impact of climate change on fish assemblages, and reports that those species at high risk of local extinction are not necessarily those bearing the most unique combination of traits. Our results for European birds show the same trend. Having used effect traits instead of response traits might explain this pattern, as there is no a priori reason to believe that particular combinations of effect traits should negatively influence the response of species to environmental change.
The projected changes we present may lead to an increase in richness of species with invertebrate diet and pick and peck feeding behaviour, which, in turn, may impact human well-being through enhancement of natural pest control31. Indeed an increase in richness of species with invertebrate diets would likely benefit pest control and associated ecosystem services, although the regions that need it most (southern European countries with economies that highly dependent on agricultural yields) are projected to experience reductions in these services32. However, our results need to be treated with caution as the overall functional diversity within the invertebrate diet group and, more specifically, the diversity of feeding behaviours and locations are not projected to change. In other words, change in the richness of species with an invertebrate diet will most likely result in an increase in predation but not in the variety of predation behaviours and locations. More importantly, some diet groups (e.g. vertebrate diet) are likely to experience an increase in mean species richness across Europe, together with a decrease in diversity of feeding behaviour and location. Other groups, such as fish-eating diet, may experience a decrease in functional diversity that is decoupled from changes in species richness. The outcome of such projected changes on complementarity require additional analyses in order to deduce regional consequences on ecosystem services. Indeed, the link between traits, ecosystem functioning and ecosystem services is far from trivial14 and is influenced by quantity of other factors not explicitly modelled here, such as community assembly rules and land use practices. Additionally, our modelling framework does not explicitly account for inter-specific competition, which could impede the increase of species richness in some groups. Projected change in species richness are thus likely to be the maximum change when competition within a guild does not influence the pure effects of climate and land use change. However, this is also important to note that at the resolution of our study (10 arc-minutes, roughly 19 km in Europe), the outcome of competitive interactions might be moderate as the spatial heterogeneity and the area of a pixel might buffer competitive exclusion within a guild.
Our study thus provides clear evidence that the repercussions of projected climate and land use change on functional diversity of European avifauna assemblages is moderate, despite the likely negative impacts of these changes on individual species ranges11. One major beneficial effect of environmental changes relates to the projected increase in species with invertebrate diets, which could ultimately influence pest control, but which could also negatively influence pollination services. These detrimental effects relate to a decrease in functional diversity in Northern Scandinavia that might ultimately reflect reduced ecosystem functioning in an arctic region. However, relatively small changes in functional diversity may be paralleled by high regional turnover of individual species that results in substantial changes in trophic relationships that accompany altered species assemblages4.
METHODS
Species distribution data
Presence-absence data for all European species were obtained from the EBCC atlas of European breeding birds33, that we further completed for Northern Africa and Eastern Europe using geo-referencing and digitizing breeding bird distribution maps from the handbooks of the birds of the Western Palaearctic34 at a 0.5° resolution. We did not consider seabirds in our analysis as climate and land cover variables may not be the most relevant drivers of the restricted terrestrial distribution of their breeding sites. Moreover, our spatial analysis has focused on projected changes in Europe. Therefore, we considered only species that have their current breeding ranges at least partly included in Europe and we removed species with less 20 occurrences for statistical modelling reasons. From the total list of European breeding and resident bird species, we finally retained 402 species. For all modelled species, we considered their whole Western Palaearctic range (including North Africa and the Middle East) in order to model the full extent of their environmental niche28.
Environmental data
Current climate was represented by five bioclimatic variables from the Worldclim database35 at 0.5° resolution for calibrating the models and 10’ resolution for projecting them. These variables were: Temperature seasonality (intra-annual standard deviation * 100), maximum temperature of the warmest month, minimum temperature of the coldest month, precipitation of the wettest month and precipitation of the driest month (Supplementary Table 3).
Future climate by 2080 (2051-2080) was represented by a set of regional climate model (RCM) runs originating from the ENSEMBLES EU project, which has physically downscaled global circulation model (GCM) data generated for the 4th assessment report of the IPCC36. We used three available SRES scenarios37 for these models, namely A1b, A2 and B1. RCMs downscale the very coarse resolution climate model output of CGMs (usually 1 – 2 ° Lat/Lon per grid cell) to a much finer spatial resolution (usually 10 – 30 ‘ Lat/Lon) on a physical process basis. To this end, an RCM is fed at the study area boundaries by the global output of GCMs in order to provide boundary conditions and global weather input for the downscaling. We have used 3 different RCMs, namely HadRM3, RCA3 and RACMO238, 39, 40, 41, fed by three different GCMs (HadCM3, ECHAM5, and CCSM3) and resulting in 4 RCM/GCM combinations (Supplementary Table 4). All RCM scenarios were interpolated to the same 10’ spatial resolution for 30-year monthly mean values of temperature and precipitation. Based on these monthly values, our five bioclimatic variables of the Worldclim database were calculated for future time steps.
Current land cover for the whole Palearctic was represented by GLOBCOVER 2009 (https://earth.esa.int/web/guest/pi-community) at 300m resolution. We up-scaled the data to the resolution of the species distributions (0.5°) and 10’ resolution for projection under current and future conditions by calculating the area fraction of each land cover type within each pixel. We used the level 1 classification (i.e. built-up areas, arable lands, permanent crops, grasslands, forests and others) that is consistent with the EU CORINE classification on which the land cover scenarios were based.
Bird species distributions are also influenced by the structure of the vegetation. Despite the fact that it is difficult to accurately represent the structure of the vegetation mosaic at 0.5° and 10’ resolutions, we estimated the Simpson diversity index using the fraction of each land cover class as a weighting scheme.
Future land cover data was taken from the EU funded ALARM and ECOCHANGE projects42, 43, 44. The ALARM land cover change scenarios provide annual fractions of land use for 8 main land use/cover categories per 10’ resolution grid cell (i.e. % built-up, % cropland, % permanent crops, % grassland, % forest, % biofuels (liquid, non-woody or woody), % land in succession) and for the period 2006-2080. We then retained the period 2051-2080 to be consistent with the climatic data. The countries covered are those of the EU25 plus Switzerland and Norway. We removed % of biofuel and % land in succession that were not available for calibrating the models (period 1961-1990).
We retained three storylines that are consistent with the climate change scenarios: 1) GRAS - Growth Applied Strategy, where deregulation, free trade, growth and globalisation will be policy objectives actively pursued by governments. Environmental policies will focus on damage repair and limited prevention based on cost benefit-calculations. There is no emphasis on biodiversity. This scenario is considered equivalent to A1b; 2) BAMBU – Business-As-Might-Be-Usual, where policy decisions already made in the EU are implemented and enforced. At the national level, deregulation and privatisation continue except in “strategic areas”. Internationally, there is free trade. Environmental policy is perceived as another technological challenge. This scenario is considered equivalent to A2; and 3) SEDG – Sustainable European Development Goal, which enhances the sustainability of societal development by integrated social, environmental and economic policy. The scenario aims for a competitive economy and a healthy environment, gender equity and international cooperation. It represents a normative scenario with stabilisation of GHG emissions. This scenario is considered equivalent to B1.
Given the land cover scenarios were only available for the EU25 plus Switzerland and Norway, species projections into the future were only carried out over those 27 countries.
In summary, models were calibrated and projected in time using 5 bioclimatic variables, 5 land cover type variables, and one land cover diversity variable under four regional climate models and three emission scenarios.
Functional trait information
Trait information for the 402 modelled birds were extracted from the Handbook of the Birds of the Western Palaearctic34. Missing species and data were gathered from species publications and Internet websites treating avifauna. The traits were: body mass, diet (invertebrates, vertebrates, vegetal, fish, carrion), feeding behaviour (pursuit (air and/or aquatic), sally, foliage-gleaning, pouncing, grazing, picking/pecking/stabbing, digging, overturning, probing), feeding location (water, mud, ground, canopy and air) and activity (nocturnal, crepuscular and diurnal). For diet, feeding behaviour, and feeding location and activity, each sub-category was expressed as a binary variable (0 or 1) to make sure a species could be assigned to several strategies. In our study, we did not consider traits that can only be measured with reference to the surrounding environment, such as nesting habitats. We did so because of the circularity in the methodology as changes in land cover (defining the surrounding environment) are implicitly accounted for in our modelling framework. We preferred to constrain our analyses to a specific set of traits that were relevant to understanding the implications of environmental change on community assembly 12.
Species distribution modelling
Species distribution models were calibrated over the whole western Palearctic biogeographic zone at a resolution of 0.5°, and then projected into the future over EU25 plus Switzerland and Norway at 10’ resolution. By this, we considered the whole Western Palaearctic range (including North Africa and the Middle East) to calibrate models for the full extent of the niches of species28 and to allow species that currently occur only around the margins of Europe to potentially migrate into the EU25 as climate becomes suitable.
An ensemble of forecasts of species distributions models (SDM45, 46) was obtained for each of the 402 species. The ensemble included projections with Generalized Additive Models, Boosting Regression Trees, Classification Tree Analysis, Multiple Adaptive Regression Splines and Random Forest. Models were calibrated for the baseline period using 65% random sample of the initial data and evaluated against the remaining 35% data, using the True Skill Statistic (TSS47). This analysis was repeated 5 times, thus providing a 5-fold internal cross validation of the models (biomod package48 in R49). The quality of the models was very high to excellent with an average AUC and TSS of 0.97 and 0.87 respectively (Supplementary Fig. 6), while for the least well-modelled species, the ensemble model quality reached an AUC of 0.93 and a TSS of 0.7, which are traditionally considered as good predictive performance47.
For each species, we projected the probability of occurrence within each 10’ resolution pixel under both current and future conditions as a weighted sum of occurrence-probability projections made by the 5 modelling techniques run over 5 sub-samples. This modest downscaling at a scale of 1:3, from models calibrated at 0.5° to 10’ projections has been shown well suitable at such spatial extent and resolution50. The weighting scheme for building ensembles was proportional to the TSS statistics for each modelling technique and cross-validation (i.e. the techniques that delivered the most accurate models had the highest weights). Probabilities of occurrence were further transformed into binary maps using the value that maximized the TSS score as a threshold.
Dispersal ability
Not all species are expected to disperse at the same rate and distance. However, the information about natal dispersal was not known for all 402 species. To estimate what could be the uncertainty associated to the non-inclusion of natal dispersal, we gathered the information on natal dispersal for 74 species from Paradis et al. (1998)51 and Barbet-Massin et al. (2012)11. For these 74 species, we then estimated the projected change in habitat suitability accounting for natal dispersal, and further compared them with the ones estimated assuming no dispersal constraints (CHS). The results for these 74 species confirmed that the non-inclusion of natal dispersal into the modelling procedure for the 402 species should not change the outcome of the analyses (Supplementary Fig. 7). For the time considered (100 years), most species should be able to reach their suitable habitats in terms of climate and land cover change.
Species sensitivity to climate and land use change
Each ensemble of species projections for current and future conditions were converted into a metric of species sensitivity27. Change in habitat suitability (CHS) measures the relative change in suitable climate and land use. It corresponds to the total suitable area projected into the future under the assumption of unlimited dispersal minus the total suitable area projected onto the current conditions, with the resulting quantity divided by the total suitable area projected onto the current conditions. There was no relationship between CHS and the predictive performance of the models (Supplementary Table 5).
The metric was averaged across Species × Model × Scenario × RCM combinations.
Statistical analyses
All analyses have been carried in the R environment49 (specific functions within specific package are indicated in brackets).
Functional distance and the functional tree of bird life
We first log-transformed and normalized body mass prior to all analyses. We used a mixed-variables coefficient of distance that generalizes Gower’s coefficient of distance to allow for the treatment of various types of variables when calculating distances52. Euclidean distance was used for body mass, while the Sorensen distance53 (S7 coefficient of Gower and Legendre54, function dist.ktab in ade4) was used for binary data types, e.g., for each sub-group of diet and feeding behaviour trait. Then, we used hierarchical clustering to build the most reliable dendrogram of all species in functional-trait space, employing an average agglomeration method (UPGMA, function hclust)55. The functional dendrogram expressed 78% of the original distances between species (Mantel correlation between the original distance matrix and the distance matrix from the dendrogram equaled 0.78, p-value < 0.001 with 9999 randomizations, function Mantel in vegan56).
Functional uniqueness and link with species’ sensitivity
We adapted the Evolutionary Distinctiveness index57, which measures the relative contributions of species to phylogenetic diversity, for use in a functional context. First, for each branch of the functional dendrogram, we estimated a value equal to its length divided by the number of species subtending the branch. The functional uniqueness of a species is simply the sum of these values for all branches from which the species is descending, to the root of the functional dendrogram (function originality in ade458). We calculated the strength of the signal between the functional tree and the measure of species sensitivity estimated for the range of climate and land use projections. We used the robust measure proposed by Abouheif to test for serial independence to detect a functional signal in species sensitivity59 (function abouheif.moran in ade4). We tested the strength of the phylogenetic signal in functional uniqueness using Pagel’s lambda statistic and its associated likelihood ratio test60, 61. To test the link between functional uniqueness and species sensitivity to climate and land use change, we calculated Pearson’s correlation between the functional uniqueness of species and their expected sensitivity to the range of climate and land use projections.
Species richness per group and functional diversity
We estimated the species richness for each category of each functional trait per pixel. We estimated the mean assemblage body mass per pixel (instead of species richness) given that body mass is a continuous variable. To calculate functional diversity, we used the mean pair-wise functional distance (MFD) between all species present in a pixel. This index is a classic metric in community ecology5, represents an unbiased estimate of the variance of the trait considered, and is not correlated with species richness (function mpd in picante62). This was calculated for all traits together (e.g. Fig. 4) and also within functional groups. For the latter, we re-calculated the functional distance matrix without the trait considered (e.g. diet) and calculated the MFD for all remaining traits (Fig. 3B) and for single trait (e.g. feeding MFD per diet type, Fig. 3C) within pixel. We analysed the variability to regional climate models for MFD for diet and showed that the results were little sensitive to this variability (Supplementary Fig. 8).
For the spatial distribution of MFD, we simply mapped the MFD onto the geographic space. Relative change in MFD between current and future conditions was estimated as equation 1:
| (1) |
Supplementary Material
Acknowledgments
The research leading to these results had received funding from the European Research Council under the European Community’s Seven Framework Programme FP7/2007-2013 Grant Agreement no. 281422 (TEEMBIO). WT and JR also acknowledged the European Commission funded project VOLANTE (FP7-ENV-2010-01 No. 265104). LZ was funded through the ANR-BiodivERsA project CONNECT (ANR-11-EBID-002), as part of the ERA-Net BiodivERsA 2010 call. The computations presented in this paper were performed using the CIMENT infrastructure (https://ciment.ujf-grenoble.fr), which is supported by the Rhône-Alpes region (GRANT CPER07_13 CIRA: http://www.ci-ra.org).
Footnotes
Conflict of interest statement
The authors declare no competing financial interests.
References
- 1.Purvis A, Agapow P, Gittleman JL, Mace GM. Nonrandom extinction and loss of evolutionary history. Science. 2000;288:328–330. doi: 10.1126/science.288.5464.328. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Brigham CA, Hoeksema JD, Lyons KG, Mills MH, van Mantgem PJ. Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia. 2000;122:297–305. doi: 10.1007/s004420050035. [DOI] [PubMed] [Google Scholar]
- 3.Díaz S, Fargione J, Chapin FS, III, Tilman D. Biodiversity loss threatens human well-being. Plos Biol. 2006;4(8):e277. doi: 10.1371/journal.pbio.0040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15(4):365–377. doi: 10.1111/j.1461-0248.2011.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annual Review of Ecology and Systematics. 2002;33:475–505. [Google Scholar]
- 6.Petchey OL, Gaston KJ. Functional diversity (FD), species richness and community composition. Ecol Lett. 2002;5:402–411. [Google Scholar]
- 7.Flynn DFB, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, et al. Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett. 2009;12:22–33. doi: 10.1111/j.1461-0248.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 8.Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality. Nature. 2007;448(7150):188–U186. doi: 10.1038/nature05947. [DOI] [PubMed] [Google Scholar]
- 9.Clavel J, Julliard R, Devictor V. Worldwide decline of specialist species: toward a global functional homogenization? Front Ecol Environ. 2010;9(4):222–228. [Google Scholar]
- 10.Dullinger S, Gattringer A, Thuiller W, Moser D, Zimmermann NE, Guisan A, et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat Clim Change. 2012;2:619–622. [Google Scholar]
- 11.Barbet-Massin M, Thuiller W, Jiguet F. The fate of European breeding birds under climate, land-use and dispersal scenarios. Glob Change Biol. 2012;18:881–890. [Google Scholar]
- 12.Wenny DG, DeVault TL, Johnson MD, Kelly D, Sekercioglu CH, Tomback DF, et al. The Need to Quantify Ecosystem Services Provided by Birds. Auk. 2011;128(1):1–14. [Google Scholar]
- 13.Whelan CJ, Wenny DG, Marquis RJ. Ecosystem services provided by birds. Year in Ecology and Conservation Biology. 2008;2008;1134:25–60. doi: 10.1196/annals.1439.003. [DOI] [PubMed] [Google Scholar]
- 14.Luck GW, Lavorel S, McIntyre S, Lumb K. Improving the application of vertebrate trait-based frameworks to the study of ecosystem services. Journal of Animal Ecology. 2012;81(5):1065–1076. doi: 10.1111/j.1365-2656.2012.01974.x. [DOI] [PubMed] [Google Scholar]
- 15.Sekercioglu CH. Increasing awareness of avian ecological function. Trends Ecol Evol. 2006;21(8):464–471. doi: 10.1016/j.tree.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Sekercioglu CH, Daily GC, Ehrlich PR. Ecosystem consequences of bird declines. Proc Natl Acad Sci USA. 2004;101(52):18042–18047. doi: 10.1073/pnas.0408049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavergne S, Mouquet N, Thuiller W, Ronce O. Biodiversity and climate change: Integrating evolutionary and ecological responses of species and communities. Annual Review of Ecology, Evolution and Systematics. 2010;41:321–350. [Google Scholar]
- 18.Van der Putten WH, Macel M, Visser ME. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos T R Soc Lon B. 2010;365(1549):2025–2034. doi: 10.1098/rstb.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekercioglu CH. Functional Extinctions of Bird Pollinators Cause Plant Declines. Science. 2011;331(6020):1019–1020. doi: 10.1126/science.1202389. [DOI] [PubMed] [Google Scholar]
- 20.Jiguet F, Gadot AS, Julliard R, Newson SE, Couvet D. Climate envelope, life history traits and the resilience of birds facing global change. Glob Change Biol. 2007;13(8):1672–1684. [Google Scholar]
- 21.Loreau M, Naeem S, Inchausti P, Grime JP, Hector A, Hooper DU, et al. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 22.Naeem S. Species redundancy and ecosystem reliability. Conserv Biol. 1998;12(1):39–45. [Google Scholar]
- 23.Triplett S, Luck GW, Spooner P. The importance of managing the costs and benefits of bird activity for agricultural sustainability. International Journal of Agricultural Sustainability. 2012;10(4):268–288. [Google Scholar]
- 24.Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, et al. Defining and measuring ecological specialization. Journal of Applied Ecology. 2010;47:15–25. [Google Scholar]
- 25.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16(5):545–556. [Google Scholar]
- 26.Bennett PM, Owens IPF. Variation in extinction risk among birds: chance or evolutionary predisposition? Proc R Soc Lon B. 1997;264(1380):401–408. [Google Scholar]
- 27.Thuiller W, Lavergne S, Roquet C, Boulangeat I, Araujo MB. Consequences of climate change on the Tree of Life in Europe. Nature. 2011;470:531–534. doi: 10.1038/nature09705. [DOI] [PubMed] [Google Scholar]
- 28.Barbet-Massin M, Thuiller W, Jiguet F. How much do we overestimate local extinction rates when restricting the range of occurrence data in climate suitability models? Ecography. 2010;33(5):878–886. [Google Scholar]
- 29.Huntley B, Green RE, Collingham YC, Willis SG. A climatic atlas of European breeding birds. Lynx Edicions. 2007 doi: 10.1371/journal.pone.0001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buisson L, Grenouillet G, Villéger S, Canal J, Laffaille P. Toward a loss of functional diversity in stream fish assemblages under climate change. Glob Change Biol. 2013;19(2):387–400. doi: 10.1111/gcb.12056. [DOI] [PubMed] [Google Scholar]
- 31.Wilby A, Thomas MB. Natural enemy diversity and pest control: patterns of pest emergence with agricultural intensification. Ecol Lett. 2002;5(3):353–360. [Google Scholar]
- 32.Civantos E, Thuiller W, Maiorano L, Guisan A, Araujo MB. Potential impacts of climate change on ecosystem services in Europe: the case of pest control by vertebrates. BioScience. 2012;62(7):658–666. [Google Scholar]
- 33.Hagemeijer WJM, Blair MJ. The EBCC atlas of European breeding birds, their distribution and abundance. Poyser; London: 1997. [Google Scholar]
- 34.BWPi . Birds of the Western Palearctic Interactive 2.0. BirdGuides; Oxford, UK: 2006. [Google Scholar]
- 35.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- 36.IPCC . The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK and New York, NY, USA: 2007. [Google Scholar]
- 37.Nakicenovic N, Swart R, editors. Emissions Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- 38.Collins M, Booth BBB, Harris GR, Murphy JM, Sexton DMH, Webb MJ. Towards quantifying uncertainty in transient climate change. Climate Dynamics. 2006;27(2-3):127–147. [Google Scholar]
- 39.Jones CG, Willen U, Ullerstig A, Hansson U. The Rossby Centre Regional Atmospheric Climate Model part 1: Model climatology and performance for the present climate over Europe. Ambio. 2004;33(4-5):199–210. [PubMed] [Google Scholar]
- 40.Jones CG, Wyser K, Ullerstig A, Willen U. The Rossby Centre regional atmospheric climate model part II: Application to the Arctic climate. Ambio. 2004;33(4-5):211–220. [PubMed] [Google Scholar]
- 41.Meijgaard E, van Ulft LH, van de Berg WJ, Bosveld FC, van den Hurk BJJM, Lenderink G, et al. The KNMI regional atmospheric climate model RACMO, version 2.1: KNMI, Postbus 201, 3730 AE. De Bilt; The Netherlands: 2008. [Google Scholar]
- 42.Dendoncker N, Bogaert P, Rounsevell M. A statistical method to downscale aggregated land use data and scenarios. Journal of Land Use Science. 2006;1:63–82. [Google Scholar]
- 43.Rounsevell MDA, Reginster I, Araújo MB, Carter TR, Dendoncker N, Ewert F, et al. A coherent set of future land use change scenarios for Europe. Agriculture, Ecosystems & Environment. 2006;114(1):57–68. [Google Scholar]
- 44.Dendoncker N, Schmit C, Rounsevell M. Exploring spatial data uncertainties in land-use change scenarios. International Journal of Geographical Information Science. 2008;22(9):1013–1030. [Google Scholar]
- 45.Araújo MB, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Marmion M, Hjort J, Thuiller W, Luoto M. Statistical consensus methods for improving predictive geomorphology maps. Computers and Geosciences. 2009;35:615–625. [Google Scholar]
- 47.Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS) Journal of Applied Ecology. 2006;43:1223–1232. [Google Scholar]
- 48.Thuiller W, Lafourcade B, Engler R, Araujo MB. BIOMOD – A platform for ensemble forecasting of species distributions. Ecography. 2009;32:369–373. [Google Scholar]
- 49.R Development Core Team, editor. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 50.Araújo MB, Thuiller W, Williams PH, Reginster I. Downscaling European species atlas distributions to a finer resolution: implications for conservation planning. Global Ecology and Biogeography. 2005;14:17–30. [Google Scholar]
- 51.Paradis E, Baillie S, Sutherland WJ, Gregory RD. Patterns of natal and breeding dispersal in birds. Journal of Animal Ecology. 1998;67:518–536. [Google Scholar]
- 52.Pavoine S, Vallet J, Dufour AB, Gachet S, Daniel H. On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos. 2009;118(3):391–402. [Google Scholar]
- 53.Dray S, Chessel D, Thioulouse J. Co-inertia analysis and the linking of ecological data tables. Ecology. 2003;84(11):3078–3089. [Google Scholar]
- 54.Gower JC, Legendre P. Metric and Euclidean properties of dissimilarity coefficients. Journal of Classification. 1986;3:5–48. [Google Scholar]
- 55.Mouchet M, Guilhaumon F, Villeger S, Mason NWH, Tomasini JA, Mouillot D. Towards a consensus for calculating dendrogram-based functional diversity indices. Oikos. 2008;117(5):794–800. [Google Scholar]
- 56.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. vegan: Community Ecology Package. R package version 2.0-7. 2013 http://CRANR-projectorg/package=vegan
- 57.Isaac NJB, Turvey ST, Collen B, Waterman C, Baillie JEM. Mammals on the EDGE: conservation priorities based on threat and phylogeny. PloS One. 2007;2:e296. doi: 10.1371/journal.pone.0000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chessel D, Dufour A-B, Thioulouse J. The ade4 package-I - one-table methods. R News. 2004;4:5–10. [Google Scholar]
- 59.Abouheif E. A method for testing the assumption of phylogenetic independence in comparative data. Evol Ecol Res. 1999;1:895–909. [Google Scholar]
- 60.Münkemüller T, Lavergne S, Bzeznik B, Dray S, Jombart T, Schiffers K, et al. How to measure and test phylogenetic signal. Methods in Ecology and Evolution. 2012;3(4):743–756. [Google Scholar]
- 61.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 62.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 63.Roquet C, Thuiller W, Lavergne S. Building megaphylogenies for macroecology: taking up the challenge. Ecography. 2013;36(1):13–26. doi: 10.1111/j.1600-0587.2012.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.