Summary
The transforming growth factor β (TGFβ) superfamily growth factors play vital roles during the development, homeostasis, and pathogenesis of multi-cellular organisms. Smad4 serves as an exclusive co-activating smad that elicits most of the transcription responses invoked by the TGFβ superfamily members. We used Cre recombinase driven by the Fsp1/S100A4 promoter to delete the Smad4 gene in fibroblasts. We show that Fsp1/S100A4 is expressed in the elastic and fibrocartilage, and demonstrate that the fsp1-Cre; Smad4 flox/ flox mutants have normal body size, but exhibit a short ear phenotype due to the deletion of the Smad4 gene in the ear chondrocytes. In contrast, TGFβ type II receptor deletion using Fsp1-cre does not lead to this phenotype, supporting the notion that non-TGFβ mediated signaling via Smad4 is essential for proper formation of ear cartilage during development. Smad4 deficiency in Fsp1+ fibroblasts leads to defective chondrocyte maturation and cartilage production, likely due to a deficiency in bone morphogenic protein 5(BMP-5) mediated signaling via Smad4. Our results emphasize the importance of BMP signaling pathways in the maturation and function of certain lineages of chondrocytes and offer an insight into the heterogeneity of the chondrocyte population in the body.
Keywords: Smad4, BMP-5, cartilage, chondrocyte, Fsp1/ S100A4
Introduction
The TGFβ superfamily growth factors regulate a wide spectrum of processes in multicellular organisms including cell growth, motility, proliferation, differentiation and apoptosis [1, 2]. Members of the TGFβ superfamily include the TGFβ family cytokines, the BMP family cytokines, Activin/inhibin, and the GDFs (growth and differentiation factors). TGFβ superfamily members signal through the core Smad mediated transcription mechanism. The binding of the ligands brings the type I and type II receptors together, which then recruit and phosphorylate the receptor-regulated Smads (R-smads, Smad 1, 2, 3, 5, 8). The type I receptors from different families of the TGFβ superfamily have distinct binding affinities to the R-Smads. However, all the R-smads pair with the same coactivator Smad (Co-Smad), Smad4, to form transcriptionally active complexes and induce the expression of their target genes. Although Smad-independent TGFβ signaling has been identified, it is still widely believed that a significant portion of the TGFβ superfamily induced cellular responses are via the Smad-dependent pathway.
The Fsp1/S100A4 protein belongs to the S100 family of calcium-binding proteins (Donato 2001). It has long been known to play prominent roles in multiple diseases such as tissue fibrosis and cancer [3, 4]. Multiple functions have been suggested of the Fsp1/S100A4 protein, the most established of which is the regulation of cell motility and contractility. The expression of Fsp1/S100A4 is generally restricted to the mesenchymal lineages. One prominent cell population that strongly expresses Fsp1/S100A4 is the stromal fibroblast [5].
Chondrocytes are generally round shaped mesenchymal cells located in the matrix cavities in the cartilage. They are specialized cells responsible for cartilage synthesis and maintenance [6, 7]. The major components in cartilage are type II collagen, aggrecan and hyaluronic acid (or HA), which play essential roles in the structural organization of the matrix and confer the tensile strength and osmotic resistance associated with cartilage.
Cartilage is further divided into three types, elastic cartilage, fibrocartilage, and hyaline cartilage, based on their unique composition and physical properties. Although heterogeneity in the chondrocyte population has rarely been reported, chondrocytes in different types of cartilages are either intrinsically different or they receive different signal input so that they are able to synthesize cartilages of distinct composition.
The generation and the differentiation of chondrocytes was studied in most detail in the hyaline cartilage. Multiple growth factors have been implicated, including the TGFβ superfamily members, fibroblast growth factors, and insulin-like growth factor I, in the differentiation of chondrocytes [6]. The behaviors of the chondrocytes have also been studied in vitro using primary chondrocyte culture and chondrogenic cell lines. Furthermore, synovial fibroblasts have also been cultured and identified as a source for chondrocytes [8], leaving open the possibility that fibroblasts in other regions of developing cartilage can also serve as precursor of chondrocytes, such as in the ear.
In this study we show that Fsp1/S100A4 is expressed in the chondrocytes associated with the elastic cartilage and fibrocartilage, likely derived from Fsp1 positive fibroblasts via fibroblast to chondrocyte differentiation. Therefore the Fsp1-cre; Smad4 flox/flox mice generated by crossing Fsp1/S100A4-cre mice with mice harboring Smad4 floxed allele [9] prove to be a good model for studying the role of the signaling mediated by TGFβ superfamily members in the maturation and function of chondrocytes. The absence of Smad4 in the ear chondrocytes results in the accumulation of immature chondrocytes, likely differentiating fibroblastic precursors, in the elastic cartilage of the ear resulting in severe retardation of ear pinna growth. We also demonstrate that this phenotype is a result of an impairment of Smad4 mediated BMP-5 signaling, and that this pathway is essential for proper differentiation of Fsp1/S100A4 positive fibroblast into chondrocytes. The impaired BMP mediated signaling via Smad4 and not TGFβ mediated action, is further supported when TGFβ type II receptor is deleted in the Fsp1 positive fibroblasts and these mice do not exhibit the short ear phenotype. Collectively these results favor the notion that BMP mediated signaling via Smad4 is important for fibroblast to chondrocyte differentiation during cartilage development associated with the outer ear.
Materials and Methods
Animals
Fsp1-cre mice were kindly provided by Dr. E. G. Neilson; TGFβRII flox mice were kindly provided by Dr. H. L. Moses; Rosa26-EYFP mice were kindly provided by B. G. Neel. The Smad4 flox/flox; fsp1-Cre+ line was maintained by crossing Smad4 flox/flox; fsp1-Cre+ mice with Smad4 flox/flox mice. The TGFβRII flox/flox fsp1-cre+ line was maintained by crossing TGFβRII flox/wt fsp1-cre+ mice with TGFβRII flox/flox mice. Mice were maintained at the Beth Israel Deaconess Medical Center animal facility under standard conditions. All animal studies were reviewed and approved by the animal care and use committee of the Beth Israel Deaconess Medical Center.
Mouse measurement and statistics
The lengths and widths of the mouse ear pinnas were measured with a digital caliper. The area of the ear pinna was calculated by the formula S=π*L*W/4 (approximating the pinna as an ellipse). The weights of the mice were measured with a digital balance. The statistics was performed using t-test.
Immunohistochemistry
Antibodies used: Smad4 (Santa Cruz), Fsp1 (kindly provided by Dr. E. G. Neilson). Formalin fixed, paraffin embedded tissue sections were deparaffinized and rehydrated. Antigen retrieval was performed at 98°C for 30 min, in 0.01 M citrate buffer (pH 6.0). The sections were blocked with diluted serum for 1 hr and then incubated over night at 4°C with primary antibody (1:50–1:200 dilution). Tissue peroxidase activity was blocked with hydrogen peroxide 3% for 10 min, before incubating with a biotinylated secondary antibody (1:200) for 30 min. The antigen-antibody complex was revealed with avidin-biotin-peroxidase for 30 min (Vectastain® ABC Kit, Vector) and stained for 1–5 min with diamino-benzidine tetrahydrochloride. The sections were then counterstained with haematoxylin, dehydrated and mounted.
Cartilage staining
For Alcian Blue staining: Sections were deparrafinized and then stained for 30 minutes with Alcian Blue Solution [1% Alcian Blue in 3% glacial acetic acid and 1% HCl (pH 1.0)]. Sections were rinsed, and then counter-stained for 5 minutes in Neutral Red Stain Solution (1% Neutral Red Stain in 0.1% glacial acetic acid).
For Toluidine Blue staining: Sections were deparrafinized and then stained for 2–3 minutes with 0.1% Toluidine blue (Sigma).
Ear fibroblast culture
Mouse ear tissues were cut into small pieces and treated with 400 unit/mL Collagenase IV (Worthington) in DMEM and incubated at 37°C in 5% CO2 for 24 hours. Next day, the medium was replaced with regular DMEM+20% heat-inactivated fetal bovine serum (FBS) and kept at 37°C in 5% CO2 under sterile tissue culture conditions. Cells were passaged when they reached 80% confluency.
BMP-5 stimulation of the ear fibroblasts
Primary ear fibroblasts were seeded at 1 million cells per well of a 6 cm culture plate. Cells were serum starved in 0.1% FBS/DMEM for 24 hours. Then the experimental plates were treated with 100 ng/mL recombinant human BMP-5 (rhBMP-5 R&D) for 48 hours. RNA was isolated with trizol (invitrogen). Reverse transcription of 1µg RNA from each sample was performed with Superscript II reverse transcriptase (invitrogen), followed by PCR (1µL template, 28 cycles). Results were confirmed in at least two pairs of ear fibroblast lines.
Sequences of the PCR primers:
Aggrecan forward: CAGCGAAGCCACCCTGGAGG.
Aggrecan reverse: GTCACCATAGCAACCTTCCC
Col II forward: GGTGGCTTCCATTTCAGCTA
Col II reverse: TACCGGTATGTTTCGTGCAG
Has II forward: TGGTGAGACAGAAGAGTCCC
Has II reverse: TGGACATCTCCTCCAACACC
Smad4 forward: GGCCAGTTCACAATGAGCTTG
Smad4 reverse: CTCGCTCTCTCAATCGCTTC
BMP-5 forward: TGACAGCAGCAGAATTCCGG
BMP-5 reverse: CTGCACAGAGCTGTAAGCCC
GAPDH forward: CTCATGACCACAGTCCATGC
GAPDH reverse: CACATTGGGGGTAGGAACAC
Pellet culture
Sub-confluent monolayer fibroblast cultures were trypsinized and counted. Pellets were formed by centrifugation of 500,000 cells per 15mL conical tube. The pellets were then supplied with serum-free DMEM supplemented with the 10ng/mL TGF-β1 (R&D) and ITS+1 premix (final concentration: 1X, Sigma). The experimental groups were also supplemented with 500 ng/mL rhBMP-5. Each condition is done in duplicate. The pellets were then incubated in the conical tubes with lids loosely fastened at 37°C in 5% CO2 for 14 days. The media was replaced every three to four days.
Pellets were harvested by rinsing in PBS twice, followed by fixation in 10% formalin for 30 minutes. Samples were embedded in paraffin, sectioned and stained with Alcian Blue.
Imaging of YFP signal
Mouse tissues were fixed at 4°C overnight in paraformaldehyde; then transferred into 30% sucrose/PBS for at least 24 hours. After being rinsed in PBS, the tissues were embedded in OCT compound and frozen in liquid nitrogen. 10µm-thin sections were directly mounted and visualized.
Results
Fsp1-Cre; Smad4 flox/flox mice have the short ear phenotype due to a deficiency in the elastic cartilage production
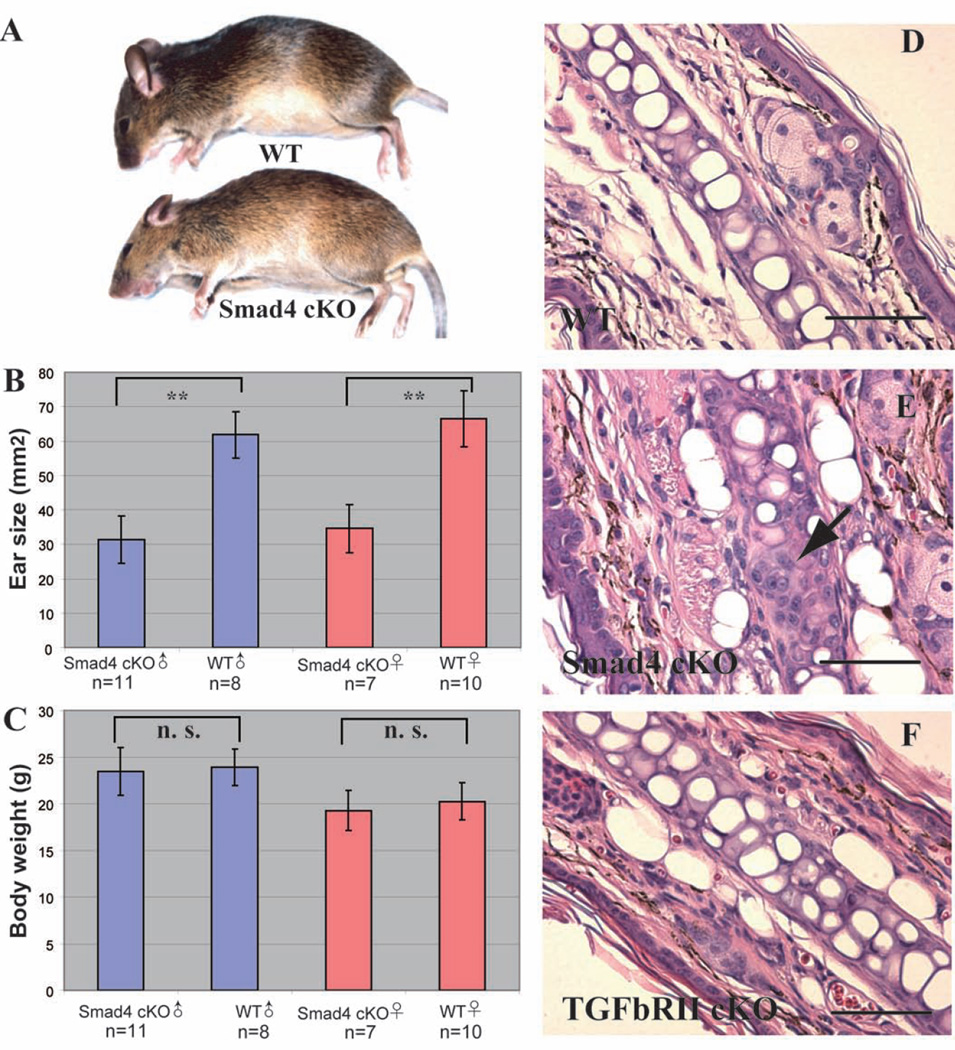
The fsp1-Cre+; Smad4 flox/flox conditional knock-out mice (henceforth referred to as Smad4 cKO) have significantly smaller ear pinnas when compared to their fsp1-cre-; Smad4 flox/flox wild-type littermates (henceforth referred to as WT), apparent since three weeks of age. Eight-week-old Smad4 cKO mice have ear pinnas about half the size of those of their WT litter mates (Figure 1A, 1B), despite the fact that the gross body size and weight of Smad4 cKO mice are not statistically different from those of their WT littermates (Figure 1A, 1C). Interestingly, the Smad4 cKO mice mimic the short ear phenotype observed also in the Bmp-5 null mice, where in a cartilage defect has been suggested as the cause [10, 11].
Figure 1. The Smad4 cKO mutants have a short ear phenotype.
A. The Smad4 cKO mouse has a similar body size when compared to WT littermate, but visibly smaller ears. B. Smad4 cKO mice have significantly smaller ears compared to their WT littermates at weeks of age. **: p< 0.01. Males and females are compared in separate groups because of the natural differences in their body size and weight. C. Smad4 cKO mice have normal body weight at 8 weeks of age. n. s.: no statistical difference. D-F: H&E staining of ear sections. D. In WT ear, chondrocytes occupy most of the space in the cartilage. E. In Smad4 cKO ear, there are clusters of immature chondrocytes in the cartilage (arrow). F. The TGFβRII cKO mice have normal chondrocytes in their ear cartilages. Scale bar: 50µm.
The Hematoxylin and Eosin (H&E) staining of ear sections from Smad4 cKO reveals thicker, more disorganized tissue organization in the ears, suggesting that an altered cartilage production in the cKO ears may result in the stunted the growth of the surrounding tissues (Figure 1E, 4C), when compared to WT ears (Figure 1D, 4B). The boundaries of the cartilage are also less defined in the Smad4 cKO ears. Furthermore, there are many more small round shaped cells in the Smad4 cKO ear cartilage that do not resemble the morphology of mature chondrocytes in the WT ear cartilage (Figure 1E, arrow). These cells likely represent immature chondrocytes or differentiating fibroblast arrested at this stage.
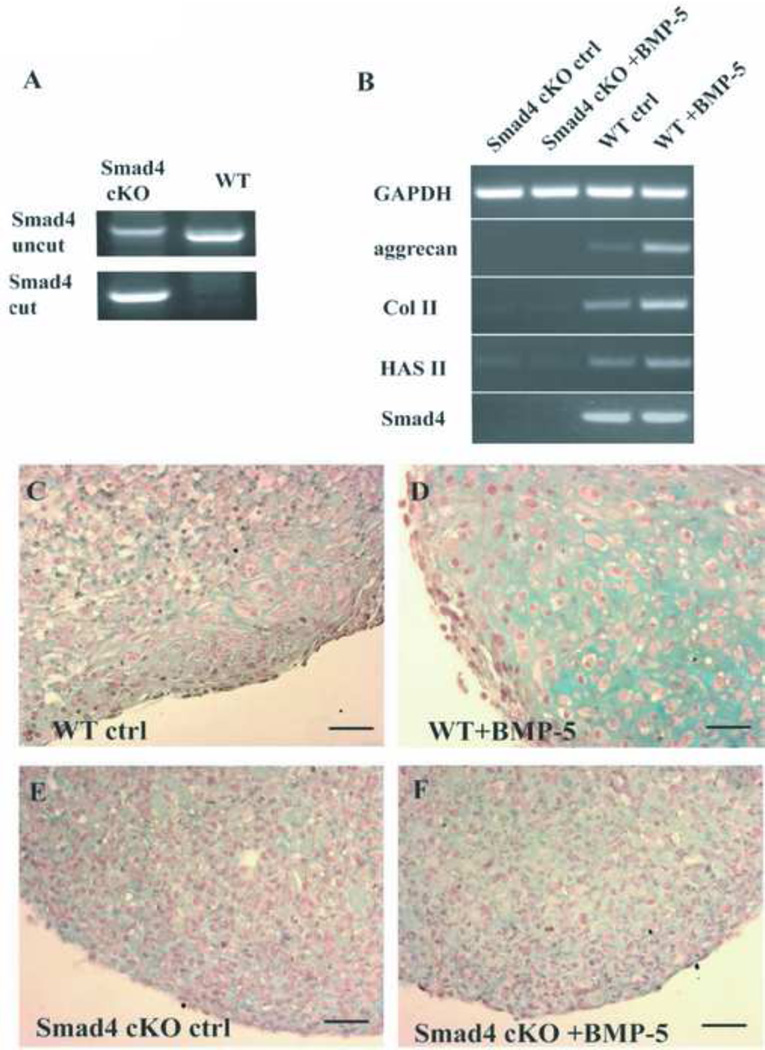
Figure 4. BMP-5 stimulates the cartilage production in WT but not the Smad4 cKO fibroblasts.
A: genotyping of the WT and the Smad4 cKO fibroblasts. The uncut lane shows the presence of intact Smad4 locus; the cut lane shows the presence of the recombined Smad4 locus (deletion of exon 9 and 10 by Cre recombinase). B: Semiquatitiative RT-PCR to determine the expression of the cartilage producing genes in the cultured Smad4 cKO and WT fibroblasts with BMP-5 stimulation. GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; Col II: type II collagen α1 chain; HAS II: hyaluronic acid synthase II. C-F: Alcian Blue stainings of the pellet cultures. C-D: Stimulation of the WT fibroblasts with BMP-5 induces chondrocyte differentiation and cartilage production in vitro (D). The control WT fibroblasts without BMP-5 treatment remain undifferentiated and make no cartilage (C). E-F: The Smad4 cKO fibroblasts do not respond to BMP-5 treatment and differentiate. Scale bar: 50µm.
However, when Fsp1-cre was used to delete the TGFβ type II receptor, the short ear phenotype was not observed. Furthermore, the ear cartilage from Fsp1-cre+; TGFβRII flox/flox mice do not exhibit the accumulation of immature chondrocytes or any other structural abnormalities as observed in Smad4 cKO ear cartilage (Figure 1F). Therefore, our results demonstrate that the TGFβ pathway is not essential in the maturation and function of ear chondrocytes, yet signaling pathway/s mediated via Samd4 are important.
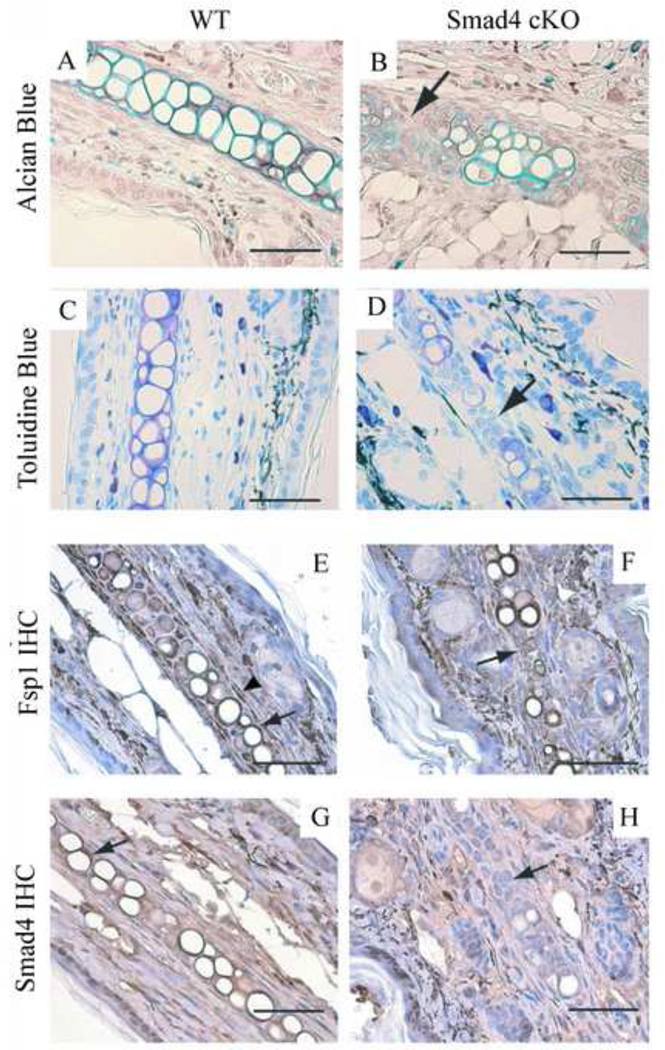
To examine the characteristics of the Smad4 cKO ear cartilage, we also utilize the Alcian Blue, which stains the cartilage in turquoise color, and the Toluidine Blue, which stains the cartilage in violet color, to directly visualize the cartilage deposition pattern. We observe that, in the Smad4 cKO mice, there is a reduction of cartilage, especially around the clusters of immature chondrocytes, further supporting our hypothesis that immature chondrocytes may be associated with defects in cartilage production (Figure 2B, 2D, arrows, compare to WT Figure 2A, 2C).
Figure 2. The Smad4 cKO ears are deficient in cartilage.
In the Smad4 cKO ears, there is less cartilage (B: Alcian blue stain: turquoise; D: Toluidine blue stain, purple), especially around the clusters of immature chondrocytes (arrow). WT control stains: A: Alcian blue stain. C: Toluidine blue stain. E-F: Fsp1 labeling. E. In WT ear tissue, Fsp1 is expressed in the fibroblasts (arrowhead) and the chondrocytes (arrow). F. In the Smad4 cKO chondrocytes, Fsp1 is also expressed in the chondrocytes. Arrow points to a cluster of immature chondrocytes that are positive for Fsp1. G-H: Smad4 labeling. G. Smad4 is expressed strongly in the WT ear chondrocytes (arrow). H. The Smad4 cKO ear chondrocytes show insignificant Smad4 staining. Arrow points to a cluster of immature chondrocytes that are negative for Smad4 staining. Scale bar: 50µm.
We next examined whether Fsp1/ S100A4 is expressed in the ear fibroblasts and chondrocytes. Immunohistochemistry (IHC) confirmed the expression of Fsp1 in the ear chondrocytes of both WT and Smad4 cKO mice (Figure 2E, 2F, arrows) and some spindle-shaped fibroblasts (Figure 2E, arrowhead). To demonstrate that the Smad4 cKO ear chondrocytes are in fact deficient in Smad4, we also performed IHC for Smad4 protein. The WT ear chondrocytes label strongly for Smad4 (Figure 2G, arrow) while ear chondrocytes from Smad4 cKO mutants exhibit insignificant Smad4 localization (Figure 2G, arrow).
Fsp1/S100A4 is expressed in the chondrocytes associated with elastic cartilage and fibrocartilage, but not in the chondrocytes associated with the hyaline cartilage
To verify the expression of Fsp1-cre in the ear chondrocytes, and to further study the presence of Fsp1/S100A4 in different types of cartilages, we also crossed Fsp1-cre mice to the Rosa26-Eyfp mice [12], which harbors a transgene consisting of Rosa26 promoter and Eyfp (Enhanced yellow fluorescent protein) gene, separated by a floxed stop codon. In the Fsp1-cre+; Rosa26-Eyfp mice, cells (or their progenitors) that express Fsp1 will have the stop codon excised from the Rosa26-Eyfp locus and hence will become irreversibly positive for YFP protein.
In correlation with the Fsp1 antibody staining (vide supra), we observed YFP expression in the ear chondrocytes of the Fsp1-cre; Rosa26-Eyfp mice (Figure 3A). Chondrocytes associated with elastic cartilages in other parts of the body were also positive for the YFP signal (data not shown).
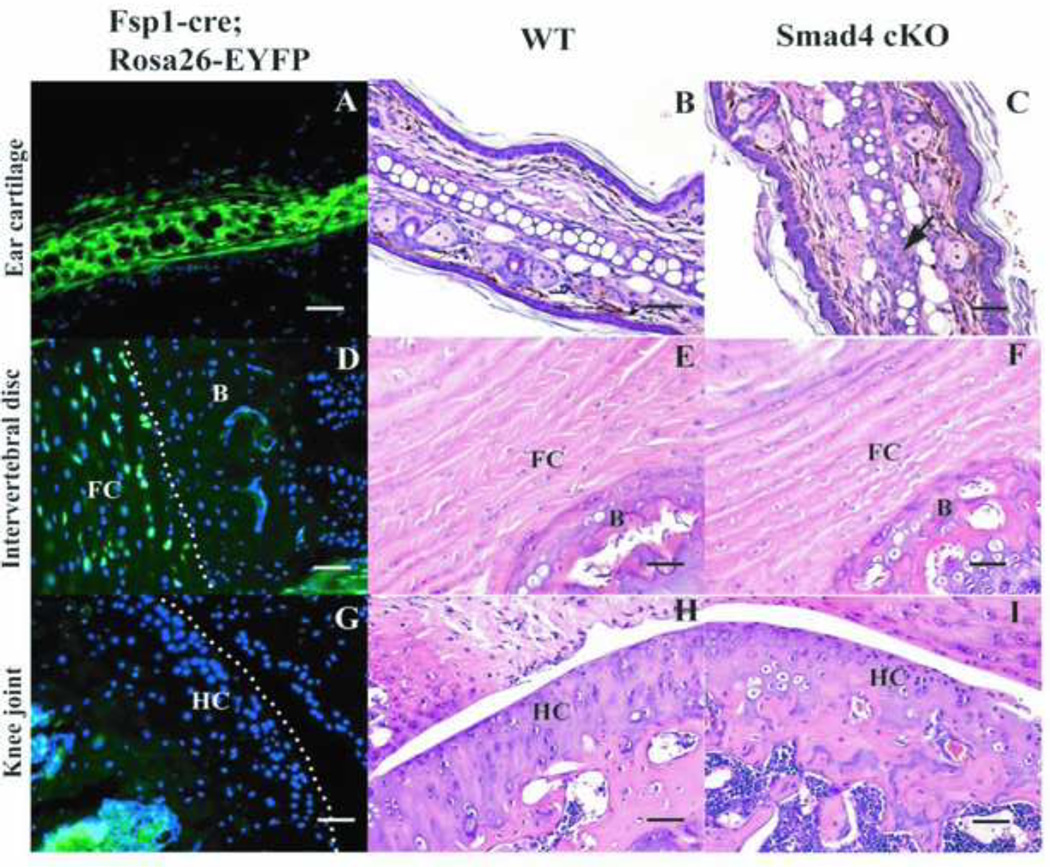
Figure 3. Expression profile of Fsp1 in different types of the cartilages. A. D. G. J. are sections of the cartilages from Fsp1-cre; Rosa26-Eyfp mice.
YFP positive cells are where the fsp1-cre has been expressed. B. C. E. F. H. I. K. L. are H&E stainings of WT and Smad4 cKO cartilages. A-C: Fsp1 is expressed in the ear chondrocytes (A) and the Smad4 cKO mouse has more immature chondrocytes in the ear cartilage (C, arrow) than the WT mouse does (B). D-F: Fsp1 is expressed in the fibrocartilage in the intervertebral discs (D). However, the Smad4 cKO (F) does not show any gross defects compared to WT (E). FC: fibrocartilage. B: bone. G-I: Fsp1 is not expressed in the hyaline cartilage (G) (knee joints are shown here as an example); therefore Smad4 cKO mice (I) have wild type chondrocytes in the hyaline cartilage as the WT mice do (H). HC: hyaline cartilage. Scale bar: 50µm.
Next we examined whether Fsp1 is expressed in any other type/s of chondrocytes. We observed YFP signal in the chondrocytes associated with fibrocartilage (Figure 3D), but not in the chondrocytes associated with hyaline cartilage (Figure 3G). Despite this, insignificant abnormalities of fibrocartilage in the Smad4 cKO mice was observed (Figure 3F compared to WT Figure 3E). The Smad4 cKO mice exhibit normal hyaline cartilage, which correlated with the absence of Fsp1 from the chondrocytes in this tissue (Figure 3I compared to WT Figure 3H). The absence of Fsp1 expression in the chondrocytes associated with hyaline cartilage also likely explains why we do not observe dwarfism in Smad4 cKO, as reported for the Col2a1-cre; Smad4 flox/flox mice, in which deletion of Smad4 in the chondrocytes associated with hyaline cartilage leads to defects in the growth of the bones [13].
BMP-5 induces the differentiation of the ear chondrocytes and the production of the cartilage
Next, we evaluate the signaling pathways via Smad4 that control the maturation of the ear chondrocytes. Among the TGFβ superfamily members, both TGFβ family and BMP family growth factors are important for the maturation and function of the chondrocytes in the hyaline cartilage. The Fsp1-cre+; TGFβRII flox/flox mice however, as described above, show neither the short ear phenotype nor any ear cartilage defects as observed in the Smad4 cKO mutants, which exclude the possibility of TGFβ regulating the differentiation and the function of chondrocytes via Smad4 in the ear. As mentioned before, the Bmp-5 null mice also exhibit the short ear phenotype as observed in the Smad4 cKO mice reported in this study, we therefore examined whether decreased BMP signaling via Smad4, particularly BMP-5 signaling, may be important for chondrocyte maturation and function.
We cultured Fsp1 positive ear fibroblasts from WT and Smad4 cKO ears and studied their chondrogenic differentiation potential. In the majority of the Smad4 cKO ear fibroblasts, exons in the Smad4 loci were floxed out (Figure 4A), with a dramatic decrease in Smad4 transcripts (Figure 4B), when compared to the WT ear fibroblasts. These results suggest that we have successfully cultured Smad4 deficient fibroblasts.
To examine whether BMP-5 promotes the maturation of the ear chondrocytes from fibroblasts, we stimulated the ear fibroblasts with recombinant human BMP-5. Using RT-PCR we demonstrate that with incubation with 100 ng/mL of BMP-5, the WT ear fibroblasts initiate transcription of genes essential for the production of cartilage, such as aggrecan, type II collagen and hyaluronic acid synthase. The Smad4 cKO fibroblasts exhibit lower basal expression of these genes, and lack any such response to BMP-5 stimulation (Figure 4B).
Next, when we incubate the WT fibroblast with BMP-5, they differentiate into chondrocytes and produce cartilage, suggesting that fibroblast to chondrocyte differentiation was likely induced by BMP-5. The cells expand in size and adopt the morphology of chondrocytes; and Alcian Blue staining demonstrates that cartilage fills the extra-cellular space between the cells (Figure 4C). WT fibroblasts without the treatment of BMP-5 do not show discernible chondrocyte differentiation from ear fibroblast (Figure 4D). The Smad4 cKO ear fibroblasts do not respond to BMP-5 treatment (Figure 4E, 4F).
Discussion
The TGFβ superfamily of growth factors have important functions in the development and maturation of chondrocytes [7]. BMP-5 has been shown to promote chondrocytes differentiation and cartilage production [8, 14]. In this study, we demonstrate that in fsp1-Cre; Smad4 flox/flox mice, ear chondrocytes deficient in Smad4 fail to acquire the morphology of typical chondrocytes and are deficient in cartilage production. BMP mediated signaling via Smad4, not TGFβ mediated signaling, is important for Fsp1 positive ear fibroblast differentiation into chondrocytes. We show that BMP-5 is able to induce chondrocyte-specific gene expression in the WT but not in the Smad4 cKO ear fibroblasts, BMP-5 stimulates fibroblast to chondrocyte differentiation and cartilage formation in the WT but not the Smad4 cKO fibroblasts. Our results demonstrate the importance of BMP signaling in the differentiation of chondrocytes, and show that Smad4 is a key mediator of this action. Moreover, the ear chondrocyte differentiation assay we developed in this study will serve as a valuable resource for the future studies designed to elucidate chondrocyte differentiation.
We also demonstrate that Fsp1/S100A4 is a marker for the mature chondrocytes in the elastic cartilage and fibrocartilage but not for the chondrocytes associated with hyaline cartilage. Such selective expression of Fsp1/S100A4 may serve to better understand distinct cartilage pathologies. For example, while synovial fibroblasts exhibit normal differentiation into chondrocytes in culture, they do not show significant mineralization [8]. Furthermore, synovial fibroblast can only repair damaged hyaline cartilage by depositing scar-like tissue [15]. These studies strongly suggest that the synovial fibroblast-derived chondrocytes have stronger tendency to synthesize fibrocartilage instead of hyaline cartilage. Further supporting evidence comes from the transcriptional profiling of synovial fibroblasts in rheumatoid arthritis [16], which demonstrates that the proliferating synovial fibroblasts have strong expression of Fsp1/S100A4. Therefore, future studies involving chondrocyte maturation and function can utilize Fsp1/S100A4 expression as a marker of how chondrocytes may behave in vivo.
In summary, our study confirms the importance of BMP signaling during the maturation of the chondrocytes, and emphasizes a distinction among the chondrocyte population in different cartilages. The identification of Fsp1/S100A4 as an elastic and fibrocartilage chondrocyte marker will aid in future attempts to illustrate the distinct signaling events involved in the production of different types of cartilages and aid in tissue/cartilage engineering studies. Lastly using two novel genetic mouse models, we establish that BMP signaling via Smad4 and not TGFβ is important for poper development of the outer ear.
Research Highlights.
FSP-1+ fibroblasts participate in elastic cartilage and fibrocartilage production
Loss of Smad4 in fibroblasts results in a short ear phenotype
Chondrocyte maturation is conducted via Smad4 signaling
BMP-5 induces differentiation of ear chondrocytes
Acknowledgements
We wish to thank Lauren Orefice for her technical assistance provide to the authors during the course of this study. This work was primarily supported by the BIDMC Department of Medicine research funds to the Division of Matrix Biology, and partially supported by NIH Grants DK 55001, DK 62987, DK 13193 and DK 61688. We also thank Dr. Michael Duncan for his useful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nature reviews. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 2.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes & development. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 3.Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases? Journal of molecular medicine (Berlin, Germany) 2008;86:507–522. doi: 10.1007/s00109-007-0301-3. [DOI] [PubMed] [Google Scholar]
- 4.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. The Journal of biological chemistry. 2006;281:677–680. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- 5.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. The Journal of cell biology. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer J, Steinhoff J, Klinger M, Fricke L, Rohwedel J. Cells differentiated from mouse embryonic stem cells via embryoid bodies express renal marker molecules. Differentiation. 2006;74:91–104. doi: 10.1111/j.1432-0436.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 7.Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Current topics in developmental biology. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- 8.Seto H, Kamekura S, Miura T, Yamamoto A, Chikuda H, Ogata T, Hiraoka H, Oda H, Nakamura K, Kurosawa H, Chug UI, Kawaguchi H, Tanaka S. Distinct roles of Smad pathways and p38 pathways in cartilage-specific gene expression in synovial fibroblasts. The Journal of clinical investigation. 2004;113:718–726. doi: 10.1172/JCI19899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, DePinho RA. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes & development. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- 11.King JA, Marker PC, Seung KJ, Kingsley DM. BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Developmental biology. 1994;166:112–122. doi: 10.1006/dbio.1994.1300. [DOI] [PubMed] [Google Scholar]
- 12.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Tan X, Li W, Wang Y, Wang J, Cheng X, Yang X. Smad4 is required for the normal organization of the cartilage growth plate. Developmental biology. 2005;284:311–322. doi: 10.1016/j.ydbio.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 14.Mailhot G, Yang M, Mason-Savas A, Mackay CA, Leav I, Odgren PR. BMP-5 expression increases during chondrocyte differentiation in vivo and in vitro and promotes proliferation and cartilage matrix synthesis in primary chondrocyte cultures. Journal of cellular physiology. 2008;214:56–64. doi: 10.1002/jcp.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. The Journal of bone and joint surgery. 1996;78:721–733. doi: 10.2106/00004623-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Masuda K, Masuda R, Neidhart M, Simmen BR, Michel BA, Muller-Ladner U, Gay RE, Gay S. Molecular profile of synovial fibroblasts in rheumatoid arthritis depends on the stage of proliferation. Arthritis research. 2002;4:R8. doi: 10.1186/ar427. [DOI] [PMC free article] [PubMed] [Google Scholar]