Abstract
Objective:
This one-year study investigated whether the Manitoba Provincial Health Contact program for congestive heart failure (CHF) is a cost-effective intervention relative to the standard treatment.
Design:
Individual patient-level, randomized clinical trial of cost-effective model using data from the Health Research Data Repository at the Manitoba Centre for Health Policy, University of Manitoba.
Methods:
A total of 179 patients aged 40 and over with a diagnosis of CHF levels II to IV were recruited from Winnipeg and Central Manitoba and randomized into three treatment groups: one receiving standard care, a second receiving Health Lines (HL) intervention and a third receiving Health Lines intervention plus in-house monitoring (HLM). A cost-effectiveness study was conducted in which outcomes were measured in terms of QALYs derived from the SF-36 and costs using 2005 Canadian dollars. Costs included intervention and healthcare utilization. Bootstrap-resampled incremental cost-effectiveness ratios were computed to take into account the uncertainty related to small sample size.
Results:
The total per-patient mean costs (including intervention cost) were not significantly different between study groups. Both interventions (HL and HLM) cost less and are more effective than standard care, with HL able to produce an additional QALY relative to HLM for $2,975. The sensitivity analysis revealed that there is an 85.8% probability that HL is cost-effective if decision-makers are willing to pay $50,000.
Conclusion:
Findings demonstrate that the HL intervention from the Manitoba Provincial Health Contact program for CHF is an optimal intervention strategy for CHF management compared to standard care and HLM.
Abstract
Objectif:
Cette étude, échelonnée sur un an, visait à déterminer si le programme pour l'insuffisance cardiaque congestive (ICC) du Centre provincial de communication en matière de santé du Manitoba constitue une intervention efficace par rapport au coût, comparé au traitement standard.
Conception:
Il s'agit d'un essai clinique aléatoire (au niveau individuel du patient) du modèle coût-efficacité effectué à l'aide du Registre de données de recherche sur la santé de la population du Centre des politiques de santé du Manitoba, à l'Université du Manitoba.
Méthodes:
Un total de 179 patients de 40 ans et plus ayant reçu un diagnostic d'ICC de niveaux II à IV ont été recrutés à Winnipeg et au Centre du Manitoba puis assignés aléatoirement à trois groupes de traitement: le premier recevant le traitement standard, le deuxième bénéficiant d'une intervention à l'aide des lignes d'information (LI) et le troisième recevant l'intervention à l'aide des lignes d'information en plus d'un service de suivi à domicile (LID). Les résultats de l'étude coût-efficacité ont été mesurés selon les années-personnes sans invalidité (QALY) tirées du questionnaire SF-36 et selon les coûts en dollars canadiens 2005. Les coûts comprenaient l'intervention et l'utilisation des services de santé. Après un rééchantillonnage par amorçage (bootstraping), les rapports du coût-efficacité différentiel ont été traités pour tenir compte de l'incertitude liée aux petits échantillons.
Résultats:
Il n'y avait pas de différence statistique entre le coût total moyen par patient (y compris le coût de l'intervention) de chacun des groupes à l'étude. Les deux interventions (LI et LID) sont moins coûteuses et sont plus efficaces que le traitement standard; le LI offre la possibilité d'un QALY supplémentaire de 2 975 $ par rapport au LID. L'analyse de sensibilité révèle qu'il y a une probabilité à 85,8 % que le LI soit efficace par rapport au coût, dans la mesure où les décideurs acceptent de payer 50 000 $.
Conclusion:
Les résultats démontrent que l'intervention à l'aide des LI dans le cadre du programme pour l'ICC du Centre provincial de communication en matière de santé du Manitoba constitue une intervention stratégique optimale pour la gestion de l'ICC, comparativement au traitement standard et au LID.
Heart failure is the most frequent indication for hospital readmission and the most frequent discharge diagnosis in Canada. An estimated 400,000 Canadians are living with congestive heart failure (CHF) (Heart and Stroke Foundation of Manitoba 2010). In Canada, cardiovascular disease is one of the most costly chronic diseases (Patra et al. 2007). As healthcare costs have increased dramatically in recent years, cost containment has become increasingly important to healthcare planners and decision-makers. Interest in the potential cost savings of telehealth has correspondingly grown. Telehealth for chronic disease management has been implemented in recent years to improve and maintain the health of patients with chronic disease. As defined by the American Telemedicine Association (2011), home telehealth is remote care delivery or monitoring in which healthcare providers deliver services to patients at home through information and communication technology. Telehealth provides new prospects for cost savings and quality of care in a community setting. Telehealth applications used in CHF interventions provide better outcomes in terms of reduction of hospitalization readmission, bed days of care for all-cause or heart failure–related events, emergency visits, mortality, better health-related quality of life and patient satisfaction (Canadian Agency for Drugs and Technologies in Health 2008).
Evidence related to the cost-effectiveness of telehealth interventions for CHF is mixed in the literature (Canadian Agency for Drugs and Technologies in Health 2008). A few international studies show that telehealth can be an effective method to reduce healthcare utilization rates and costs as well as improve quality of life for people with CHF (Clark et al. 2007; Jennett et al. 2003; Noel et al. 2004; Schmidt et al. 2010; Seto 2008; Wooden et al. 2008). Other studies show telehealth to be associated with unchanged or increased costs (Smith et al. 2008). However, there is a paucity of research in Canada examining both costs and effectiveness of telehealth interventions for CHF.
In Manitoba, the TeleCARE program applying information technology is intended to help patients with chronic disease, such as CHF or type 2 diabetes, manage their condition through combining a nursing call centre with a home monitoring strategy. The service is provincewide and available 24 hours, 7 days a week to all Manitobans. Nurses and other healthcare providers who are specialists in CHF self-management provide care and assessment via the telephone according to an established patient call schedule. During the phone calls, an assessment of the patient's health is made, and the healthcare provider monitors symptoms and gives professional advice about the disease in a timely manner. In addition, the healthcare provider offers education and self-monitoring tools, including blood pressure monitors and body weight scales, for patients to monitor risk factors believed to have a correlation with the illness, such as diet, BMI, blood pressure, stress levels and physical activity. Moreover, Health Lines nursing staff communicate regularly with primary care physicians to discuss the health status and care management strategies most appropriate for individual patients, including such factors as the person's health, living environment and availability of informal supports. This communication is considered fundamental to the intervention, as it helps to ensure an aspect of care continuity to patients with CHF and reflects the need for an interdisciplinary and holistic approach to provide timely and ongoing care to the chronically ill. The use of technology in combination with this strategy of joint care provision is an essential component of healthcare reform.
The purpose of this study was to determine whether the Manitoba Provincial Health Contact program for CHF is cost-effective relative to standard care. That is, what are the costs and effects of the intervention compared to usual care?
Methods
Study Design
This economic evaluation is piggy-backed onto a 2005 effectiveness study, Testing the Effectiveness of Health Lines in Chronic Disease Management of Congestive Heart Failure (hereafter, Health Lines study) (Katz and Doupe 2009). Patients aged 40 and older living in Winnipeg and Central Manitoba with a diagnosis of CHF New York Heart Association (NYHA) levels II, III and IV were recruited. A total of 179 patients were randomized into three groups. Group 1 received standard care. Group 2 received standard care plus Health Lines (HL): that is, nurses were available on the telephone to provide suggestions about the patient's daily management of the disease. Group 3 received standard care plus Health Lines plus in-house monitoring (HLM): that is, they were provided with monitoring devices and instructions on how to use them. Patients in this study enrolled between April 25, 2005 and April 12, 2006. The last day of the study was September 25, 2006. Patient health outcome status surveys were conducted by mail, with follow-up over the phone to participants at baseline and at three, six and 12 months of the active intervention. The survey instruments included the Short-Form 36 (SF-36) Health Survey (McHorney et al. 1993), Revised Self-Care Behavior Scale (Artinian et al. 2002) and Client Satisfaction Questionnaire (Attkisson and Zwick 1982); these were used to assess the general effectiveness of the intervention. The SF-36 assessed health-related quality of life. The Revised Self-Care Behavior Scale assessed activities that patients with CHF must perform to some extent so that they can continue to function in their daily life. The Client Satisfaction Questionnaire consists of eight questions, each of which has four response choices; 1 indicates the lowest rating of degree of satisfaction and 4 indicates the highest degree of satisfaction. This questionnaire measured patient satisfaction with the telehealth services received.
Patients were recruited with the consent of their primary care doctors, who were involved from the outset. No patients were recruited where the primary care doctors did not buy in to the program. All patients' contacts were reported to the primary care doctor by fax. Medication changes were made by the primary care doctors rather than the nurses. A key component of this intervention was the integration of the Health Lines interventions with regular primary care services. All advice provided to patients was also communicated to the patient's primary care physician to ensure continuity of care. None of the study patients saw private physicians outside of the provincial health plan.
The economic costs of the telehealth program interventions depend upon the perspective adopted. Because we conducted this analysis from the perspective of the healthcare system, only direct costs are included. The intervention costs include all expenses from the healthcare sector associated with the program. Specific cost items included equipment and technology cost, personnel wages, technician assistance, travel expenses, administrative supports and supplies. We also measured healthcare utilization costs. A discount rate of 0% for intervention costs and health outcomes was applied in the analysis because the time horizon of the study was 12 months. Costs in this study were measured based on 2005 Canadian dollars. Costs were not adjusted for the present value because of the study's short time frame.
This study was approved by the Health Research Ethics Board at the University of Manitoba (Ethics reference number: H2010:164). Because the data contain personal health information, Health Information Privacy Committee (HIPC) approval was sought and granted from Manitoba Health (File number: 2010/2011-09).
Measuring Healthcare Utilization Costs
Healthcare utilization included family physician visits, physician specialist visits, cardiac physician visits, internist specialist visits and hospital in-patient days. We excluded emergency department (ER) visits in the study, because ER data in Central Manitoba were incomplete. Costs were associated with each category of care. Healthcare utilization cost data were obtained from enrolment to the intervention completion date from the Health Research Data Repository at the Manitoba Centre for Health Policy, University of Manitoba. The Data Repository holds records for virtually all contacts with the provincial healthcare system, including physicians, hospitals, personal care homes, home care and pharmaceutical prescriptions of all registered individuals (MCHP 2009). Physician costs were read directly from the Data Repository.
Hospital costs included cost per weighted case (CPWC) value (Finlayson et al. 2001). CPWC is a method of estimating hospital costs that applies Case Mix Groups (CMG™) to homogeneous groups of hospital administrative records with respect to length of stay and measures of intensity of resource use. This approach is necessary because administrative data do not track detailed resource use by individual admission. CPWC is a relative, average cost to a “standard” hospital patient by summing the weights assigned to all cases treated by a hospital and dividing this number into the hospital's total in-patient expenditure. It is used for describing and comparing the cost of care, as it removes the effects of differences in the acuity, severity and complexity of the populations served in different hospitals on the cost of providing care, and permits the assignment of a cost to each case that is discharged from a hospital.
Two main types of healthcare utilization cost data were included: healthcare utilization for all reasons and healthcare utilization specifically for CHF. The CHF-specific utilization data were categorized if there was a diagnosis of CHF. (Note that whether a physician visit carries a particular diagnosis may depend on the billing practice of the physician, and therefore the CHF-specific data will underestimate total costs for CHF-specific visits. Therefore, we conducted the analyses in terms of both CHF-specific costs and total healthcare costs.) These healthcare service costs are used to determine whether Health Lines reduced overall healthcare utilization costs compared with standard treatment.
Quality-adjusted Life-years
The SF-36 Health Survey was not originally designed to calculate preference-based utilities, which are used to derive quality-adjusted life-years (QALYs). To obtain QALYs, a conversion formula developed by Brazier and colleagues (2002) was used to calculate the SF-6D utility score (QALYs) from SF-36 data. The SF-6D is a system for classifying health state derived from a selection of SF-36 items. It is composed of six multilevel dimensions. Any patient who completes the SF-36 can be uniquely classified according to the SF-6D. We chose this method for our study because it is based on the well-validated and commonly used SF-36 (McHorney et al. 1993). SF-36 scores in the eight domains were converted to a single preference-based utility score indicating the value that would be placed on a health state. The SF-6D algorithm was used to convert SF-36 responses and generate a utility score for each subject.
Statistical Methods
The healthcare utilization cost was non-normally distributed owing to skewness from several high-cost outliers. Therefore, non-parametric tests were used to determine whether there was a statistically significant difference in costs across three study groups. The mixed-effects repeated measures models were used to test for statistically significant differences in effectiveness in terms of SF-6D utility, SF-36 domain scores and self-behaviour scales between study groups over time. A mixed-effects model incorporates fixed and random effects, with different interpretations and analysis for the two types. The fixed-effects model compares the interventions, and the random effects determine individual differences in response to an effect. The mixed-effects design for repeated measurements was chosen because this approach allows a wide variety of correlation patterns (variance–covariance structures) without violating important regression assumptions. Alpha was set at p<0.05. The robustness of the study to variations in assumptions was examined through the sensitivity analysis. A non-parametric bootstrap with replacement method and 1,000 replications was used to estimate the confidence interval for cost and effect differences (Drummond et al. 2005). Data manipulation programming and all statistical analyses were performed using SAS (version 9.2, SAS Institute, Inc., Cary, NC).
Results
Data were analyzed for patients who enrolled in the Health Lines study between April 25, 2005 and April 12, 2006. The last day of the Health Lines study was September 25, 2006; therefore, the intervention period ranged from 166 to 518 days, meaning that some of the later enrollees have fewer outcome measures. The data were elicited from a total of 179 patients who participated in the Health Lines study. Data cleaning was achieved based on the following criteria: (a) patients under 40 years old were deleted; (b) patients who did not have clear enrolment dates were deleted; and (c) patients whose completion dates were earlier than their enrolment dates were deleted. This study filtered five invalid records, and a total of 174 patients' records were used for the analysis.
Patient characteristics at baseline are shown in Table 1. Approximately one-third of the total study population was randomly allocated to each study group. The average age of all patients was 75 (SD, 12) years. Seventy-three patients were 80 years or older. The participants include 90 females and 84 males. Sixty per cent of patients resided in the Winnipeg Health Region, while 40% were from the Manitoba Central Health Region. More than 45% of all study patients had moderate-stage (NYHA level III) CHF, while 31% had an advanced stage (NYHA level IV).
TABLE 1.
Patient characteristics across three study groups at baseline
| Overall (N=174) | Control (n=55) | HL (n=61) | HLM (n=58) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 90 (52%) | 24 (44%) | 32 (52%) | 34 (59%) |
| Female | 84 (48%) | 31 (56%) | 29 (48%) | 24 (41%) |
| Age group | ||||
| 40–59 | 23 (13%) | 8 (15%) | 7 (12%) | 8 (14%) |
| 60–69 | 33 (19%) | 17 (27%) | 10 (17%) | 16 (27%) |
| 70–79 | 44 (25%) | 15 (23%) | 17 (28%) | 12 (21%) |
| 80 and older | 73 (42%) | 25 (45%) | 26 (43%) | 22 (38%) |
| Geography | ||||
| Winnipeg Health Region | 104 (60%) | 34 (62%) | 36 (59%) | 34 (59%) |
| Manitoba Central Health Region | 70 (40%) | 21 (38%) | 25 (41%) | 24 (41%) |
| CHF severity | ||||
| NYHA level II | 38 (22%) | 11 (20%) | 14 (23%) | 13 (22%) |
| NYHA level III | 82 (47%) | 27 (49%) | 30 (49%) | 25 (43%) |
| NYHA level IV | 54 (31%) | 17 (31%) | 17 (28%) | 20 (35%) |
Compared to the control group, healthcare service utilization was lower in both intervention groups, although this finding was not significantly different between groups (p=0.3893). The number of deaths in each group during the intervention period was small. Patients in the control group had more all-reasons hospital in-patient days than both intervention groups, but the differences were not significant (p=0.4865). However, hospital in-patient days for CHF were significantly higher for the intervention groups relative to the control group (p<0.05).
Mean per-patient costs are shown in Table 2 by treatment groups. Results revealed that although costs were higher for both hospitalization and physician/specialist visits for the control group, the differences were not significant. While there was very little difference between groups in physician/specialist service cost, the high health utilization cost was driven by the cost of hospitalization in each study group. The difference in hospitalization cost between the highest (control) group to the lowest (HLM) group was $2,519 per patient.
TABLE 2.
Mean per-patient costs (Canadian dollars, 2005) by study group (SD)
| Control (N=55) | HL (n=61) | HLM (n=58) | p | |
|---|---|---|---|---|
| Healthcare utilization | ||||
| Hospitalization | $5,640 (17,361) | $3,342 (8,944) | $3,121 (8,174) | 0.9316 |
| Physician/specialist visits | $1,511 (1,549) | $1,234 (1,414) | $1,082 (936) | 0.3487 |
| Subtotal | $7,151 (18,106) | $4,576 (9,996) | $4,203 (8,651) | 0.7583 |
| Intervention | ||||
| Staffing salary | – | $1,766 | $1,766 | |
| Setting-up and operating costs | – | $88 | $342 | |
| Subtotal | – | $1,854 | $2,108 | |
| Total | $7,151 (18,106) | $6,430 (9,966) | $6,311 (8,651) | 0.7765 |
Source: Manitoba Provincial Health Contact Centre, 2010; Manitoba Centre for Health Policy, 2005/06.
The cost of the program intervention was estimated from a healthcare provider's perspective using an accounting approach. All direct costs were allocated to each patient in the intervention groups over a one-year period. The expected life of a telemonitoring device was estimated at five years, and the cost of purchasing the telemonitoring items has been depreciated over this period using a straight-line method. The total cost for delivering the telehealth intervention program for the CHF patients was $235,397 – that is, the average intervention costs for HL and HLM groups were $1,854 and $2,108, respectively. Compared to the control group, the total saving from averted healthcare utilization costs through the interventions was $28,307, or $238 per capita. The total healthcare costs per patient, including intervention cost for the three study groups, were $7,151 (control group), $6,430 (HL) and $6,311 (HLM). The difference in total per-patient mean costs (including interventions costs) across study groups is insignificant (p=0.7765).
Neither healthcare utilization nor costs associated with healthcare utilization differed significantly among the three study groups, but outcomes measured in terms of QALYs did differ. We found that the domain scores of SF-36 physical functioning and role limitation (physical) were significantly different over time among groups (p<0.05). In particular, physical functioning was observed to be significantly different among groups over time (p=0.0011). The domain scores of bodily pain and role limitation (emotional) were also significantly different among groups (p<0.05). Although the mortality was small in each cohort, the QALY calculations were adjusted to reflect mortality. SF-6D utility scores were higher in the intervention groups at all measurements. The differences were also statistically significant among groups and over time (see Table 3, page 50).
TABLE 3.
SF-36 mean (SD) domain scores and SF-6D mean (SD) utility score by group
| SF-36 | Baseline | Follow-up Survey 1 | Follow-up Survey 2 | Follow-up Survey 3 | Time p | Study Group p | Time* Study Group p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=44) | HL (n=47) | HLM (n=40) | Control (n=44) | HL (n=47) | HLM (n=40) | Control (n=31) | HL (n=32) | HLM (n=29) | Control (n=19) | Control (n=19) | HL (n=18) | ||||
| Physical functioning | 40.18 (27.61) | 37.66 (28.85) | 40.93 (27.62) | 35.83 (23.79) | 45.22 (29.84) | 41.88 (28.77) | 35.67 (26.90) | 32.50 (26.09) | 41.72 (29.80) | 41.58 (29.30) | 53.44 (23.36) | 42.50 (29.67) | 0.0113 | 0.5200 | 0.0011 |
| Role, physical | 28.29 (36.19) | 37.41 (28.85) | 33.13 (38.56) | 25.61 (34.68) | 42.02 (40.41) | 33.13 (38.56) | 19.17 (29.86) | 30.47 (35.77) | 26.72 (35.31) | 38.16 (45.16) | 39.06 (38.70) | 38.89 (43.91) | 0.0317 | 0.2537 | 0.8337 |
| Bodily pain | 53.30 (30.75) | 64.28 (24.95) | 56.00 (28.38) | 50.93 (27.48) | 66.04 (25.65) | 54.56 (27.31) | 54.27 (26.64) | 61.78 (29.44) | 49.14 (27.86) | 53.05 (26.82) | 80.67 (28.38) | 60.33 (32.62) | 0.1107 | 0.0017 | 0.2323 |
| General health | 44.61 (23.30) | 45.93 (19.47) | 44.73 (17.79) | 46.10 (21.61) | 49.13 (18.28) | 45.85 (21.29) | 47.43 (21.25) | 46.13 (17.68) | 42.52 (23.26) | 49.05 (19.59) | 55.63 (27.51) | 40.17 (23.38) | 0.2087 | 0.5341 | 0.5300 |
| Vitality | 39.20 (20.88) | 42.17 (23.28) | 42.50 (20.29) | 39.64 (23.44) | 44.36 (22.71) | 41.67 (24.50) | 38.17 (22.22) | 39.69 (25.71) | 41.72 (21.85) | 41.84 (22.68) | 45.67 (23.74) | 36.11 (23.49) | 0.9763 | 0.9205 | 0.4495 |
| Social functioning | 61.08 (30.29) | 73.91 (26.06) | 73.13 (25.72) | 62.80 (29.54) | 75.00 (27.21) | 71.88 (23.64) | 62.08 (31.74) | 69.53 (28.56) | 66.38 (30.45) | 63.82 (34.33) | 77.50 (26.81) | 73.61 (27.75) | 0.5313 | 0.0598 | 0.9811 |
| Role, emotional | 49.61 (41.39) | 62.77 (40.71) | 57.50 (43.35) | 55.69 (46.19) | 72.34 (38.90) | 61.67 (40.33) | 53.33 (46.81) | 69.79 (43.47) | 60.92 (44.60) | 59.65 (47.89) | 77.78 (34.88) | 75.93 (35.80) | 0.0994 | 0.0396 | 0.9563 |
| Mental health | 68.64 (21.81) | 75.48 (19.63) | 71.25 (16.30) | 71.19 (21.07) | 78.38 (20.49) | 73.85 (20.86) | 70.67 (21.10) | 79.25 (23.82) | 75.17 (19.67) | 73.82 (17.98) | 78.93 (21.51) | 81.78 (12.96) | 0.1005 | 0.2882 | 0.4743 |
| SF-6D utility | 0.60 (0.13) | 0.65 (0.11) | 0.61 (0.10) | 0.60 (0.11) | 0.67 (0.12) | 0.63 (0.11) | 0.59 (0.12) | 0.64 (0.12) | 0.62 (0.10) | 0.63 (0.12) | 0.70 (0.10) | 0.65 (0.11) | 0.0247 | 0.0452 | 0.8993 |
Domain scores range 0–100.
Table 4 reveals that the Health Lines program is an effective intervention for helping patients with CHF improve self-maintenance so that they can continue to function in daily life. The result also illustrated a significant improvement in self-care behaviour in the intervention groups over time (p<0.05). The patient satisfaction survey indicated that patients generally felt good about the quality of the services received and thought these helped them deal more effectively with their health problems.
TABLE 4.
Mean (SD) score for Self-Care Behavior Scale survey
| Control | HL | HLM | |
|---|---|---|---|
| Baseline | 98.48 (19.19) | 105.90 (17.80) | 101.90 (19.65) |
| Follow-up survey 1 | 101.00 (15.43) | 108.59 (20.70) | 104.60 (19.29) |
| Follow-up survey 2 | 103.31 (17.70) | 106.06 (16.75) | 102.61 (19.72) |
| Follow-up survey 3 | 105.18 (19.00) | 120.77 (17.80) | 110.57 (17.52) |
Incremental Cost-effectiveness
The incremental cost-effectiveness ratios (ICERs) were calculated based on the first follow-up survey where there was a statistically significant difference in the health effects among groups. Table 5 shows the ICERs for the interventions. In order to support decision-making of mutually exclusive intervention programs, we ranked the program according to effectiveness, and then calculated the incremental cost-effectiveness ratio for each successively more effective program (e.g., incremental cost per incremental gain in QALYs). The standard approach to care was strongly dominated by HL and HLM because it was the most costly and least effective. Compared to HLM, HL was more costly and more effective. We estimated the ICER for HL compared to HLM by dividing these incremental costs by incremental effectiveness. The HL was associated with an ICER of $2,975 in generating additional QALYs.
TABLE 5.
Incremental cost-effectiveness ratio
| Program | Cost (Canadian Dollars, 2005) | Effectiveness (QALYs) | Incremental Cost-Effectiveness Ratio |
|---|---|---|---|
| HLM | $6,311 | 0.63 | |
| HL | $6,430 | 0.67 | $2,975 |
Sensitivity Analysis
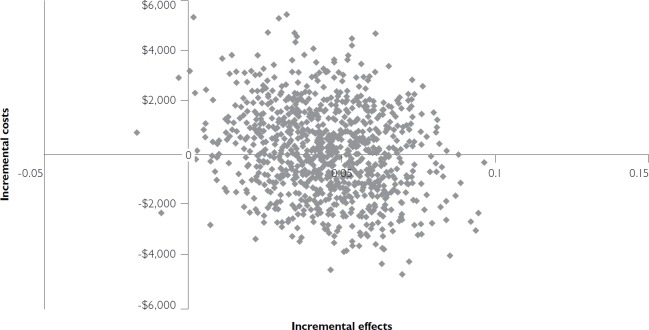
The sensitivity analysis was performed to investigate the effects of uncertainty in costs and outcomes of the intervention. A non-parametric bootstrap with replacement method was used to create 1,000 resamples of the cost and effectiveness data from all four survey points for replacement. By using this method, 1,000 replications were generated to estimate the precision of the cost-effectiveness calculation. Figure 1 demonstrates that uncertainty in ICER was estimated through bootstrapping. As 48% of replications fell in the bottom-right quadrant, illustrating that HL produced beneficial effects with lower costs, and 52% of resamples fell within the top-right quadrant of the plane, this indicates a likelihood of HL's having higher cost and better outcomes in terms of QALYs for CHF management. The simulation shows that the mean incremental cost of HL relative to HLM was $85 (95% CI: -$3,088, $3,336) once we took into account savings from healthcare utilization averted. The mean incremental effect of HL was 0.04 (95% CI: 0.01, 0.08) compared to HLM. Therefore, even though the incremental mean cost was not significant, HL produced significantly better outcomes for CHF patients.
FIGURE 1.
Incremental cost-effectiveness plane from bootstrap sampling for HL vs. HLM
The cost-effectiveness acceptability curve (CEAC) in Figure 2 shows the probability that an intervention is cost-effective compared to the alternative. The CEAC is derived from the joint distribution for incremental costs and incremental effects from the bootstrapping result and shows the probability that the decision evaluated is cost-effective (the y-axis), given joint uncertainty in model parameters for different values of the decision-maker's willingness to pay for health benefit (the x-axis). The CEAC shows a 95.4% probability that HL will be considered cost-effective if decision-makers are willing to pay up to $100,000 for an additional QALY. The most often-used threshold in the literature is $50,000/QALY (Grosse 2008); at this point, a decision to adopt the HL intervention over HLM has an 85.8% probability of being cost-effective. HL has a greater than 50% likelihood of being the more cost-effective intervention if the “willingness to pay” value is placed at $10,000.
FIGURE 2.
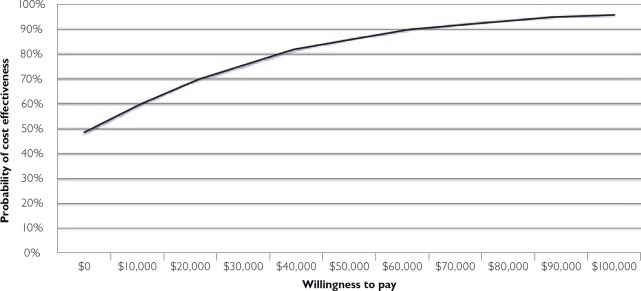
Cost-effectiveness acceptability curve for HL vs. HLM
Discussion
The results suggest that both interventions generated net health system savings through reduced utilization. Differences in costs were not significant among groups, largely because the sample size was too small. Cost-effectiveness analysis allows us to compare the benefits that patients derive from a program with the costs of offering the program. Our cost-effectiveness analysis was also limited by sample size. We measured patient satisfaction with the Client Satisfaction Questionnaire, and found that patients in all three groups were very satisfied with their treatment. There were no statistically significant differences among groups (p=0.4211). We used the SF-36, a generic health-related quality-of-life survey, to measure subjective health. Patients receiving either of the two interventions reported significantly better scores in physical functioning, physical pain, emotional health and overall health utility compared to the control group. Using an algorithm supplied by the University of Sheffield, we converted the SF-36 scores into QALYs and found that there were statistically significant differences in QALYs generated by the three programs at the time of the first survey. Our cost-effectiveness analysis demonstrated that HL is a more effective intervention for CHF than HLM, but it comes at a cost. A standard cost-effectiveness calculation demonstrated that the HL intervention could generate an additional QALY for $2,975. Finally, we conducted a sensitivity analysis to take into account the uncertainty associated with small sample sizes, and to try to generate advice helpful to decision-makers. Sensitivity analysis allows us to simulate outcomes to better estimate the probability that an intervention will be cost-effective.
When we took into account the increased QALYs generated by both interventions at all four survey points using mixed-effects repeated measures models, and combined apparent health system savings with program costs to generate a net cost, the analysis suggested that HL still generated better outcomes than HLM. Assuming that a decision-maker would be interested in implementing HL intervention, our sensitivity analysis suggests that the more important a patient's subjective quality of life becomes to the decision-maker, the more cost-effective the HL strategy becomes. The threshold of “willingness to pay” analyses aims to indicate an upper limit for cost-effectiveness; the findings indicated a greater than 95% likelihood that HL would cost no more than an additional $100,000 for additional effectiveness (in QALYs).
Conclusion
This study provides evidence that HL is more likely to be cost-effective in the management of CHF compared to the standard and HLM interventions. The cost-effectiveness of HL depends on how much decision-makers are willing to pay for an additional QALY; whether this incremental improvement in outcome represents good value for money considering the likelihood of higher healthcare costs is a value judgment. These findings add to the growing body of evidence that telehealth interventions for CHF patients have positive effects on outcomes. Despite the limitations of the data sample size in this study, our results suggest that HL is cost-effective for CHF management, assuming a willingness to pay a threshold of $10,000 for an additional QALY. On the basis of these findings, this study will guide health-care providers and policy makers who are responsible for integrating telehealth into chronic disease management, funding telehealth programs and creating policies that encourage the use of communication technology to support healthcare services and improve quality of care.
Acknowledgements
The authors thank the Manitoba Centre for Health Policy and Manitoba Health for providing data (Health Information Privacy Committee file number: 2010/2011-09). The results and conclusions presented are those of the authors. No official endorsement by the Manitoba Centre for Health Policy and Manitoba Health is intended or should be inferred.
Contributor Information
Yang Cui, PhD Student, Department of Community Health Sciences, Faculty of Medicine, University of Manitoba, Winnipeg, MB.
Malcolm Doupe, Associate Professor, Department of Community Health Sciences, Senior Research Scientist, Manitoba Centre for Health Policy, University of Manitoba Winnipeg, MB.
Alan Katz, Professor, Departments of Family Medicine and Community Health Sciences, Associate Director for Research, Manitoba Centre for Health Policy, University of Manitoba Winnipeg, MB.
Paul Nyhof, Chief Executive Officer, Providence Place, Faculty of Business Administration, I.H. Asper School of Business, University of Manitoba, Winnipeg, MB.
Evelyn L. Forget, Professor, Department of Community Health Sciences, Faculty of Medicine, University of Manitoba, Winnipeg, MB.
References
- American Telemedicine Association. 2011. “What Is Telemedicine?” Retrieved September 25, 2013. <http://www.americantelemed.org/i4a/pages/index.cfm?pageid=3333>.
- Artinian N., Magnan M., Sloan M., Lange M. 2002. “Self-Care Behaviors among Patients with Heart Failure.” Heart & Lung 31(3): 161–72 [DOI] [PubMed] [Google Scholar]
- Attkisson C., Zwick R. 1982. “The Client Satisfaction Questionnaire. Psychometric Properties and Correlations with Service Utilization and Psychotherapy Outcomes.” Evaluation and Program Planning 5: 233–37 [DOI] [PubMed] [Google Scholar]
- Brazier J., Roberts J., Deverill M. 2002. “The Estimation of a Preference-Based Measure of Health from the SF-36.” Journal of Health Economics 21: 271–92 [DOI] [PubMed] [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health. 2008. Home Telehealth for Chronic Disease Management. Retrieved September 25, 2013. <http://www.cadth.ca/media/pdf/H0475_Home_Telehealth_tr_e.pdf>. [PubMed]
- Clark R.A., Inglis S.C., McAlister F.A., Cleland J.G., Stewart S. 2007. “Telemonitoring or Structured Telephone Support Programmes for Patients with Chronic Heart Failure: Systematic Review and Meta-analysis.” British Medical Journal 334: 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M.F., Sculpher M.J., Torrance G.W., O'Brien B.J., Stoddart G.L. 2005. Methods for the Economic Evaluation of Health Care Programmes (3rd ed.). Oxford: Oxford University Press [Google Scholar]
- Finlayson G., Roos N.P., Jacobs P., Watson D. 2001. Using the Manitoba Hospital Management Information System: Comparing Average Cost per Weighted Case and Financial Ratios of Manitoba Hospitals. The Next Step. Winnipeg: Manitoba Centre for Health Policy and Evaluation: Retrieved September 25, 2013. <http://mchpappserv.cpe.umanitoba.ca/reference/mis2.pdf>. [Google Scholar]
- Grosse S.D. 2008. “Assessing Cost-Effectiveness in Healthcare: History of the $50,000 per QALY Threshold.” Expert View of Pharmacoeconomics Outcomes Research 8: 165–78 [DOI] [PubMed] [Google Scholar]
- Heart and Stroke Foundation of Manitoba. 2010. “Statistics.” Retrieved September 25, 2013. <http://www. heartandstroke.mb.ca/site/c.lgLSIVOyGpF/b.3661109/k.34F4/Statistics.htm>.
- Jennett P.A., Hall L.A., Hailey D., Ohinmaa A., Anderson C., Thomas R. et al. 2003. “The Socio-Economic Impact of Telehealth: A Systematic Review.” Journal of Telemedicine and Telecare 9: 311–20 [DOI] [PubMed] [Google Scholar]
- Katz A., Doupe M. 2009. “Preliminary Report of Research Findings from the Chronic Disease Management of Congestive Heart Failure through Health Lines Initiative.” Unpublished manuscript. [Google Scholar]
- Manitoba Centre for Health Policy. 2009. “Population Health Research Data Repository.” Retrieved September 25, 2013. <http://umanitoba.ca/faculties/medicine/units/mchp/resources/repository/index.html>.
- McHorney C.A., Ware Jr. J.E., Raczek A.E. 1993. “The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and Clinical Tests of Validity in Measuring Physical and Mental Health Constructs.” Medical Care 31(3): 247–63 [DOI] [PubMed] [Google Scholar]
- Noel H.D., Vogel D.C., Erdos J.J., Cornwall D., Levin F. 2004. “Home Telehealth Reduces Healthcare Costs.” Telemedicine and e-Health 10: 170–83 [DOI] [PubMed] [Google Scholar]
- Patra J., Popova S., Rehm J., Bondy S., Flint R., Giesbrecht N. 2007. “Economic Cost of Chronic Disease in Canada 1995–2003.” Toronto: Ontario Chronic Disease Prevention Alliance and the Ontario Public Health Association [Google Scholar]
- Schmidt S., Schuchert A., Krieg T., Oeff M. 2010. “Home Telemonitoring in Patients with Chronic Heart Failure: A Change to Improve Patient Care?” Deutsches Ärzteblatt International 107(8): 131–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E. 2008. “Cost Comparison between Telemonitoring and Usual Care of Heart Failure: A Systematic Review.” Telemedicine and e-Health 14(7): 679–86 [DOI] [PubMed] [Google Scholar]
- Smith B., Hughes-Cromwick P.F., Forkner E., Galbreath A.D. 2008. “Cost-Effectiveness of Telephonic Disease Management in Heart Failure.” American Journal of Managed Care 14(2): 106–15 [PubMed] [Google Scholar]
- Wooden A.K., Sherrard H., Fraser M., Stuewe L., Cheung T., Struthers C. 2008. “Telehome Monitoring in Patients with Cardiac Disease Who Are at High Risk of Readmission.” Heart & Lung 37(1): 36–45 [DOI] [PubMed] [Google Scholar]