Abstract
The simultaneous association of gastric carcinoma with omental mucosa-associated lymphoid tissue (MALT) lymphoma is a rare event that has not been reported previously. We focused on the hypothetic pathogenetic mechanisms, diagnosis and treatment of this rare condition. A 55-year-old woman with Helicobacter pylori infection underwent distal gastrectomy in our hospital. Three independent early gastric cancers and a mass near the cecum were diagnosed preoperatively. Pathological review of the resected stomach showed three independent early signet ring cell gastric carcinomas, and the mass in the omentum near the cecum was shown to be omental MALT lymphoma. Due to the nature of the patient's disease, she was started on medical eradication of H. pylori. Synchronous gastric adenocarcinoma and omental MALT lymphoma is a rare event. Special attention given to H. pylori-associated gastric cancer patients can avoid misdiagnosis and lead to adequate treatment.
Key Words: Early gastric carcinoma, Mucosa-associated lymphoid tissue lymphoma, Helicobacter pylori, Simultaneous, Omentum
Introduction
Gastric cancer is one of the most common malignancies which counts for more than 90% of gastric cancers [1] whereas the incidence of primary mucosa-associated lymphoid tissue (MALT) lymphoma is approximately 7.6% of all non-Hodgkin's lymphomas [2]. MALT lymphoma is an indolent, low-grade, mature small B cell non-Hodgkin's lymphoid neoplasm that represents the paradigm for the association between tumorigenesis and a chronic inflammatory stimulus. Although rare, these neoplasms are clinically relevant due to their unique place in the oncology spectrum as a malignancy that, in many cases, can be cured with antibiotic therapy [3]. A close association has been suggested between gastric cancer and chronic Helicobacter pylori infection; also, H. pylori infection is now considered to be a cause of gastric MALT lymphoma. The inflammatory state caused by this microbacterium is presumed to be the pathogenesis of both neoplasms [4]. The simultaneous association of gastric carcinoma with omental MALT lymphoma is a rare event. Primary gastric carcinoma and MALT lymphoma are both thought to be associated with H. pylori infection [5]. There have been several reports in the literature on the association of gastric carcinoma and gastric MALT lymphoma, but the occurrence of these two neoplasms in the same patient is a rare event [6]. As far as we know, synchronous occurrence of gastric adenocarcinoma and primary omental MALT lymphoma has not been reported. The purpose of this article is to report a case of the simultaneous association of gastric carcinoma and omental MALT lymphoma and to focus on hypothetic pathogenetic mechanisms, diagnosis and treatment of this rare condition.
Case Report
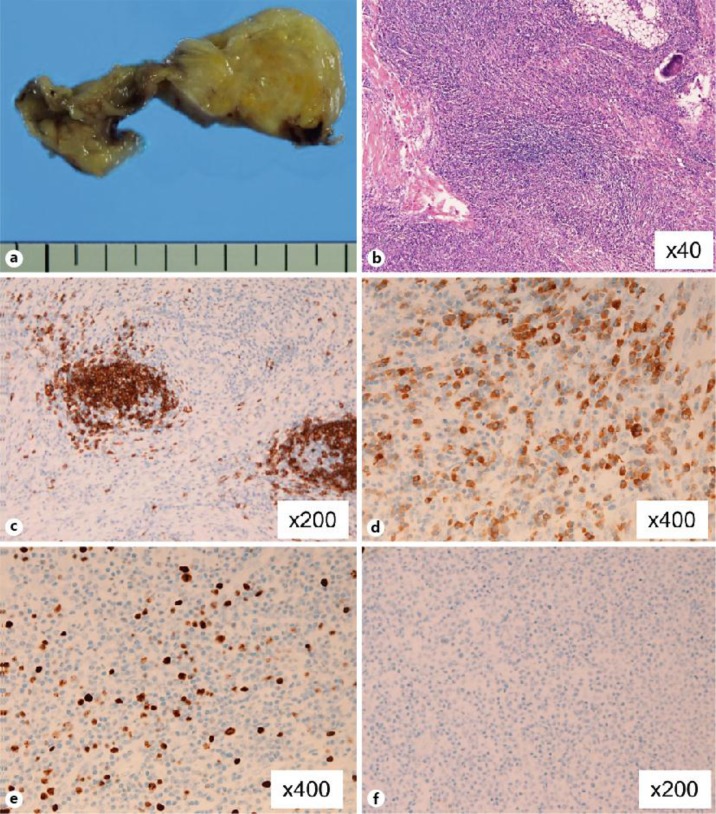
A 55-year-old woman was referred to our hospital with a history of appetite loss, right inguinal pain and weight loss. Endoscopy revealed a type 0-IIc tumor in the forecourt of the pylorus and entire wall thickening in the pylorus. Biopsy showed it to be signet ring cell carcinoma. H. pylori antigen in the urine was positive. Laboratory tests including blood counts, liver and renal function tests, tumor markers such as carcinoembryonic antigen and carbohydrate antigen 19-9 were within normal limits. Upper gastrointestinal series showed the tumor limited in the stomach. Computed tomography showed no lymph node swelling or distant metastasis. Therefore, distal gastrectomy with D2 lymph node resection was performed under the diagnosis of gastric carcinoma T1N0M0 (fig. 1). During operation a mass 2.0 × 3.0 cm in size was observed in the omentum near the cecum. The mass was also resected for pathological examination. The postoperative course was uneventful, and the patient was discharged on the 16th postoperative day. Pathological review of the surgical specimen showed three independent early gastric cancer (No. 1: 0-IIc, T1b(sm), sig.; No. 2: 0-IIc, T1b(sm), sig.; No. 3: 0-IIc, T1a(m), sig.) (fig. 2). No regional lymph node metastases were seen. Grossly, the peritoneal lesion showed a tumorous growth measuring 2.0 × 3.0 cm (fig. 3a). Microscopically, the lesion was composed of tumorous proliferation of small lymphocytes (fig. 3b). The lymphoid cells were composed of centrocytic and monocytic lymphoma. Little atypia was seen. A few mitotic figures were present. No lymphoepithelial lesions were seen because it was a peritoneal lesion. Plasmacytic differentiation was noted. Immunohistochemically, the lymphoid cells were positive for CD20 (+++) (fig. 3c), CD79a (++), CD138 (++) (fig. 3d), Ki67 (labeling = 10%) (fig. 3e), CD45RO (+), l-chain (+++), k-chain (+) and CD15 (+++), and negative for cytokeratin AE1/3, cytokeratin CAM5.2, CD3, CD30 (fig. 3f), CD10, CD34, CD56 and p53. The stainings of the light chains suggested positive light chain restriction. The diagnosis of primary omental MALT lymphoma was made. Due to the nature of this patient's disease, she was started on medical eradication of H. pylori.
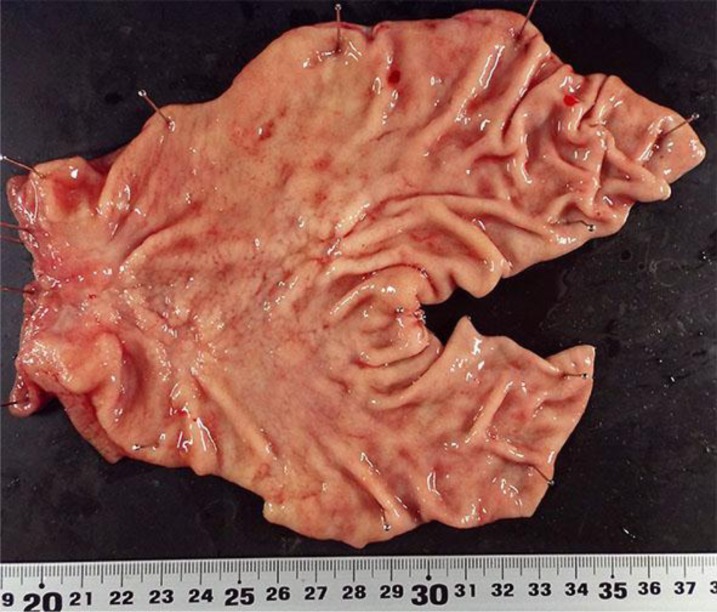
Fig. 1.
The resected specimen revealed three independent type 0-IIc early carcinomas in the forecourt of the pylorus and in the pylorus.
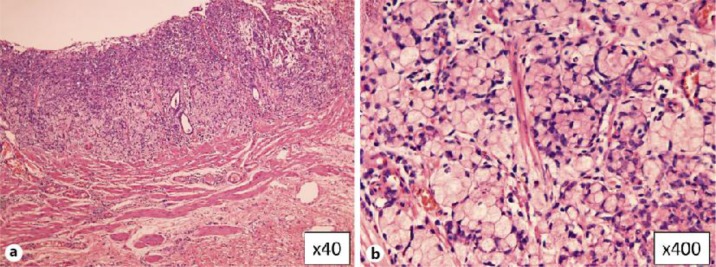
Fig. 2.
Histological findings of the resected specimen from the type 0-IIc early gastric carcinoma within the submucosa (a). High-power photomicrography revealed signet ring cell carcinoma (b).
Fig. 3.
Grossly, the peritoneal lesion showed a tumorous growth measuring 2.0 × 3.0 cm (a). Microscopically, the lesion was composed of tumorous proliferation of small lymphocytes (b). The lymphoid cells were composed of centrocytic and monocytic lymphoma. Little atypia was seen. A few mitotic figures were present. No lymphoepithelial lesions were seen. Plasmacytic differentiation was noted. Immunohistochemically, the lymphoid cells were positive for CD20 (+++) (c) and CD138 (++) (d), and Ki67 labeling was 10% (e). CD30 was negative for staining (f).
Discussion
An important question in the present case is the pathogenicity of the two different malignancies. H. pylori infection is thought to be an important aspect of developing gastric carcinoma. H. pylori infection leads to chronic gastritis: it activates neutrophils which produce nitric oxide, superoxides and hydroxyl ions, resulting in DNA damage and mutations and eventually gastric carcinogenesis [7]. On the other hand, H. pylori helps T cell activation, lymphoid follicle formation and B cell proliferation [8]. It is thought that the H. pylori antigen interacts with CD4 cells binding to B cells in the marginal zone, causing hyperproliferation of B cells. MALT lymphoma can arise from various MALT organs by expressing special homing receptors and adhesion molecules, or by either microbial antigens or autoantigens [9]. The omentum is an active immunologic organ full of lymphoid tissue. The inflammatory or cancer-bearing state of the gastric tissue could have allowed passage of the bacterium itself into the interperitoneal space, leading to a hyperlymphoproliferative lesion in the omentum. In a related note, the lymphoproliferative state in a distant organ can be explained as an aberrant autoimmune response induced by molecular mimicry due to H. pylori infection [10].
Since it is a rare phenomenon, preoperative diagnosis of omental MALT lymphoma is difficult. CT findings of peritoneal lymphomatosis closely mimic diffuse carcinomatosis [11], as experienced in the present case. A biopsy and fluid samples could have been useful in the present case if ascites had been observed preoperatively. Awareness is important when a peritoneal mass is observed in patients with H. pylori and gastric cancer. Pathological diagnosis during surgery should be examined carefully because the surgical procedures may change depending on it.
Clear guidelines for management have not yet been established for synchronous adenocarcinoma and MALT lymphoma, as the condition is rare. Eradication of H. pylori, has been proved to reduce gastric cancer and gastric MALT lymphoma [12, 13]. We speculate that H. pylori eradication would be effective in omental MALT lymphoma because H. pylori helps T cell activation, lymphoid follicle formation and B cell proliferation, leading to lymphomatogenesis.
In conclusion, synchronous gastric adenocarcinoma and omental MALT lymphoma is a rare event. Non-gastric MALT lymphomas are radiologically and clinically unspecific and can be misdiagnosed as cancer dissemination. Special attention given to H. pylori-associated gastric cancer patients can avoid misdiagnosis and lead to adequate treatment.
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology. 1982;82:228–231. [PubMed] [Google Scholar]
- 2.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 3.Pereira MI, Medeiros JA. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J Gastroenterol. 2014;20:684–698. doi: 10.3748/wjg.v20.i3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasbarrini A, Franceschi F, Cammarota G, Pola P, Gasbarrini G. Vascular and immunological disorders associated with Helicobacter pylori infection. Ital J Gastroenterol Hepatol. 1998;30:115–118. [PubMed] [Google Scholar]
- 5.Bandar A, Crowe SE. Helicobacter pylori in gastric malignancies. Curr Gastroenterol Rep. 2012;14:489–496. doi: 10.1007/s11894-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 6.Chan AO, Chu KM, Yuen ST, Leung SY, Lam SK, Wong J. Synchronous gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma in association with Helicobacter pylori infection: comparing reported cases between the East and West. Am J Gastroenterol. 2001;96:1922–1924. doi: 10.1111/j.1572-0241.2001.03895.x. [DOI] [PubMed] [Google Scholar]
- 7.Saito M, Suzuki K, Maeda T, Kato T, Kamiyama H, Koizumi K, Miyaki Y, Okada S, Kiyozaki H, Konishi F. The accumulation of DNA demethylation in Sat? in normal gastric tissues with Helicobacter pylori infection renders susceptibility to gastric cancer in some individuals. Oncol Rep. 2012;27:1717–1725. doi: 10.3892/or.2012.1718. [DOI] [PubMed] [Google Scholar]
- 8.D'Elios MM, Amedei A, Manghetti M, Costa F, Baldari CT, Quazi AS, Telford JL, Romagnani S, Del Prete G. Impaired T-cell regulation of B-cell growth in Helicobacter pylori-related gastric low-grade MALT lymphoma. Gastroenterology. 1999;117:1105–1112. doi: 10.1016/s0016-5085(99)70395-1. [DOI] [PubMed] [Google Scholar]
- 9.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 10.Mackay IR, Rose NR. Autoimmunity and lymphoma: tribulations of B cells. Nat Immunol. 2001;2:793–795. doi: 10.1038/ni0901-793. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y, Cho O, Song S, Lee H, Rhim H, Koh B. Peritoneal lymphomatosis: CT findings. Abdom Imaging. 1998;23:87–90. doi: 10.1007/s002619900292. [DOI] [PubMed] [Google Scholar]
- 12.Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, Grilli D, Bazzoli F. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–128. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 13.Bayerdörffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]