Abstract
Implication for health policy/practice/research/medical education:
The current literature indicates that maintaining adequate vitamin D levels may be an important consideration in the treatment of hypertension, especially in individuals with vitamin D insufficiency and deficiency.
Keywords: Vitmain D, Renin-angiotensin system, Blood pressure, Hypertension
In humans, 80-90% of required vitamin D is made in the skin upon sun exposure and the remaining 10-20% is ingested in fish, eggs and fortified dairy products. In the liver, vitamins D2(ergocalciferol) and D3(cholecalciferol) are converted into 25-hydroxyvitamin D2 (25[OH]D2) and 25-hydroxyvitamin D3 (25(OH]D3), respectively (1). In the kidney, 25(OH)D is converted to its biologically active form 1,25-dihydroxyvitamin D (1,25[OH]2D) by 1α-hydroxylase. The serum level of 25(OH)D is used to determine vitamin D status and normally ranges between 30-100 ng/mL (75–250 nmol/L). Vitamin D insufficiency and deficiency are defined as serum 25(OH)D levels of 20-30 mg/dL (50-75 nmol/L) and <20 ng/mL (50 nmol/L), respectively (2). The critical role of 1,25(OH)2D in calcium and phosphorus homeostasis is well established (1,2). Accumulating evidence indicates that vitamin D also plays an important role in regulating the blood pressure.
A recent randomized, double-blind, placebo controlled study conducted by Nasri et al. demonstrated that oral supplementation of vitamin D led to a decrease in the blood pressure in individuals with diabetes mellitus (3). Sixty individuals were randomized to receive either vitamin D3 50,000 IU per week (n=30) or placebo (n=30) for 12 weeks. Five individuals (8.3%) had vitamin D deficiency and 27 others (45%) had vitamin D insufficiency. Vitamin D supplementation increased serum 25(OH)D levels from 84±52 nmol/L to 164±57 nmol/L (P= 0.001). Vitamin D supplementation led to a decrease in both systolic (121±13 to 110±9 mm Hg; P= 0.001) and diastolic blood pressure (81±8 to 76±7 mm Hg, P= 0.046). No statistically significant change in blood pressure was observed in the placebo group.
The pivotal role of the renin-angiotensin system in the regulation of the blood pressure is well established (Figure 1). Renin is secreted by the juxtaglomerular cells of the kidney in response to decreased renal blood flow. It converts plasma angiotensinogen to angiotensin I, which is then converted to angiotensin II by angiotensin-converting enzyme. Angiotensin II leads to increased water and sodium reabsorption in the kidney and vasoconstriction. Mounting evidence indicates that vitamin D regulates the renin-angiotensin system. An inverse relationship between the blood pressure and serum 25(OH)D levels has been documented in a number of the epidemiological studies (4,5). In a cross-sectional study, Forman et al. explored the relation between 25(OH)D and the renin-angiotensin system in 184 individuals with normal blood pressure (6). Compared with vitamin D-sufficient individuals, those with vitamin D deficiency and insufficiency had greater plasma angiotensin II levels and a trend for higher plasma renin activity. In addition, the activity of the renin-angiotensin system in the kidney, as measured by the renal plasma flow in response to angiotensin II infusion, was greater in vitamin D-deficient than in vitamin D-sufficient individuals. These results suggested that decreased plasma 25(OH)D levels were associated with increased activity of the renin angiotensin system. Resnick et al. investigated the relation between the plasma renin activity and calcium-regulating hormones including calcitonin, 1,25(OH)2D3 and parathyroid hormone in 51 individuals with essential hypertension (7). An inverse relation of the serum 1,25(OH)2D3 level to the plasma renin activity (r= −0.65, P< 0.001) was observed. This study corroborated a link between calcium homeostasis, vitamin D metabolism and the renin-angiotensin system.
Figure 1 .
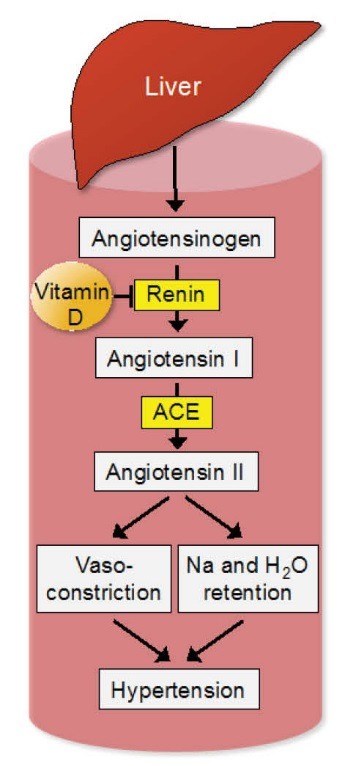
The effects of vitamin D on the renin-angiotensin system. 1,25-dihydroxyvitamin D suppresses renin gene expression, thereby inhibiting the renin-angiotensin system. ACE, angiotensin-converting enzyme; H2O, water; Na, sodium.
To unravel the molecular mechanisms involved in vitamin D-mediated regulation of the renin-angiotensin system, mice with loss-of-function mutations involving vitamin D receptor gene (VDR-null mice) were examined. The VDR-null mice develop hypocalcemia at 3 weeks of age, hyperparathyroidism, rickets and osteomalacia (8). Li et al. demonstrated that renin gene expression in the kidney and angiotensin II levels in the plasma were substantially increased in the VDR-null mice leading to hypertension and cardiac hypertrophy (9). In wild-type mice, pharmacological inhibition of 1,25(OH)2D3 synthesis led to an increase in renin gene expression, whereas 1,25(OH)2D3 treatment suppressed renin expression. Similarly, 1,25(OH)2D3 markedly suppressed renin gene expression in cultured cells by a VDR-mediated mechanism. These investigators also demonstrated that the effect of vitamin D on renin gene expression was independent of calcium metabolism. The authors concluded that 1,25(OH)2D3 is a negative regulator of the renin-angiotensin system. Subsequently, it was shown that 1,25(OH)2D3 suppressed renin gene expression by binding to the transcription factor cAMP response element-binding protein (CREB), thereby suppressing the activity of the cAMP response element in the renin gene promoter (10).
To determine whether the inhibitory effects of 1,25(OH)2D on the renin-angiotensin system is dependent on calcium or phosphorous, Zhou el al. examined the effects of dietary interventions in 1α-hydroxylase knockout mice. Phenotypically resembling VDR-null mice, 1α-hydroxylase knockout mice demonstrate undetectable serum 1,25(OH)2D levels and develop hypocalcemia, hypophosphatemia, secondary hyperparathyroidism, growth retardation and skeletal abnormalities characteristic of rickets (11). Zhou et al. demonstrated that 1α-hydroxylase knockout mice developed hypertension and cardiac hypertrophy associated with the activation of the renin-angiotensin system in the kidney and heart (12). Despite the normalization of the serum calcium and phosphorus levels, a calcium- and phosphorus-fortified diet neither normalized the blood pressure nor suppressed the activity of the renin-angiotensin system in the 1α-hydroxylase knockout mice. However, 1,25(OH)2D treatment led to the normalization of the activity of the renin-angiotensin system and blood pressure.
In summary, the current literature indicates that maintaining adequate vitamin D levels may be an important consideration in the treatment of hypertension, especially in individuals with vitamin D insufficiency and deficiency.
Authors’ contributions
All authors wrote the paper equally.
Conflict of interests
Authors declare no conflicts of interest.
Funding/Support
This work received no funding from public, commercial or not-for-profit organizations.
Please cite this paper as: Ajabshir S, Asif A, Nayer A. The effects of vitamin D on the renin-angiotensin system. J Nephropathol. 2014; 3(2): 41-43. DOI: 10.12860/jnp.2014.09
References
- 1.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Nasri H, Behradmanesh S, Ahmadi A, Rafieian-Kopaei M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial . J Nephropathol. 2014;3(1):29–33. doi: 10.12860/jnp.2014.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20(7):713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Hintzpeter B, Mensink GB, Thierfelder W, Müller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62(9):1079–89. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 6.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin systemin humans. Hypertension. 2010;55(5):1283–8. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resnick LM, Müller FB, Laragh JH. Calcium-regulating hormones in essential hypertensionRelation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105(5):649–54. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 8.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R. et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE. et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282(41):29821–30. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 11.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN. et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98(13):7498–503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–9. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]


