Abstract
Background: Chronic allograft nephropathy (CAN) is a common cause of delayed allograft failure throughout the world. Its prevalence and risk factors vary depending on a number of factors. There is little information on the prevalence and risk factors for early CAN in live related renal transplant patients.
Objectives: We aimed to determine the prevalence and the risk factors of early CAN in our setup.
Patients and Methods: The study was conducted at Sindh Institute of Urology & Transplantation (SIUT), Karachi, from 2002 to 2005 on patients who had live related kidney transplantation and underwent at least one allograft biopsy within 18 months of transplantation. The biopsies were performed and prepared in accordance with established indications and guidelines. The Banff 97 classification and its updates were used to diagnose and categorize the biopsy pathology. Patients were divided into two groups depending on the presence or absence of CAN on biopsies. Following parameters were compared among the groups: age, sex, human leukocyte antigen (HLA) match, immunosuppression used, acute rejection (AR) episodes, urinary tract infections (UTIs), viral infections, cyclosporine levels, early and late graft function monitored by serum creatinine.
Results: A total of 164 patients fulfilled the study inclusion criteria. The mean age of recipients and donors was relatively young. The majority of the donors were siblings. The overall prevalence of CAN was 25.6% (42/164), between 3 and 18 months post transplantation. The median time to the appearance of CAN was 9 months post-transplant. The prevalence of CAN increased as post-transplant duration increased. In 39 (92.8%) subjects, CAN was detected on the second or subsequent graft biopsy. Only 3 (7.2%) patients showed CAN on the first graft biopsy. The majority of cases belonged to moderate degree or grade II CAN. The mean serum creatinine values were higher in the CAN group at the time of discharge and all times post-transplantation.
Conclusions: In conclusion, the results show that serum creatinine at the time of discharge is a useful predictor of later development of chronic changes in the allograft. Further studies are needed to identify the risk factors for the early development of chronic changes in living related renal transplant program.
Keywords: Delayed graft failure, Graft biopsy, Live related, Rejection, Transplant outcome
1. Introduction
Kidney transplantation is the treatment of choice for patients with end-stage renal disease (ESRD) (1). The overall outcome of kidney transplantation has improved significantly during the last few decades due to improved surgical techniques, better medical care, prevention and treatment of infections, and above all, due to advances in the immunosuppressive regimens (2). In this context, the contribution of the new immunosuppressive agents has been very important, since the incidence of acute rejection (AR) during the first few months after transplantation has fallen dramatically by as much as 15-20%. Even though the graft outcome has improved markedly during the first year (90-95%), the long-term survival has not changed much, for two main reasons; the continuous loss of grafts after the first year, and the death of patients with a functioning graft, as a result of cardiovascular involvement (3,4).
Chronic allograft nephropathy (CAN), now renamed as interstitial fibrosis/tubular atrophy (IFTA), is the major cause of graft loss after the first year post transplant, and is followed by the death of the patient with a functioning graft. It is currently the main problem for kidney transplant recipients (KTRs). The importance of this lesion can also be judged from the fact that chronic allograft failure is one of the main causes of chronic renal insufficiency in many countries (5-10). In fact, nearly 20% of all transplants carried out in the USA are for patients who have already had one or two previous transplants (3,4).
The overall prevalence and incidence of CAN in renal allograft biopsies depends on the timing and the indication of the graft biopsies. In protocol biopsies, it prevalence has been reported as high as 94% (Grade I) in first year post-transplantation and up to 100% after 10 years (5-10). The causes and the natural history of CAN vary depending on a number of factors, including the donor source and age (11-15). There are very few studies on the prevalence of CAN in a live related renal transplant setting, especially from the developing countries (16). We have earlier reported an overall prevalence of CAN in the largest renal allograft biopsy series of 29.8% in such a setting (17).
2. Objectives
In this study, we aimed to determine the prevalence and the risk factors for early CAN in our KTRs in a live related renal transplant program.
3. Patients and Methods
This was a retrospective observational study carried out at Sindh Institute of Urology & Transplantation (SIUT), Karachi, from January 2002 to December 2005. Those patients were included who had live related donor transplantation in the same period of time and had undergone at least one allograft biopsy within 18 months of transplantation. The Banff 97 classification and its updates were used to diagnose and categorize the lesions (18-20). The biopsies were performed when there was unexplained rise in serum creatinine >20% above baseline value in accordance with established indications and procedures (17). Two cores of renal graft biopsy were obtained percutaneously with the help of an automatic biopsy gun under ultrasound guidance from the lower pole of the allograft. A third core was also obtained for C4d study in cases of suspicious humoral rejection. The cores were examined immediately under the dissection microscope by a histotechnician to ascertain the presence of cortex and glomeruli in the specimen. The tissue for light microscopy (LM) was transferred immediately into a bottle containing 10% neutral buffered formalin and fixed for 1 hour prior to urgent processing in autoprocessor. The urgent processing takes 2 hours to complete, after which paraffin blocks were made and cut at 3-4 um thickness. Ten serial sections were obtained. Levels 1, 6 and 10 were stained with hematoxylin and eosin (H&E) stain, level 8 and 11 with PAS, level 7 with trichrome and level 9 with silver stain, as described in our earlier report (17). Further sections were cut and stained for any special stain if requested by the pathologist. The sections were examined by two renal pathologists first independently and then jointly and classified according to the Banff criteria.
The tissue for C4d was snap-frozen for immunofluorescence (IF) examination. Sections were at a thickness of 5-6 um and stained for C4d by the indirect IF method, using commercially available anti-C4d antibody (Quidel, San Diego, CA, USA). The stained slides were examined under the epifluorescence microscope and interpreted according to the Banff 2007 classification (20).
Early CAN was arbitrarily defined as chronic fibrosing changes occurring within one and a half year of transplantation. Patients were divided into two groups depending on the presence or absence of CAN on graft biopsy. Following parameters were analyzed and compared among the groups: age, sex, human leukocyte antigen (HLA) match, immunosuppression used, numbers of AR episodes, episodes of urinary tract infection (UTI), viral infections, cyclosporine exposure, early and late graft functions monitored by serum creatinine on the day of discharge, at 4, 8, and 12 weeks, and 6, 9, and 12 months.
The immunosuppression was based on triple regimen comprising steroids, azathioprine or mycophenolate mofetil (MMF) and cyclosporine (CsA). Methylprednisolone was given as a bolus dose of 15 mg/kg during surgery followed by oral prednisolone 0.5 mg/kg/day from first post-operative day and decreased to 0.15 mg/kg/day at the end of third month post-transplant. CsA was initially started at 8 mg/kg/day and was later titrated according to trough blood levels. The area under curve (AUC) was recorded in the first week of post transplantation. Azathioprine was given in a dose of 1.5 mg/kg/day and MMF was given in a dose of 500-750 mg twice a day. Induction therapy was used in a total of 21 patients and the agents used were antithymocyte globulin (ATG) or simulect.
For CsA monitoring, C0 and C2 were measured in whole blood sample. The sample was taken 5 minutes prior to the next dose for C0 and 2 hours after the morning dose for C2 levels. CsA levels were performed on Abbot Tdx analyzer by fluorescence polarization immunoassay (FPIA) technique. To calculate AUC level, samples were obtained at zero, two, three, and six hours of the next dose and twelve hours peak was substituted by trough level, then the result was calculated by trapezoid rule.
Cytomegalovirus (CMV) antigenemia assay was done by indirect IF test. BK virus detection was done by quantitative real time polymerase chain reaction (PCR) using Qiagen® QIA amp DNA mini extraction kit. Three samples of urine and one sample of serum were tested.
3.1. Tissue typing
HLAs in each donor and recipient pair were assessed by serological techniques. Briefly, after aseptic precautions, 20 ml of peripheral blood was drawn from donor and recipient pair. The lymphocytes were isolated from the blood by adding lymphocyte preparing medium (lymphodex). From the separated lymphocytes, T enriched and B enriched cell populations were separated by using immunomagnetic beads. The separated B and T cells were used for HLA A and B and HLA DR typing, respectively, by utilizing National Institute of Health (NIH) standard complement dependent micro-cytotoxicity (CDC) technique on one LAMDA commercial trays for both antigens. Finally, HLA class I and II antigens were screened on inverted fluorescence microscope. The lymphocyte cross-match was performed by utilizing standard NIH method.
3.2. Data analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS) version 10.0 computer program (SPSS, Chicago, IL, USA). Descriptive statistics such as mean ± SD were used for continuous variables and numbers (percentages) for categorical data. Differences between groups were examined using χ2 -test for demographic variables and student’s t-test for quantitative variables. Results were considered significant at a p value of <0.05.
4. Results
A total of 164 patients fulfilled the study inclusion criteria. The key patient demographic and clinical characteristics of the study subjects are shown in Table 1. The mean age of KTRs was 28.0±9.1 years and of donors, 31.4±7.2 years. It is evident that the mean age of recipients and donors in our setup was relatively young. Males were greatly predominant in recipients (78.6% vs. 21.4% females). On the other hand, among donors they only slightly outnumbered females (52.4% vs. 47.5% females). The majority of cases of ESRD belonged to the unknown category (75%) and majority of the donors are siblings (53.6%). Table 2 lists the main immunological risk factors and ischemia times, showing overall good antigen match and brief ischemia times. The vast majority of recipients (85.36%) shared at least one haplotype.
Table 1. The main demographic, clinical and laboratory characteristics of 164 renal transplant recipients and their donors included in the present study.
| Recipients | |
| Mean age±SD, in years | 28.0 ± 9.1 |
| Sex, n (%) | |
| Male | 129 (78.6%) |
| Female | 35 (21.4%) |
| Causes of ESRD, n (%) | |
| Unknown | 123 (75%) |
| Glomerulopathies | 5 (3%) |
| Stones | 30 (18.3%) |
| Diabetes mellitus | 6 (3.7%) |
| Donors | |
| Mean age±SD, in years | 31.4 ± 7.2 |
| Sex, n (%) | |
| Male | 86(52.4%) |
| Female | 78(47.5%) |
| Relations, n (%) | |
| Siblings | 88 (53.6%) |
| Parents | 49 (29.9% |
| Offspring | 4 (2.4%) |
| Spouse | 8 (4.9%) |
| Cousins | 15 (9.1% |
|
Serum creatinine clearance (ml/min) |
120.6301±19.7126 |
Table 2. Some immunological and technical parameters of 164 renal transplant patients included in the study.
| Antigen matching | |
| HLA matches | |
| ≥ 3 Antigen | 140 (85.36%) |
| < 3 Antigen | 24 (14.63%) |
|
Ischemia times and delayed graft
function |
|
|
Cold ischemia time, Mean ± SD (in minutes) |
141.9 ± 55.2 |
|
Warm ischemia time, Mean ± SD (in minutes) |
10.4 ± 21.3 |
| Delayed graft function | 11 (6.7%) |
|
Immunosuppression and
rejection rates |
|
| Immunosuppression drugs | |
| Prednisone | 164 (100%) |
| Cyclosporine A | 164 (100%) |
| Azathioprine | 153 (93.3%) |
| Mycophenolate mofetil | 11 (6.7%) |
| Acute rejection | 38 (23.2%) |
AR was found in 38 (23.2%) cases on renal allograft biopsies done for graft dysfunction and the overall prevalence of CAN was 25.6%, being found in 42 of the biopsies performed between 3 and 18 months post-transplantation. The median time to the appearance of CAN was 9 months post-transplant. Figure 1 shows the rise in the prevalence of CAN as post-transplant duration increased. In 39 (92.8%) subjects, CAN was detected on the second or subsequent graft biopsy. Only 3 (7.2%) patients showed CAN on the first graft biopsy. Table 3 shows the grades and types of CAN. As is evident from this table, the majority of cases (59.5%) belonged to moderate degree or grade II CAN and the “a” subtype (76.2%), the later indicating no specific etiology. Table 4 shows a comparison of numerous variables among the transplant patients with CAN and those without CAN with corresponding p values. Only recipients’ gender and CMV and polyoma virus infections showed statistically significant differences in the two groups. Figure 2 shows the values of mean serum creatinine at different time periods post-transplant in the two groups. It is apparent that the patients in the CAN group had higher mean values of serum creatinine at all times post-transplantation.
Figure 1.
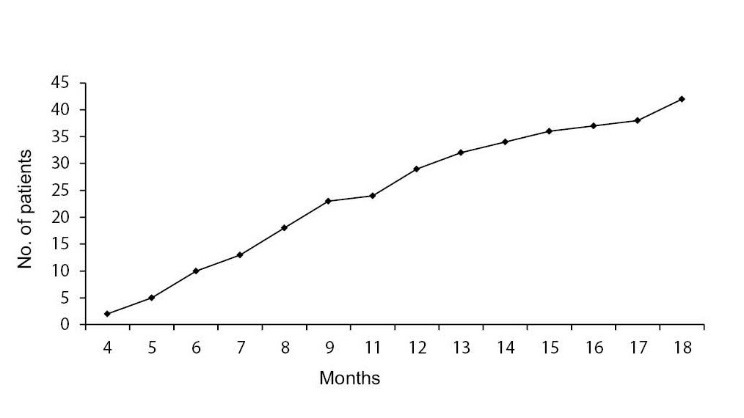
The rise in the prevalence of chronic allograft nephropathy (CAN) diagnosis as post-transplant duration increases.
Table 3. Grading of chronic allograft nephropathy (CAN) according to the Banff 97 classification .
| CAN Grades | Number of patients | Percentage |
| Ia | 12 | 28.6 |
| IIa | 15 | 35.7 |
| IIb | 10 | 23.8 |
| IIIa | 05 | 11.9 |
| Total | 42 | 100 |
Table 4. The comparison of main demographic, clinical and laboratory features among early chronic allograft nephropathy (CAN) positive (n=42) and CAN negative (n=122) groups.
| CAN | No CAN | p value | |
| Recipients’ age, mean±SD, in years | 27.6±8.0 | 28.1±9.5 | 0.73 |
| Recipients’ sex, n(%) | |||
| Male | 39 (90.9%) | 91 (74.4%) | 0.02 |
| Female | 4 (9.5%) | 31 (25.1%) | |
| Donors’ sex, n(%) | |||
| Male | 39 (90.9%) | 91 (74.4%) | 0.72 |
| Female | 4 (9.5%) | 31 (25.1%) | |
| Human leukocyte antigen (HLA) match, n(%) | |||
| >3 antigen | 19(45.2%) | 70 (57.4%) | 0.17 |
| ≤ 3 antigen | 23(54.8%) | 52 (42.3%) | |
| Antithymocyte globulin (ATG), n(%) | 3 (7.1%) | 9 (7.4%) | |
| Immunosuppressive regimen, n(%) | |||
| Pred, CyA, Aza | 40 (95.2%) | 113 (92.6%) | 0.55 |
| Pred, CyA, MMF | 2 (4.8%) | 9 (7.4%) | |
| Donors’ creatinine clearance (ml/min) | 121.8878±21.1883 | 120.9445±20.0328 | 0.72 |
| Delayed graft function (DGF), n(%) | 4 (9.5%) | 7 (5.7%) | 0.39 |
| Early graft function, n(%) | 38 (90.5%) | 115 (94.3%) | 0.92 |
| ATG for induction/DGF, n(%) | 3 (7.1%) | 9 (7.4%) | |
| Secondary surgery, n(%) | 6 (11.9%) | 7 (5.7%) | 0.19 |
| Acute tubular necrosis on early biopsy, n(%) | 9 (21.4%) | 26 (21.3%) | 0.98 |
| Dialysis required in early period, n(%) | 3 (7.1%) | 8 (6.6%) | 0.96 |
| Acute cellular rejection, n(%) | 6 (14.3%) | 18 (14.4%) | 0.94 |
| Acute vascular rejection, n(%) | 6 (14.3%) | 8 (6.6%) | 0.12 |
| Cyclosporin A (CyA) toxicity, n(%) | 5 (11.9%) | 17 (13.9%) | 0.73 |
| AUC of CyA (Mean±SD) | 6293±2431 | 6716±2012 | 0.28 |
| Cytomegalovirus infection, n(%) | 5 (13.2%) | 1 (0.8%) | 0.001 |
| Polyoma virus infection, n(%) | 6 (14.3%) | 1 (0.8%) | 0.0001 |
| Urinary tract infections, n(%) | 10 (23.8%) | 24 (19.7%) | 0.56 |
Figure 2 .
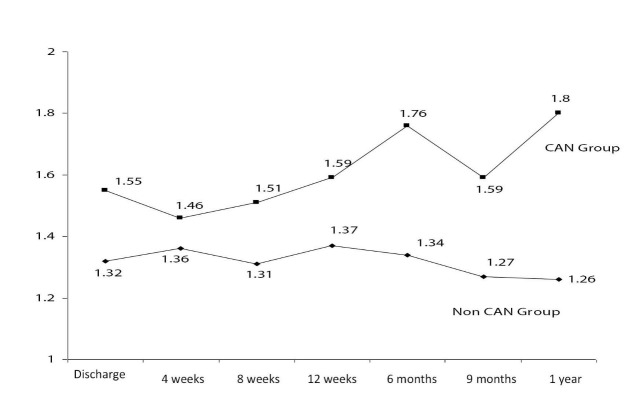
The mean serum creatinine at different time points post-transplant in the two groups is shown. Patients who subsequently showed early chronic allograft nephropathy (CAN) on biopsy had higher serum creatinine at first hospital discharge and all time periods before or after the detection of CAN.
5. Discussion
Although one-year kidney graft survival has changed dramatically with the introduction of modern immunosuppressive agents to more than 90%, but the longevity of the graft has not changed much. The main culprit responsible for this dismal long-term outcome is the CAN, the prevalence of which approaches 100% of the grafts after 10 years. Traditionally, it was thought that, once the diagnosis of CAN has been made, it is difficult to alter the course of progression and the final outcome. However, more recently, with the availability of new immunosuppressants with antifibrotic properties, it appears that this process can be stopped and even reversed if diagnosed early. We designed this study to investigate the prevalence of early CAN and to determine the potential risk factors in a setting of live related renal transplantation.
The overall prevalence of CAN was 25.6% of all biopsies in this study. This is low as compared with the other studies, mostly from the western world. This may be because of the younger age of both the recipients and donors in our program. According to US scientific renal transplant registry, 1990-1998, the risk of graft loss is double for recipients of kidneys from donors older than 55 years (10-15). The effect of donor age explains about 30% of variance in kidney transplant outcome beyond 1 year (10). Kidneys from donors older than 55 years have an increased risk of chronic graft failure. These findings are ascribed to the reduced renal mass, leading to glomerular hypertension, or more recently, to accelerated senescence (11,14). Furthermore, it has been suggested that the higher rate of AR episodes in kidneys from older donors reflects increased immunogenicity. Elderly donors have age dependent progressive reduction of glomerular filtration rate and renal reserve (14). In this study, the mean age of both the donors and recipients was low with no significant difference in the mean values between the two groups (p= 0.45). The United Network of Organ Sharing (UNOS) data also shows that the results are worse for recipients above 50 years. The main cause of graft failure is death with a functioning graft, and as expected, the older the recipient age, higher the risk of death. It is assumed that the risk of graft failure caused by acute or chronic rejection tends to decrease with age (3,4). In our study, the recipients were also of younger age group with no significant difference in the mean age of the two groups (p= 0.730). In addition, we included only graft biopsies performed within 18 months of transplantation. It is well known that the prevalence of CAN increases as the post-transplantation duration increases.
Graft survival is better in female recipients of male donor kidneys, an effect usually ascribed to nephron-dose, although factors relating to patient survival, underlying diseases, sensitization and other factors complicate such analyses. Females have more active immune responses and pregnancy could affect female responsiveness to alloantigens through sensitization, tolerance or persistent microchimerism (11). The gender of the living donors in the USA is more frequently male consisting of 60% of the live donor population (3,4). Female renal transplant recipients have an increased relative risk for AR compared with male renal transplant recipients. In contrast, women have a decreased relative risk for the development of chronic allograft failure. This decreased risk for chronic allograft failure is age dependent, with the younger patients demonstrating little difference between men and women (3,4). In our study, out of 42 patients who developed CAN, 38 (90.5%) were male recipients. Only 4 (9.5%) were female as compared to 31 (25.6%) female recipients in non-CAN group and male recipients 91 (74.4%; p= 0.02), suggesting male gender recipient as a risk factor for CAN. Donor sex did not exert any effect on the development of CAN (p= 0.72).
HLA matching is one of the most important predictors for survival of the graft. HLA matched grafts have an estimated half-life of 12.4 years as compared with 8.6 years for mismatched grafts (21-25). HLA DR matching has been shown to have the earliest and most beneficial effect on graft outcome (11,21-25). HLA A and HLA B matching also positively impact graft survival (25). In our study, 42.3% were matched for <3 antigens and 57.7% matched ≥3 antigens in the CAN group as compared to 54.6% with <3 antigens in CAN group (p= 0.931). The fact that three-fourths of our transplant population had at least three antigen match, might have masked the deleterious effect of poor matching on the development of CAN. Moreover, the beneficial effect of matching may be more prominent in the long-term period. In addition, the role of HLA typing in the era of modern immunosuppression has become a matter of controversy. While there is evidence that long- term survival is better for transplants with no antigen mismatch than for mismatched transplants (25), the impact of HLA mismatches is not linear over the entire range of zero to 6 mismatches, in that progressive increase in the number of mismatches from one to 6 have only small effect on survival as compared with the large benefit afforded by the use of a graft with no mismatch (22,23).
Prolonged cold ischemia time (CIT) increases the risk of graft loss; this is mostly a problem in the setting of cadaver transplantation. Increased organ ischemia time leads to delayed graft function (DGF), increased AR, enhanced CAN, and reduced long-term allograft survival. The mechanism by which ischemia reperfusion injury (IRI) predisposes to AR and CAN are unknown. As a result of IRI, there is up regulation of major histocompatibility complex (MHC) antigen expression. We had documentation of CIT in 105 patients in non-CAN group with mean time of 141.06 minutes, while, in CAN group with 27 patients, the mean time was 138.58 minutes. Warm ischemia mean time in non-CAN population was 9.7413 minutes in 105 patients while mean time for warm ischemia in CAN group was 15.55 minutes.
Many investigators have shown that the occurrence and numbers of AR episodes are powerful immunologic predictors both of CAN and late graft loss (26,27). In one study of 1706 adult renal transplants (1995 to 2003) with a functioning graft for at least 1 year and receiving CsA, the results showed that an AR episode occurring within 3 months post transplantation had no effect on either death-censored long term graft failure (p= 0.2157) or CAN (p= 0.9331). However, an AR episode occurring at 1 year or later post-transplantation was significantly associated with death censored long term graft failure (p< 0.0001) and CAN (p< 0.0001) (28,29).
In a study of 63045 primary renal transplant recipients collected from United States Renal Data Systems (USRDS) from 1988 to 1997 to see the impact of AR on chronic allograft failure in recent era showed that AR episode within first 6 months after transplantation is a negative prognostic factor for the development of chronic allograft failure. AR episodes with partial or no functional recovery exert a more detrimental effect on long-term outcome than AR episodes with complete functional recovery (30). Acute vascular rejection is an adverse prognostic feature compared with tubulointerstitial rejection (31). A higher incidence of AR episodes is associated with sensitization as a result of previous blood transfusion or transplantation (32). In a study of 264 renal transplant recipients, anti-HLA antibody (by ELISA), and panel reactive antibodies (PRA) by CDC method were used to categorize the patients into immunological risk groups. Group 1, non-sensitized (PRA 1 to 10%), group 2 and group 3 sensitized (PRA= 11-50% and >50% respectively). Incidence of AR episodes among non-sensitized and sensitized groups was 35% vs. 84%. Graft survival was poor (63%) among patients having anti-HLA IgG antibodies as compared with those who had negative test (33). In our study most of the rejection episodes occurred within 3 months post-transplantation and the number of AR episodes was similar in the two groups. There were proportionately more episodes of vascular rejection in the CAN group which were however not statically significant.
The introduction of cyclosporine significantly reduced AR rates but intrinsic nephrotoxicity and its contribution to CAN have greatly diminished the enthusiasm for its long term use (28,29). The advent of newer potent immunosuppressive medications such as MMF and sirolimus has enabled the minimization, withdrawal, or avoidance of cyclosporine altogether. However, the results of calcineurin inhibitors (CNI)-sparing regimens have been inconclusive and CNI toxicity may be accepted as a trade-off for early graft survival (34,35). Specific histological features of CNI toxicity are identified as early as 3 months post-transplant and are one of the major contributors to the development of CAN, with 100% of adult transplant recipients showing some histological evidence of CNI toxicity by 10 years of post-transplant (5).
Some improvement of renal function has been achieved by replacing CNIs with MMF (34) or sirolimus (35). However, even with graft biopsy it is not easy to exclude immunological activation in these cases of CAN. A number of patients are therefore exposed to the risk of late irreversible rejection after stopping the CNIs (35). In our study, 5 (11.9%) and 17 (13.9%) patients had cyclosporine toxicity in CAN and non CAN group while 37 (88.1%) and 105 (86.1%) did not showed cyclosporine toxicity in CAN and non CAN group respectively (p= 0.73).
In this study population, 6 patients (14.3%) in CAN group had polyoma virus infection, 4 were picked on graft biopsy and 2 on urine and blood PCR; one (0.8%) patient in non CAN group was positive, while 36 (85.7%) and 121 (99.2%) had no detectable polyomavirus infection in CAN and non CAN group, respectively (p= 0.0001).
CMV is a major cause of morbidity, costs and even mortality in organ transplant recipients (12). CMV may also enhance the development of CAN. The evidence for the role of CMV in CAN is somewhat limited, and controversial results have been reported. Infection with CMV can also increase the risk of acute and chronic rejection through overproduction of mediators, cytokines, chemokines and growths factors (12). In this study, CMV turned out to be a significant risk factor for CAN development (p= 0.001). Five (13.2%) patients had CMV infection in the CAN group, while in non-CAN group, only 1 (0.8%) patient was positive for CMV infection. CMV infection was detected on antigenemia in all patients.
Several studies have shown that UTIs are an important risk factor for the onset of chronic rejection (36). The mechanism remains largely unclear. On the one hand, infectious agents may directly destroy grafts, while, on the other hand, microbial agents stimulate the secretion of tumor necrosis factor alpha, interferon gamma and interleukin-6. These cytokines may induce processes similar to those observed in chronic rejection (36). Muller et al., (36) reported in a retrospective study conducted between 1972-1991 that, UTIs are an important risk factor for the onset of chronic rejection. The study included 225 patients with histologically proven chronic rejection and 351 patients without apparent signs of chronic rejection. The result showed that, chronic rejection patients always had more UTIs than controls and this difference gained statistical significance in 3 years after transplantation. The rate of UTI was highest in those patients who had an early onset of chronic rejection. Iqbal et al. reported in a study that UTI can lead to graft dysfunction and may induce graft loss in severe pyelonephritis (37).
Our results did not show UTIs as a significant risk factor for CAN development (p= 0.56). This may be due to the prompt diagnosis and treatment of the UTIs in the posttransplant period with regular surveillance for UTIs.
New therapeutic strategies in kidney transplantation are difficult to evaluate because the usual end-points are poor tools in the long run, and time-to-event of potentially useful end-points such as graft loss is too long. Consequently, short-term variables predicting long-term evolution of kidney transplant would be very useful for patients’ management. Early serum creatinine may be a good marker of long-term survival, but published studies are based on registry data and five years survival. This predictive capacity improves with serum creatinine at 3, 6 and 12 months. Mild differences in serum creatinine such as between 1.5 and 1.6-2.0 mg/dl are associated with highly significant impact in long-term survival (38).
In our study, serum creatinine at the time of discharge showed a higher value in the CAN group than in the non-CAN group. The difference of serum creatinine widened further at 3 months, 6 months and 1 year. Discharge serum creatinine remained high in CAN group even before the diagnosis of CAN and later on further creeped up at 3 months, 6 months and 1 year irrespective of any other factor in study.
6. Conclusions
The results of our study show that serum creatinine at the time of discharge is a useful predictor of later development of chronic changes in the allograft. Further studies are needed to identify the risk factors for the early development of chronic changes in living related renal transplant program.
Author contributions
HK, MM and TA designed and conducted the research, analyzed the data, and prepared the primary draft. EA, SFA, JK, SAN, and SAH critically reviewed and gave the final approval. All authors contributed equally to data acquisition.
Conflict of interests
The authors declared no competing interests.
Funding/Support
None.
Implication for health policy/practice/research/medical education:
Chronic allograft nephropathy (CAN) is a common cause of delayed allograft failure throughout the world. Its prevalence andrisk factors vary depending on a number of factors. Traditionally, it was thought that, once the diagnosis of CAN has beenmade, it is difficult to alter the course of progression of the scarring process and the final outcome. However, more recently,with the availability of new immunosuppressants with antifibrotic properties, it appears that this process can be stopped andeven reversed if diagnosed early. This study is an attempt to identify CAN at an early stage and to identify the risk factors underlyingCAN in a live related renal transplant program. The study is an important contribution to the scanty literature in this area.
Please cite this paper as: Khan H, Mubarak M, Aziz T, Ahmed E, Akhter SF, Kazi J, et al. Prevalence and risk factors for early chronicallograft nephropathy in a live related renal transplant program. J Nephropathol. 2014; 3(2): 69-79. DOI: 10.12860/jnp.2014.15
References
- 1.Pascual M, Theruvath T, Kawai T, Tolkaff-Rubin N, Cusimi AB. Strategies to improve long term out come after renal transplantation. New Engl J Med. 2002;346(8):580–90. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 2.Gjertson DW. A multi-factor analysis of kidney graft outcomes at one and five years post-transplantation: 1996 UNOS Update. Clin Transpl. 1996;??:343–60. [PubMed] [Google Scholar]
- 3.Cecka JM. The UNOS Scientific Renal Transplant Registry. Clin Transpl. 1998;???:1–16. [PubMed] [Google Scholar]
- 4.Cecka JM. The UNOS renal transplant registry. Clin Transpl. 2001;????:1–18. [PubMed] [Google Scholar]
- 5.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 6.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol. 1999;10(1):167–81. doi: 10.1681/ASN.V101167. [DOI] [PubMed] [Google Scholar]
- 7.Cornell LD, Colvin RB. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2005;14(3):229–34. doi: 10.1097/01.mnh.0000165888.83125.07. [DOI] [PubMed] [Google Scholar]
- 8.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605–12. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 9.Campistol JM, Boletis IN, Dantal J, de Fijter JW, Hertig A, Neumayer HH. et al. Chronic allograft nephropathy--a clinical syndrome: early detection and the potential role of proliferation signal inhibitors. Clin Transplant. 2009;23(6):769–77. doi: 10.1111/j.1399-0012.2009.01057.x. [DOI] [PubMed] [Google Scholar]
- 10.Swanson SJ, Hypolite IO, Agodoa LY, Batty DS Jr, Hshieh PB, Cruess D. et al. Effect of donor factors on early graft survival in adult cadaveric renal transplantation. Am J Transplant. 2002;2(1):68–75. doi: 10.1034/j.1600-6143.2002.020112.x. [DOI] [PubMed] [Google Scholar]
- 11.Humar A, Hassoun A, Kandaswamy R, Payne WD, Sutherland DE, Matas AJ. Immunologic factors: the majo;r risk for decreased long-term renal allograft survival. Transplantation. 1999;68(12):1842–6. doi: 10.1097/00007890-199912270-00004. [DOI] [PubMed] [Google Scholar]
- 12.Tong CY, Bakran A, Peiris JS, Muir P, Herrington CS. The association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation. 2002;74(4):576–8. doi: 10.1097/00007890-200208270-00026. [DOI] [PubMed] [Google Scholar]
- 13.Kasiske BL, Andany MA, Danielson B. A thirty percent chronic decline in inverse serum creatinine is an excellent predictor of late renal allograft failure. Am J Kidney Dis. 2002;39(4):762–8. doi: 10.1053/ajkd.2002.31996. [DOI] [PubMed] [Google Scholar]
- 14.Isoniemi H, Nurminen M, Tikkanen MJ, Von Willebrand E, Krogerus L, Ahonen J. et al. Risk factors predicting chronic rejection of renal allografts. Transplantation. 1994;57(1):68–72. doi: 10.1097/00007890-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Stoves J, Newstead CG. Variability of cyclosporine exposure and its relevance to chronic allograft nephropathy: a case-control study. Transplantation. 2002;74(12):1794–7. doi: 10.1097/00007890-200212270-00027. [DOI] [PubMed] [Google Scholar]
- 16.Massy ZA, Guijarro C, Kasiske BL. Clinical predictors of chronic renal allograft rejection. Kidney Int. 1995;52:S85–S88. [PubMed] [Google Scholar]
- 17.Kazi J, Mubarak M. Biopsy findings in renal allograft dysfunction in a live related renal transplant program. J Transplant Tech Res. 2012;2:108. [Google Scholar]
- 18.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T. et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 19.Solez K, Colvin RB, Racusen LC, Siss B, Halloran PF, Birk PE. et al. Banff ’05 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (CAN) Am J Transplant. 2007;7(3):518–26. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 20.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M. et al. Banff’ 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 21.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69(3):319–26. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Worthington JE, Martin S, Al-Husseini DM, Dyer PA, Johnson RW. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation. 2003;75(7):1034–40. doi: 10.1097/01.TP.0000055833.65192.3B. [DOI] [PubMed] [Google Scholar]
- 23.Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL. et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74(8):1192–4. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 24.Piazza A, Poggi E, Borrelli L, Servetti S, Monaco PI, Buonomo O. et al. Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: posttransplant analysis using flow cytometric techniques. Transplantation. 2001;71(8):1106–12. doi: 10.1097/00007890-200104270-00017. [DOI] [PubMed] [Google Scholar]
- 25.Opelz G. Impact of HLA compatibility on survival of kidney transplants from unrelated live donors. Transplantation. 1997;64(10):1473–5. doi: 10.1097/00007890-199711270-00017. [DOI] [PubMed] [Google Scholar]
- 26.Matas AJ, Humar A, Gillingham KJ, Payne WD, Gruessner RW, Kandaswamy R. et al. Five preventable causes of kidney graft loss in the 1990s: a single-center analysis. Kidney Int. 2002;62(2):704–14. doi: 10.1046/j.1523-1755.2002.00491.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuypers DR, Chapman JR, O’Connell PJ, Allen RD, Nankivell BJ. Predictors of renal transplant histology at three months. Transplantation. 1999;67(9):1222–30. doi: 10.1097/00007890-199905150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Marcen R, Pascual J, Teruel JL, Villafruela JJ, Rivera ME, Mampaso F. et al. Outcome of cadaveric renal transplant patients treated for 10 years with cyclosporine: is chronic allograft nephropathy the major cause of late graft loss? Transplantation. 2001;72(1):57–62. doi: 10.1097/00007890-200107150-00013. [DOI] [PubMed] [Google Scholar]
- 29.Oberbauer R, Segoloni G, Campistol JM, Kreis H, Mota A, Lawen J. et al. Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transplant Int. 2005;18(1):22–8. doi: 10.1111/j.1432-2277.2004.00052.x. [DOI] [PubMed] [Google Scholar]
- 30.Mueller A, Schnuella P, Waldhere R, Vander Wounde FJ. Impact of the Banff’ 97 classification for histological diagnosis of rejection on clinical outcome and renal function Parameters after kidney transplantation. Transplantation. 2000;69(6):1123–7. doi: 10.1097/00007890-200003270-00017. [DOI] [PubMed] [Google Scholar]
- 31.van Saase JL, van der Woude FJ, Thorogood J, Hollander AA, van Es LA, Weening JJ. et al. The relation between acute vascular and interstitial renal allograft rejection and subsequent chronic rejection. Transplantation. 1995;59(9):1280–5. [PubMed] [Google Scholar]
- 32.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3(6):665–73. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 33.Panigrahi A, Deka R, Bhowmik D, Tiwari SC, Mehra NK. Immunological monitoring of post-transplant allograft sensitization following living related donor renal transplant. Transplant Proc. 2004;36(5):1336–9. doi: 10.1016/j.transproceed.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 34.Ojo AO, Meier-Kriesche HU, Hanson JA, Leichtman AB, Cibrik D, Magee JC. et al. Mycophenolate mofetil reduces late renal allograft loss independent of acute rejection. Transplantation. 2000;69(11):2405–2409. doi: 10.1097/00007890-200006150-00033. [DOI] [PubMed] [Google Scholar]
- 35.Mota A, Arias M, Taskinen EI, Paavonen T, Brault Y, Legendre C. et al. Sirolimus-Based Therapy Following Early Cyclosporine Withdrawal Provides Significantly Improved Renal Histology and Function at 3 Years. Am J Transplant. 2004;4(6):953–961. doi: 10.1111/j.1600-6143.2004.00446.x. [DOI] [PubMed] [Google Scholar]
- 36.Müller V, Becker G, Delfs M, Albrecht KH, Philipp T, Heemann U. Do urinary tract infections trigger chronic kidney transplant rejection in man? J Urol. 1998;159(6):1826–9. doi: 10.1016/S0022-5347(01)63165-3. [DOI] [PubMed] [Google Scholar]
- 37.Iqbal T, Naqvi R, Akhter SF. Frequency of urinary tract infection in renal transplant recipients and effect on graft function. J Pak Med Assoc. 2010;60(10):826–9. [PubMed] [Google Scholar]
- 38.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62(1):311–318. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]


