Abstract
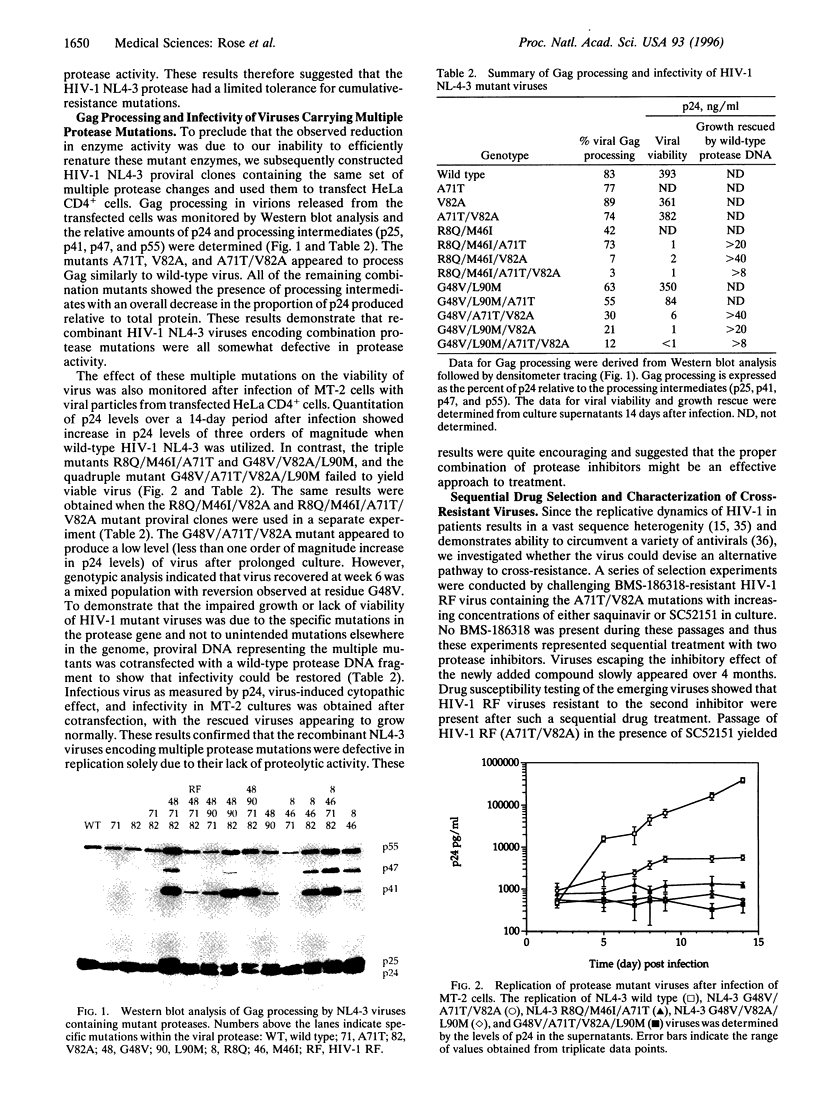
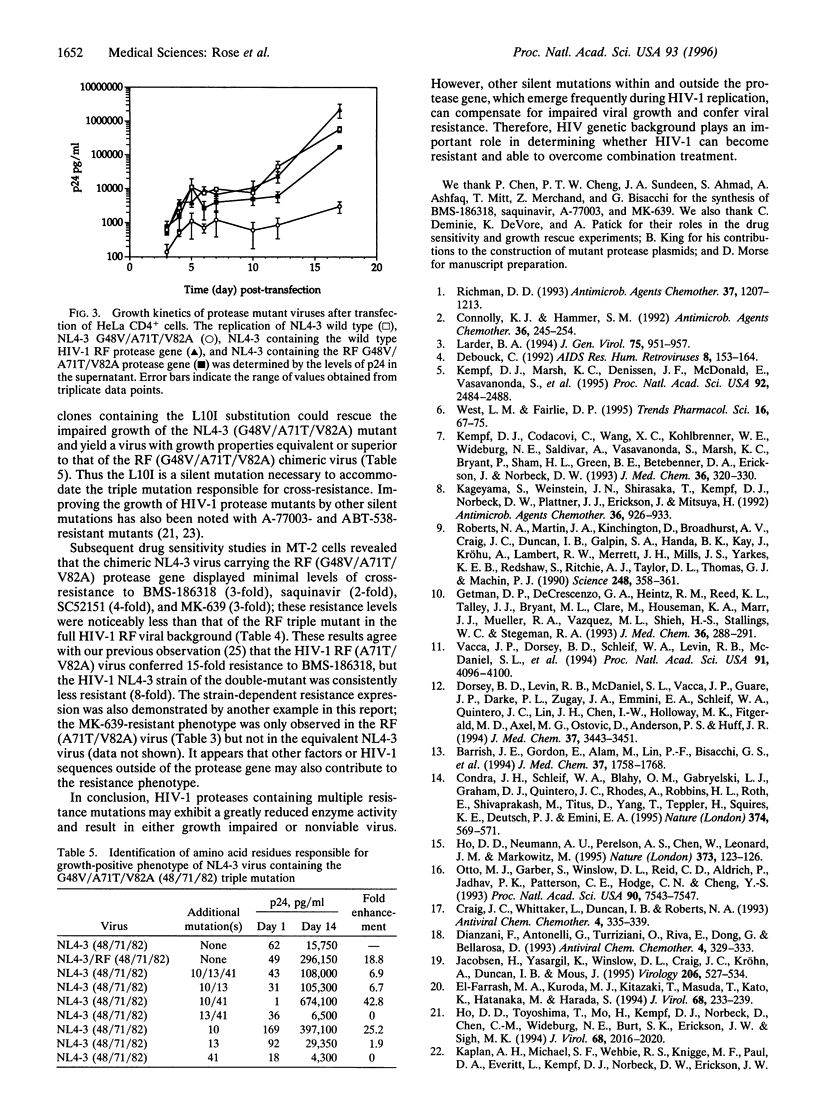
The observed in vitro and in vivo benefit of combination treatment with anti-human immunodeficiency virus (HIV) agents prompted us to examine the potential of resistance development when two protease inhibitors are used concurrently. Recombinant HIV-1 (NL4-3) proteases containing combined resistance mutations associated with BMS-186318 and A-77003 (or saquinavir) were either inactive or had impaired enzyme activity. Subsequent construction of HIV-1 (NL4-3) proviral clones containing the same mutations yielded viruses that were severely impaired in growth or nonviable, confirming that combination therapy may be advantageous. However, passage of BMS-186318-resistant HIV-1 (RF) in the presence of either saquinavir or SC52151, which represented sequential drug treatment, produced viable viruses resistant to both BMS-186318 and the second compound. The predominant breakthrough virus contained the G48V/A71T/V82A protease mutations. The clone-purified RF (G48V/A71T/V82A) virus, unlike the corresponding defective NL4-3 triple mutant, grew well and displayed cross-resistance to four distinct protease inhibitors. Chimeric virus and in vitro mutagenesis studies indicated that the RF-specific protease sequence, specifically the Ile at residue 10, enabled the NL4-3 strain with the triple mutant to grow. Our results clearly indicate that viral genetic background will play a key role in determining whether cross-resistance variants will arise.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrish J. C., Gordon E., Alam M., Lin P. F., Bisacchi G. S., Chen P., Cheng P. T., Fritz A. W., Greytok J. A., Hermsmeier M. A. Aminodiol HIV protease inhibitors. 1. Design, synthesis, and preliminary SAR. J Med Chem. 1994 Jun 10;37(12):1758–1768. doi: 10.1021/jm00038a005. [DOI] [PubMed] [Google Scholar]
- Condra J. H., Schleif W. A., Blahy O. M., Gabryelski L. J., Graham D. J., Quintero J. C., Rhodes A., Robbins H. L., Roth E., Shivaprakash M. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature. 1995 Apr 6;374(6522):569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- Connolly K. J., Hammer S. M. Antiretroviral therapy: reverse transcriptase inhibition. Antimicrob Agents Chemother. 1992 Feb;36(2):245–254. doi: 10.1128/aac.36.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C. The HIV-1 protease as a therapeutic target for AIDS. AIDS Res Hum Retroviruses. 1992 Feb;8(2):153–164. doi: 10.1089/aid.1992.8.153. [DOI] [PubMed] [Google Scholar]
- Dorsey B. D., Levin R. B., McDaniel S. L., Vacca J. P., Guare J. P., Darke P. L., Zugay J. A., Emini E. A., Schleif W. A., Quintero J. C. L-735,524: the design of a potent and orally bioavailable HIV protease inhibitor. J Med Chem. 1994 Oct 14;37(21):3443–3451. doi: 10.1021/jm00047a001. [DOI] [PubMed] [Google Scholar]
- Getman D. P., DeCrescenzo G. A., Heintz R. M., Reed K. L., Talley J. J., Bryant M. L., Clare M., Houseman K. A., Marr J. J., Mueller R. A. Discovery of a novel class of potent HIV-1 protease inhibitors containing the (R)-(hydroxyethyl)urea isostere. J Med Chem. 1993 Jan 22;36(2):288–291. doi: 10.1021/jm00054a014. [DOI] [PubMed] [Google Scholar]
- Hammer S. M., Kessler H. A., Saag M. S. Issues in combination antiretroviral therapy: a review. J Acquir Immune Defic Syndr. 1994;7 (Suppl 2):S24–S37. [PubMed] [Google Scholar]
- Heimbach J. C., Garsky V. M., Michelson S. R., Dixon R. A., Sigal I. S., Darke P. L. Affinity purification of the HIV-1 protease. Biochem Biophys Res Commun. 1989 Nov 15;164(3):955–960. doi: 10.1016/0006-291x(89)91762-2. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Toyoshima T., Mo H., Kempf D. J., Norbeck D., Chen C. M., Wideburg N. E., Burt S. K., Erickson J. W., Singh M. K. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J Virol. 1994 Mar;68(3):2016–2020. doi: 10.1128/jvi.68.3.2016-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen H., Yasargil K., Winslow D. L., Craig J. C., Kröhn A., Duncan I. B., Mous J. Characterization of human immunodeficiency virus type 1 mutants with decreased sensitivity to proteinase inhibitor Ro 31-8959. Virology. 1995 Jan 10;206(1):527–534. doi: 10.1016/s0042-6822(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Kageyama S., Weinstein J. N., Shirasaka T., Kempf D. J., Norbeck D. W., Plattner J. J., Erickson J., Mitsuya H. In vitro inhibition of human immunodeficiency virus (HIV) type 1 replication by C2 symmetry-based HIV protease inhibitors as single agents or in combinations. Antimicrob Agents Chemother. 1992 May;36(5):926–933. doi: 10.1128/aac.36.5.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Michael S. F., Wehbie R. S., Knigge M. F., Paul D. A., Everitt L., Kempf D. J., Norbeck D. W., Erickson J. W., Swanstrom R. Selection of multiple human immunodeficiency virus type 1 variants that encode viral proteases with decreased sensitivity to an inhibitor of the viral protease. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5597–5601. doi: 10.1073/pnas.91.12.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf D. J., Codacovi L., Wang X. C., Kohlbrenner W. E., Wideburg N. E., Saldivar A., Vasavanonda S., Marsh K. C., Bryant P., Sham H. L. Symmetry-based inhibitors of HIV protease. Structure-activity studies of acylated 2,4-diamino-1,5-diphenyl-3-hydroxypentane and 2,5-diamino-1,6-diphenylhexane-3,4-diol. J Med Chem. 1993 Feb 5;36(3):320–330. doi: 10.1021/jm00055a003. [DOI] [PubMed] [Google Scholar]
- Kempf D. J., Marsh K. C., Denissen J. F., McDonald E., Vasavanonda S., Flentge C. A., Green B. E., Fino L., Park C. H., Kong X. P. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2484–2488. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A. Interactions between drug resistance mutations in human immunodeficiency virus type 1 reverse transcriptase. J Gen Virol. 1994 May;75(Pt 5):951–957. doi: 10.1099/0022-1317-75-5-951. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kellam P., Kemp S. D. Convergent combination therapy can select viable multidrug-resistant HIV-1 in vitro. Nature. 1993 Sep 30;365(6445):451–453. doi: 10.1038/365451a0. [DOI] [PubMed] [Google Scholar]
- Loeb D. D., Swanstrom R., Everitt L., Manchester M., Stamper S. E., Hutchison C. A., 3rd Complete mutagenesis of the HIV-1 protease. Nature. 1989 Aug 3;340(6232):397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- Louis J. M., McDonald R. A., Nashed N. T., Wondrak E. M., Jerina D. M., Oroszlan S., Mora P. T. Autoprocessing of the HIV-1 protease using purified wild-type and mutated fusion proteins expressed at high levels in Escherichia coli. Eur J Biochem. 1991 Jul 15;199(2):361–369. doi: 10.1111/j.1432-1033.1991.tb16132.x. [DOI] [PubMed] [Google Scholar]
- Markowitz M., Mo H., Kempf D. J., Norbeck D. W., Bhat T. N., Erickson J. W., Ho D. D. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J Virol. 1995 Feb;69(2):701–706. doi: 10.1128/jvi.69.2.701-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli T., Rusconi S., Merrill D. P., D'Aquila R. T., Moonis M., Chou T. C., Hirsch M. S. Alternating versus continuous drug regimens in combination chemotherapy of human immunodeficiency virus type 1 infection in vitro. Antimicrob Agents Chemother. 1994 Apr;38(4):656–661. doi: 10.1128/aac.38.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. J., Garber S., Winslow D. L., Reid C. D., Aldrich P., Jadhav P. K., Patterson C. E., Hodge C. N., Cheng Y. S. In vitro isolation and identification of human immunodeficiency virus (HIV) variants with reduced sensitivity to C-2 symmetrical inhibitors of HIV type 1 protease. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7543–7547. doi: 10.1073/pnas.90.16.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patick A. K., Rose R., Greytok J., Bechtold C. M., Hermsmeier M. A., Chen P. T., Barrish J. C., Zahler R., Colonno R. J., Lin P. F. Characterization of a human immunodeficiency virus type 1 variant with reduced sensitivity to an aminodiol protease inhibitor. J Virol. 1995 Apr;69(4):2148–2152. doi: 10.1128/jvi.69.4.2148-2152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D. Resistance of clinical isolates of human immunodeficiency virus to antiretroviral agents. Antimicrob Agents Chemother. 1993 Jun;37(6):1207–1213. doi: 10.1128/aac.37.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Vacca J. P., Dorsey B. D., Schleif W. A., Levin R. B., McDaniel S. L., Darke P. L., Zugay J., Quintero J. C., Blahy O. M., Roth E. L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4096–4100. doi: 10.1073/pnas.91.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995 Jan 12;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- West M. L., Fairlie D. P. Targeting HIV-1 protease: a test of drug-design methodologies. Trends Pharmacol Sci. 1995 Feb;16(2):67–75. doi: 10.1016/s0165-6147(00)88980-4. [DOI] [PubMed] [Google Scholar]
- el-Farrash M. A., Kuroda M. J., Kitazaki T., Masuda T., Kato K., Hatanaka M., Harada S. Generation and characterization of a human immunodeficiency virus type 1 (HIV-1) mutant resistant to an HIV-1 protease inhibitor. J Virol. 1994 Jan;68(1):233–239. doi: 10.1128/jvi.68.1.233-239.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]




