Abstract
Background:
Snakebite in Iran has been a health concern. However, management of snakebite is not standardized and varies from center to center. This study is aimed at devising an evidence-based comprehensive protocol for snakebite management in Iran, to reduce unnecessary variations in practice.
Materials and Methods:
A narrative search in electronic databases was performed. Fifty peer-reviewed articles, guidelines, and textbooks were reviewed and practical details were extracted. Our currently used protocol in the Mashhad Toxicology Center was supplemented with this information. Consequently an improved wide-range protocol was developed. The protocol was then discussed and amended within a focus group comprised of medical toxicologists and internal medicine specialists. The amended version was finally discussed with expert physicians specialized in different areas of medicine, to be optimized by supplementing other specific considerations.
Results:
During a one-year process, the protocol was finalized. The final version of the protocol, which was designed in six steps, comprised of three components: A schematic algorithm, a severity grading scale, and instructions for supportive and adjunctive treatments. The algorithm pertains to both Viperidae and Elapidae snakebite envenomations and consists of a planned course of action and dosing of antivenom, based on the severity of the envenomation.
Conclusion:
Snakebite envenomation is a clinical toxicologic emergency, which needs to be treated in a timely and organized manner. Hence, a multi-aspect protocol was designed to improve the clinical outcomes, reduce unnecessary administration of antivenom, and help physicians make more proper clinical judgments.
Keywords: Clinical protocols, emergency treatment, Iran, snake bite, therapeutics
INTRODUCTION
Snakebite has been a health concern in Iran.[1,2] According to the National Health Statistics, 4500-6500 individuals are affected annually, with three to nine deaths.[2,3] Three-quarters of Iran's ecological regime is arid and semi-arid, which is especially suitable for different species of desert snakes to live and breed.[4,5] The major envenomating species endemic to Iran are Vipera lebetina obtusa (a Viperidae), Echis carinatus sochureki (a Viperidae), Pseudocerastes persicus persicus (a Viperidae) and Naja naja oxiana (an Elapidae).[4,5] Although not very common, sea snakebite envenomations may happen in the southern borders of Iran (Persian Gulf and Gulf of Oman).[4,5,6] Hydrophis cyanocinctus and Hydrophis lapemoides were reported to be the most common sea snake species in these areas.[6]
Bites, which mostly occur during the hot seasons,[7,8] are considered to be neglected diseases causing high morbidity rates and healthcare costs.[9] Therefore, the World Health Organization (WHO), in 2009, had given priority to the improvement of healthcare quality delivered to snakebite victims.[10] Nevertheless, management strategies for snakebite envenomation in Iran are still not standardized, and they are mostly modeled from subjective experiences and even adapted from other countries. Snake species from different parts of the world, however, are inconsistent with each other and also the quality of venom of the same species can be influenced by the geographical conditions, such as, humidity and temperature.[11] In addition, antivenoms produced in different countries may be chemically dissimilar to each other.[12] Therefore, applying a protocol which is efficient in other parts of the world does not seem to be judicious.
In this respect, a unified management strategy that encompasses all aspects of snakebite envenomation and suits the medical capacity in Iran must be devised. This can be achieved by improving the available algorithms and protocols[13,14,15,16,17,18,19,20,21,22,23,24,25] according to newer valid and local data. Hence, this study was designed to elaborate our currently used protocol in the Mashhad Toxicology Center (MTC),[13] by complying with scientifically accepted information and amending some neglected aspects.
MATERIALS AND METHODS
To design an instructive in-hospital protocol for the management of snakebite, a narrative search in electronic databases including MEDLINE, EMBASE, Scopus, and TOXNET was performed, during the period of May 2011 to April 2012, using the following search terms: Snakebite AND algorithm, snakebite AND protocol, snakebite AND treatment. In total, 50 articles, three guidelines, and related topics to snakebite in six textbooks were reviewed. The quality of all the literature was evaluated by two researchers, to identify the articles that were published in peer-reviewed journals, guidelines and textbooks that were published by reputable institutions.
In the first phase of the study, useful details from the articles, guidelines, and textbooks were extracted and summarized. Our current protocol was then supplemented with this information and details. Hence, a simple, wide-ranged descriptive protocol was developed.
In the second phase, the initial version of the protocol was discussed within a focus group comprised of medical toxicologists and Internal Medicine specialists. Thus, the second version of the protocol was provided, with some elaborations to fit our medical capacity in Iran.
In the final phase, the second version of the protocol was discussed with expert physicians specialized in Immunology, Hematology, Infectious Disease and Tropical Medicine, Neurology, and Orthopedics, to be optimized and augmented with other specific considerations. The final version of the protocol was released, to be practiced for snakebite cases.
RESULTS
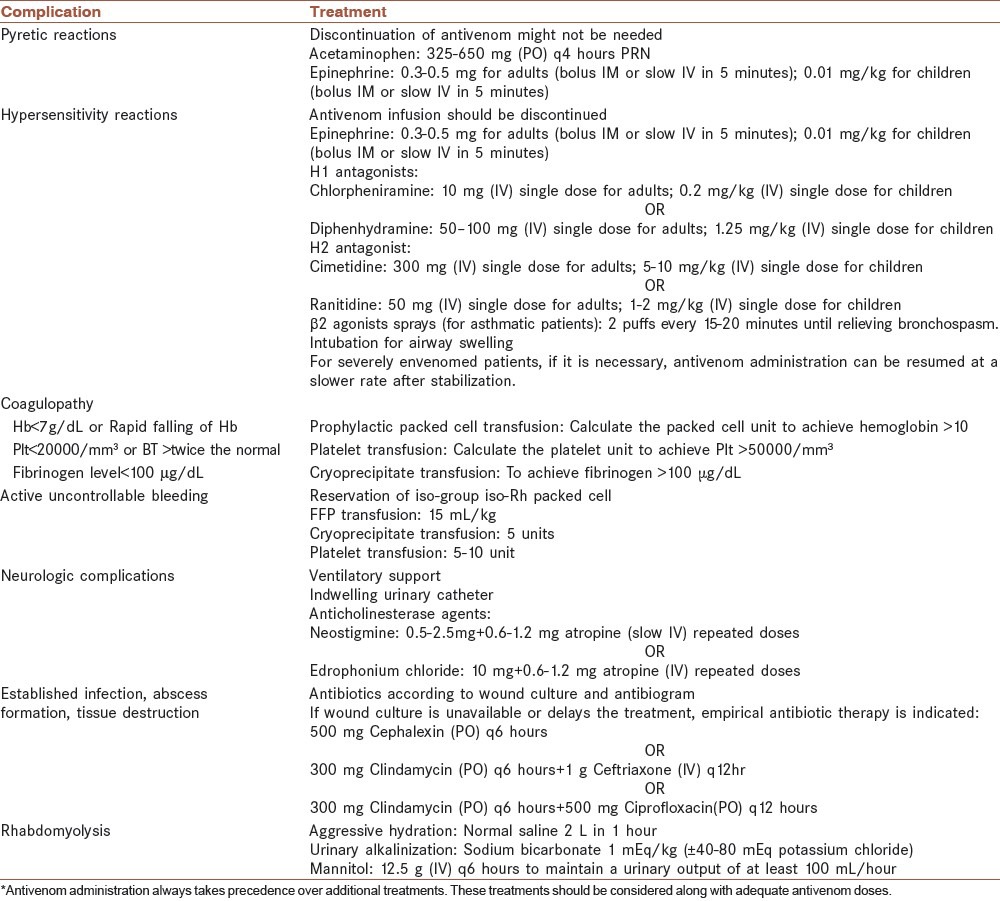
The final version of the protocol comprised of three components, a schematic algorithm, a severity grading scale, and instructions for supportive and adjunctive treatments. The algorithm which is illustrated in Figure 1 pertains to both Viperidae and Elapidae snakebite envenomations and consists of the planned course of action and dosing of antivenom, based on the severity of envenomation. The snakebite envenomation severity scale (SESS), which was developed and enhanced during this study, is an instrument for grading the severity of envenomation according to the clinical manifestations and laboratory findings [Table 1]. It is mostly based on 30 years of experience on treating snakebite victims in MTC and has been fine-tuned according to peer-reviewed literatures.[3,13,14,15,16,17,18,19,20,26] In addition to antivenom, some patients may need supportive and adjunctive treatments, which are displayed in a separate table [Table 2].[3,13,14,15,16,17,18,19,20,21,22,23,24,25,26] The protocol was designed in six steps. Each step includes some actions, in order. The necessity, details, and controversies of some actions are further explained under the section of ‘Discussion”.
Figure 1.
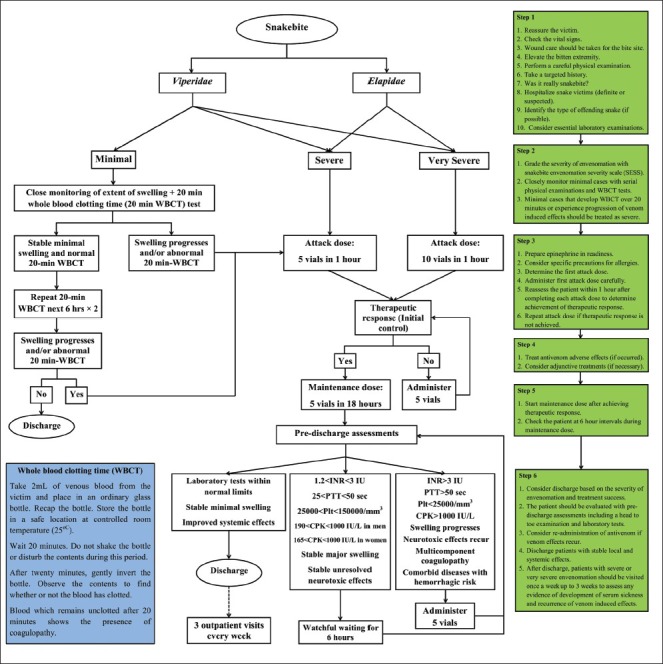
Algorithm for the management of snakebite envenomation in Iran
Table 1.
Snakebite envenomation severity scale

Table 2.
Supportive and adjunctive treatments in snakebite envenomation*
Step 1 (Initial assessment and stabilizing the patient)
The victim should be reassured if agitated
Immediate assessment of vital signs should be performed[16,21]
The bite site should be washed with sterile saline or clean water.[15,17,18] Any constriction bands, pressure bandages, jewelry, watches, and rings adjacent to the bite site should be removed. Sterile dressing and other wound care should be considered in case of incised or manipulated wounds and extensive tissue destruction.[22,23] General directions of tetanus immunization should be taken into account.
The bitten extremity should be immobilized and slightly elevated. Circulation of the bitten limb should be evaluated and the edge of edema should be marked with a pen.[15,16,21,22,23]
A careful physical examination, including assessment of the neurologic status and a search for evidence of bleeding should be considered.
A brief, but targeted history, including description of the offending snake, history of allergies and tetanus immunization should be obtained.
Based on the history and physical examination, the physician should try to determine whether the offending animal was a snake or not.
Based on the history and the patient's clinical manifestations, the offending snake brought with the victim, and available local information, the type of offending snake (Viperidae or Elapidae) can be identified.[11,12,13] An atlas of regional snakes (if available) would be helpful in such cases.
Essential laboratory examinations including complete blood count (CBC), electrolytes, creatinine, creatine phosphokinase (CPK), coagulation tests (prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR)), and urinalysis should be obtained.[13,14,15,16,17,18,19,20,21,22,23,24,25] In case of definite Viperidae snakebites, ABO and Rh typing, and fibrinogen level test (if available) may also be useful.
Step 2 (Grading the severity of envenomation)
Using the SESS, the severity of envenomation should be graded [Table 1].
Patients with minimal Viperidae envenomation can be managed with watchful waiting. These patients should be closely monitored by serial checking the clinical manifestations (evidence of bleeding and/or edema progression) every 30 minutes and performing the 20-minute whole blood clotting time (20-min WBCT) test every six hours.[18,22,23,24,25,27,28]
A case graded initially as minimal envenomation that develops WBCT for over 20 minutes or progression of other venom-induced effects in later stages, should be treated as a severe case.
Elapidae envenomations cannot be graded as minimal. Antivenom should be administered for all Elapidae envenomed cases.[14,15,22,23]
Step 3 (Antivenom administration-attack dose)
Antivenom is indicated for patients with clinical manifestations attributable to snake venom who are graded as severe and very severe according to the SESS. Furthermore, for patients who were initially graded as minimal Viperidae envenomation, but with subsequent abnormal WBCT test or progression of venom-induced effects, antivenom is also indicated.
Epinephrine should be drawn up in readiness for all patients.[13,22,23]
Specific precautions for allergies including β2 agonist inhaler (two puffs) for asthmatic patients and administration of hydrocortisone (100 mg intravenously (IV)), and chlorpheniramine (10 mg IV) for severely atopic patients and patients previously sensitized to biologic products should be considered, prior to antivenom administration.[13,22,23]
The first attack dose should be determined based on the severity of the envenomation.
The first attack dose for severe envenomation (Viperidae or Elapidae) is recommended to be five vials of Razi™ Polyvalent Antivenin and for very severe envenomation, it should be doubled to ten vials.[3,13,14]
Subsequent attack doses for very severe envenomation are recommended to be five vials, same as severe cases.
If the type of offending snake could not be identified and the patient is without any apparent clinical manifestation (unknown envenomation), administration of a five-vial attack dose is recommended. Administration of the next doses should be considered according to the patient's subsequent clinical condition.
Each dose of antivenom is recommended to be diluted in 5-10 mL/kg (250-500 mL) of isotonic fluid (normal saline) and should be administered via IV line in the unaffected extremity.[13,21,22,23]
The first dose of antivenom should be infused slowly and vigilantly during the first 20 minutes to avoid any adverse reaction. If the antivenom is tolerated by the patient, the rate of infusion should be regulated in an incremental trend to administer the whole dose in one hour. Subsequent attack doses need no titration of infusion rate and should be infused in one hour.[14,15,18]
The patient should be reassessed within one hour after each attack dose, to determine treatment success (achievement of therapeutic response). The attack dose should be repeated until achievement of therapeutic response.[13,14,15,16,17,18,19,20,21,22,23,24,25]
Except some rare cases, including uncontrolled bleeding and critically ill patients, the general recommendation is to limit the maximum attack dosing to 20 vials.[21]
Step 4 (Supportive and adjunctive treatments)
In case of hypersensitivity reactions during antivenom infusion, antivenom should be discontinued immediately and a second IV line should be established. Epinephrine, H1 antagonists, such as, chlorpheniramine or diphenhydramine, and H2 antagonist such as cimetidine or ranitidine should be administered [Table 2].[13,14,15,16,18,19,20,21,22,23,24,25] The need for continuing antivenom infusion should be re-evaluated based on the severity of the envenomation and the seriousness of the reactions. The infusion can be restarted in lower rates after the patient has stabilized.
If pyretic reactions occur, discontinuation of antivenom can be postponed, while deceleration of antivenom infusion and administration of epinephrine and antipyretic agents (acetaminophen) are recommended [Table 2].[22,23]
For bites in the head and neck region, which are associated with the risk of airway compromise, active airway management should be considered.[22,23]
Administration of blood products along with adequate doses of antivenom should be considered for patients with active uncontrollable bleeding, spontaneous bleeding together with hemodynamic instability, necessary surgical procedures, and major trauma [Table 2].[15,16,19,22,23,29,30]
In addition to adequate doses of antivenom, anticholinesterase agents, including neostigmine or edrophonium chloride, along with atropine should be administered for neurotoxin-induced paralysis.[14,15,22,31,32,33,34] Moreover, in cases with severe paralysis, mechanical ventilation is necessary.
Antibiotics are only recommended in case of the following conditions: Fever (axillary temperature over 38°C), incised or manipulated wounds, major swelling unresponsive to antivenom, abscess formation or tissue necrosis.[18,22,23,35,36,37]
Due to the potential threat of nonsteroidal anti-inflammatory drug (NSAID)-induced platelet dysfunction, only opioids are theoretically safe to be recommended for reducing the local pain.
If symptoms are suggestive of the compartment syndrome, constrictive dressings (if present) should be removed. The patient should be kept under serial examination of the affected limb. Urinalysis, CPK, and creatinine should be checked.[38] Supportive splinting is recommended to ease the pain. The affected extremity should be elevated (less than 5-10 degrees or at the level of the heart).[18,22,23,38,39] Diuresis with mannitol should be administered. Intracompartmental pressure should be determined with direct measurement.[18,21,22,23,38,39] Fasciotomy is only indicated for high intracompartmental pressure (over 30 mmHg in children or over 45 mmHg in adults) unresponsive to antivenom and mannitol diuresis.[18,22,23,39] Fasciotomy should not be done unless the coagulopathy is corrected.[18,22,23,38,39]
Rhabdomyolysis should be treated with early aggressive intravenous hydration and correction of electrolyte imbalance.[40,41,42] The serum electrolyte (sodium, potassium, calcium, and magnesium), serum and urea creatinine, blood urea nitrogen, urinalysis, acid-base status, uric acid and CPK should be checked. Diuresis with mannitol should be administered.[40,41] In case of a urine pH less than 6.5, urinary alkalinization is recommended. If acute kidney injury (volume overload, oliguria, symptomatic hyperkalemia) occurs, hemodialysis should be taken into account.[40]
Step 5 (Antivenom administration — maintenance dose)
To prevent recurrence of venom-induced effects, a maintenance dose is indicated.[13,14,20,22] After achieving therapeutic response or exceeding 20 vials as the attack dosing (whichever occurs earlier), the maintenance dose should be initiated.
Five vials in 250-500 mL isotonic fluid are recommended to be slowly infused during 18 hours.
The patient should be checked six hourly during the maintenance dose with targeted physical examinations.[21]
Step 6 (Discharge)
After completion of the maintenance dose, a pre-discharge evaluation including a targeted physical examination (to re-evaluate the venom effects) and laboratory tests (CBC, coagulation tests, and CPK) should be performed for all patients.
For a small proportion of patients who experience recurrence of venom effects after the maintenance dose, close monitoring and serial examination every six hours are recommended. Re-administration of a five-vial dose of antivenom is recommended for patients with renewed swelling progression 24 hours post the bite, INR over 3, PTT over 50 seconds, platelet count less than 25000/mm3, fibrinogen less than 50 μg/mL, multicomponent coagulopathy, comorbid conditions with hemorrhagic risk, spontaneous internal or mucosal bleeding, and re-manifestation of neurologic symptoms (in neurotoxic snakebites).[18,43]
In case of minimal Viperidae envenomation, the patient can be discharged after three normal subsequent WBCT tests.
In case of severe and very severe Viperidae or Elapidae envenomation, the patient is safe to be discharged after 24 hours following therapeutic response with stable minimal swelling, with laboratory results (coagulation parameters, platelet count, hemoglobin, creatinine, and CPK) within normal limits, and clinical improvement of systemic manifestations.[13,14,15,21,44]
In case of an unknown snakebite envenomation, with no apparent clinical manifestation, the patient can be discharged after three normal subsequent WBCT tests.
Age, comorbidities, bite site, and social support should be considered prior to discharge.[21]
After discharge, three consecutive follow-up visits are recommended every week to check recurrence of manifestations and development of serum sickness.
DISCUSSION
Devising a treatment protocol for snakebite envenomation seems challenging, as clinical trial studies in this scope are difficult to conduct. Moreover, most of the information used for designing the current guidelines and algorithms are derived from retrospective studies. Hence, the validity and applicability of each protocol are necessary to be evaluated in prospective studies. Furthermore, it should be noted that our proposed protocol is not idealized for every situation, while the clinical judgment of the treating physician, based on the patient's condition and available medication resources, remains the keystone. Moreover, this is an in-hospital protocol and does not include recommendations of pre-hospital care. Details and scientific basis of some practices in this protocol are discussed under the following topics.
Bitten extremity elevation
The bitten extremity should be immobilized, but should not be kept below heart level to prevent extensive swelling and the compartment syndrome.[16,17,21,22,23] Elevation of the bitten extremity should not exceed more than 5-10 degrees, to avoid arterial compromise and poor capillary perfusion.[22] The best approach is to place the upper extremities above the chest in relative extension (less than 45°flexion) of the elbow joint,[21] and to put two pillows beneath the lower extremities.
Identification of the offending snake
In some cases, the bite occurs during the night or in a place with low light conditions. As the bite of a black widow spider and different species of scorpions are common in Iran,[7,13] it seems necessary to rule out these kinds of envenomations before administration of snake antivenom.
Identifying the type of offending snake is the basic step for making a proper clinical decision and planning for further treatments. This is especially crucial when the patient is symptomless and the treating physician is not sure whether the offending snake was an Elapidae, a Viperidae (with minimal envenomation) or a nonvenomous snake.[16,17,18,19] The genus or at least the type of offending snake can be identified either with the snake morphological characteristics,[22,23] or the patient's clinical manifestations. Although not a definitive method, the morphological information of the offending snake can be gathered from the patient's history, local epidemiological reports, and the live or dead snake brought by the victim. Hence, it is necessary to prepare an atlas of regional snakes containing high-resolution images in all medical toxicology centers and emergency departments. However, capturing the offending snake due to the potential risk of more bites and also causing a delay in treatment is not recommended.[15] A more definitive and quicker method to identify the offending snake is application of snake venom detection kits, which are only available in a limited number of countries.[24]
Severity grading
Viperidae envenomation can cause severe local effects including intense pain and swelling, coagulopathy (increase of coagulation tests and/or thrombocytopenia) and hemorrhage.[3,9,13,14,15,16,17,18,19,20,21,22,23,26,27,45,46] However, swelling may be limited and coagulopathy only occur in some patients, while active bleeding is even less common.[3,9,27,45] Therefore, administration of antivenom is not recommended for all cases and the need for antivenom should be evaluated according to the severity of envenomation. The severity of envenomation can be judged based on our proposed severity grading scale, SESS. Nevertheless, snakebite envenomation is known to be a dynamic process.[9,21,22,23] Hence, a patient who is initially graded as a minimal case and typically does not need antivenom might deteriorate to a severe case requiring antivenom. This suggests constant scheduled physical and laboratory examinations for these patients, as described in the algorithm [Figure 1].
On the other hand, in Elapidae envenomation, which manifests with neurotoxic and myotoxic complications, including cranial neuropathies, respiratory paralysis, bulbar palsy, and rhabdomyolysis; swelling and local effects may be lacking or be limited, especially in bites of North American coral snakes and sea snakes.[13,14,15,26] Furthermore, the onset of systemic manifestations might be delayed up to even more than 12 hours.[13,14,15,26,44] Thus, it is not prudent to grade any definitive Elapidae snakebite as a minimal case, even when the victim has only fang marks without any pain or local signs.[14] Therefore, administration of antivenom should be pursued in accordance with the algorithm for all Elapidae victims.
Although Viperidae envenomation is mostly known for coagulopathy and Elapidae envenomation for neurotoxic and myotoxic effects, certain Viperidae species such as north American rattlesnake can cause neurotoxicity and/or rhabdomyolysis,[21,47] and some Elapidae species such as Australo-Papuan Elapidae can induce coagulopathy.[27]
Pre-antivenom administration care
As a clinical routine, but with no reliable scientific background, skin testing has been practiced for many years to determine the potential allergy to antivenom. It has been ascertained that this test can be misleading and non-predictive, while it delays the crucial time for antivenom administration.[9,21] Moreover, most hypersensitivity reactions are minor and can be satisfactorily treated with anti-allergic medications.[21,22,23,48] Thus, this test is not advocated anymore.[9,12,21,22,23] However, abrupt hypersensitivity reactions are constant threats during the administration of all biological products. Hence, for all patients, epinephrine should be ready for immediate injection.[22,23] In addition, some studies showed that subcutaneous injection of low-dose epinephrine prior to antivenom administration was effective in preventing immediate allergic reactions.[49,50,51] However, this remains a controversy, as in some other studies no association was found between epinephrine pretreatment and prevention of these kinds of reactions.[23,48]
Nevertheless, for high-risk patients, including patients previously sensitized to biologic products, asthmatic patients, and patients with a strong history of other atopic diseases, specific supportive treatments are necessary.[22,23]
Antivenom attack dose
Administration of antivenom should be initiated in a clinical setting, with skilled staff and prepared medications. Antivenom should be administered with serial attack dose(s) until achievement of the therapeutic response. The attack dose of a specific brand can be determined by the manufacturer through the premarketing process and studies.[13,52]
The only available antivenom for treatment of snakebite envenomation in Iran is Razi™ Polyvalent Antivenin, which has been approved by the Iranian Ministry of Health and Medical Education.[12,13,53] It is an equine F(ab’)2 antivenom, capable of neutralizing the venom of six of the most common snakes in Iran, including Echis carinatus sochureki, Vipera lebetina obtusa, Vipera albicornuta, Agkistrodon halys, Pseudocerastes persicus, and Naja naja oxiana.[12,53] The manufacturer has recommended one to two vials of antivenom as the attack dose. However, local experience from the past four decades in MTC has revealed that treatment success is less achievable with such low attack doses, causing higher morbidities.[3,13]
Moreover, in a recent study, it was shown that the median of antivenom vials used for achieving therapeutic response in northeast Iran was five.[3] Consequently, in our proposed protocol, five vials were recommended as the attack dose. In addition, it was demonstrated that at least 10 vials were required to help in subsiding the life-threatening venom-induced manifestations such as respiratory distress and spontaneous bleeding.[3] Thus, the first attack dose for very severe manifestations [Table 1], was recommended to be doubled.[3,13,14]
Therapeutic response
Therapeutic response, which is also known as ‘Initial control’ is the arrest of venom-induced effects and damages, and improvement of the patient's clinical condition.[3,14,18,20,21,22,23] This means that the antivenom has reached enough concentration to neutralize the injected venom in the body. Therapeutic response can be judged based on the local and systemic manifestations. For all snakebite envenomation, cessation of progression of swelling and/or other local manifestations can be considered as the therapeutic response.[3,14,21,22,23] To judge according to systemic manifestations, improvement in coagulation tests and platelet count for hemotoxic snakebite envenomation, reversal of cranial neuropathies, respiratory paralysis, and bulbar palsy for neurotoxic snakebite envenomation and reversal of pain and myoglobinuria, decrease of creatinine and CPK for myotoxic snakebite envenomation should be regarded as the goal.[3,14,18,20,21,22,23,24,26] For evaluation of the therapeutic response in the first 24 hours, it is more pragmatic to consider systemic manifestations rather than local effects, as local effects in the first 24 hours might progress even when the antivenom has reached enough concentration to block the venom.[18] Nevertheless, it should be kept in mind that neurotoxic features, especially following presynaptic blockade of the neuromuscular junction (NMJ) are poorly reversible and may last days to weeks, even when the envenomation has been totally resolved.[14,22,23,24,33]
Maximum attack dose
Although in most cases, one or two attack doses are enough for achieving the therapeutic response, few patients may experience progression of venom-induced effects after such doses.[3,21,54] It has recently been found that the presence of major swelling, coagulopathy, respiratory distress, spontaneous bleeding, and neurologic effects on admission are the significant risk factors of difficulty in the achievement of the therapeutic response.[3,54] Patients with these clinical manifestations require over 10 to 12 vials of antivenom.[3,54] However, in some studies it has been reported that patients who do not respond to 20 vials of antivenom will not benefit from more doses.[21] Moreover, presynaptic NMJ blockade may potentially persist for some days.[14,22,23,24,33] Hence, administration of over 15 to 20 vials as the attack dosing is not advocated (unless the patient is critically ill or has uncontrollable bleeding). Thus, in such conditions, close expectant observation, initiating the maintenance dose, and adjunctive treatments, in addition to consultation with a physician — expert are recommended.[14,15,16,17,18,19,20,21]
Adverse reactions to antivenom
Two major immediate adverse reactions following antivenom administration are pyretic reactions and acute hypersensitivity reactions.[9,19,22,23,55] The pyretic reactions, which manifest with fever (oral temperature over 38°C), shaking chills, vasodilatation, and a fall in blood pressure are due to contamination of the antivenom with the components of the killed bacteria during the production process.[9,22,23,55] The acute hypersensitivity reactions, which manifest with pruritus, urticaria, flushing, dry cough, wheezing, abdominal colic, nausea and vomiting, tachycardia, fever, and in few cases with hypotension, bronchospasm, and angioedema, are usually due to complement activation caused by the Fc component of antivenom protein.[9,55] In case of pyretic reactions, antivenom infusion can be continued; while following hypersensitivity reactions, antivenom must be discontinued immediately. Specific supportive treatments must be administered in both situations [Table 2]. After stabilizing the patient, the need for antivenom should be re-evaluated based on the severity of envenomation and the seriousness of the reactions. In these conditions, consultation with a physician — expert is recommended.
Coagulopathy
A set of decreased fibrinogen levels, elevated coagulation tests, and a high D-dimer are common features, especially following Viperidae and Australo-Papuan Elapidae envenomations, which resemble the picture of disseminated intravascular coagulation (DIC).[15,27,56,57] However, recent studies showed that DIC does not occur following snakebite, as the significant features of DIC, such as, systemic microthrombi and end-organ failure are absent in these victims.[45,56,57] In hemotoxic envenomation, the coagulation factors are depleted through a different mechanism, and thus, it is suggested that it be called venom-induced consumption coagulopathy (VICC).[56] Accordingly, the administration of heparin is ineffective and prohibited for these patients.[27]
Besides, most coagulation abnormalities following snakebite envenomation are not clinically significant and do not result in active bleeding.[3,14,24,27,45] Therefore, in case of thrombocytopenia and VICC, administration of antivenom must always take precedence over transfusion of blood components.[21,24,27,56] Immediate administration of blood products along with additional doses of antivenom must be considered for patients with active uncontrollable bleeding, spontaneous bleeding with hemodynamic instability, necessary surgical procedures, and major trauma [Table 2].[16,19,22,23,29,30,56,57]
Neurotoxin-induced paralysis
In the first stages of neurotoxic envenomation, diplopia, ptosis, and ophtalmoplegia may occur. However, the greater concern is when bulbar palsy (impairment of the IX, X, XI, and XII cranial nerves, which causes dysphagia, slurred speech, nasal speech, nasal regurgitation, choking, drooling, absence of gag reflex, and tongue fasciculation) or respiratory muscle paralysis develops. Hence, in the presence of diplopia, ptosis, ophtalmoplegia, and the first signs of respiratory paralysis, including dyspnea and use of accessory muscles of respiration, administration of anticholinesterase agents, including neostigmine or edrophonium chloride, along with atropine, is strongly recommended.[13,21,22,23,30,31,32,33] Although these treatments can reverse postsynaptic NMJ blockade, established presynaptic paralysis is poorly reversible.[21,22,23,32] In this respect, administration of 3,4-diaminopyridine may be helpful, as its effect on the Lambert-Eaton myasthenic syndrome, which manifests with extreme muscle weakness due to presynaptic NMJ blockade, is shown to be promising.[58]
Local wound infection
Administration of antibiotics is not generally recommended for a snakebite victim.[21,22,23] In most cases swelling resolves after proper antivenom administration.[3,14,21] Moreover, it is known that the snake venom is antibacterial in nature.[37] However, in cases with major swelling unresponsive to antivenom, it is not clear whether the swelling is due to venom-induced inflammation or secondary infection.[18] Hence, in these cases, if fever (axillary temperature over 38°C) exists or infection is suspected, antibiotics covering Staphylococcus aureus and Enterobacteriaceae bacteria might help the patient.[18,35,36] Furthermore, in case of abscess formation and extensive tissue necrosis, antibiotics are recommended according to culturing of infected wounds and their antibiograms.[37]
Compartment syndrome
Severe pain at rest, pain on passive stretching of the compartment muscles, hypoesthesia of compartment skin, tenseness, and discoloration of the compartment are highly suggestive of the compartment syndrome in a patient who is bitten in finger, hand, foot or anterior part of the tibia, or has severe extensive swelling, or has tied tight tourniquet around the bite site for a long time prior to admission.[18,21,22,23] However, in most cases, these manifestations only mimic the compartment syndrome and they are completely resolvable with adequate antivenom administration. Furthermore, classic treatment with fasciotomy may put the patient at more risks and complications, such as, considerable local bleeding, residual disfiguring skin scars, and function loss of the affected extremity.[22,23,39] Therefore, indication of fasciotomy in snakebite is limited to the presence of an intercompartmental pressure of over 30 mmHg in children, or 45 mmHg in adults, refractory to mannitol diuresis and antivenom.[18,21,22,23,39]
Rhabdomyolysis
Rhabdomyolysis following snakebite is mostly seen following Elapidae (sea snake) envenomation and rarely as a consequence of a severe compartment syndrome.[9,18,23,39] It is characterized by increased CPK (over 1000 U / L), myalgia, weakness, and tea-colored urine.[40,59] The treatment must be focused on protecting the kidneys from myoglobinuria-induced damages [Table 2].[40,59]
Maintenance dose
After achieving therapeutic response, maintaining a stable concentration of antivenom is essential to prevent recurrence of clinical manifestations.[14,43,52] The recurrence of venom effects is supposed to be due to the decrease of antivenom concentration and redistribution of venom from the bite site and surrounding tissues, resulting in venom — antivenom mismatch.[43] For Razi™ antivenin, a maintenance dose has not been instructed in its package insert.[53] However, from local experience, recurrence of clinical manifestations is possible without administration of a maintenance dose.[13] Hence, slow infusion of five vials in 18 hours after achieving initial control is recommended.[13,52]
Dose adjustment
The antivenom should confront as much venom as has entered the body. Therefore, for children and pregnant women, dose adjustment is not recommended.[60,61,62,63] However, for children less than 10 kg, the total volume of fluid for dilution of antivenom should be adjusted. In pregnancy, envenomation may cause miscarriage, and also venom or antivenom can cross the placenta. Nevertheless, the risk/benefit ratio generally favors antivenom administration to pregnant women with significant venom effects.[62,63]
CONCLUSIONS
Snakebite envenomation is a clinical toxicologic emergency and needs to be treated in a timely and organized manner. Hence, a multi-aspect evidence-based protocol has been designed to improve the clinical outcomes, reduce unnecessary administration of antivenom, and help physicians to make better and proper clinical judgments. The effectiveness of the protocol forms the subject for debate in future prospective studies. The authors recommend pursuing this protocol in toxicology centers around Iran, to move toward a national consensus with the help of medical toxicology experts.
ACKNOWLEDGMENTS
The authors express their gratitude to Dr. E. J. Lavonas; medical toxicologist, from the Rocky Mountain Poison and Drug Center, for his valuable comments. The authors are also thankful to Dr. M. Mahmoudi; immunologist, Dr. R. Boostani; neurologist, Dr. Z. Mozaheb; hematologist, Dr. M. M. Kooshyar; hematologist, Dr. F. Abedi, infectious disease and tropical medicine specialist, Dr H. Khosrojerdi; medical toxicologist, and Dr. A. R. Kachooie; Orthopedics, from the Mashhad University of Medical Sciences, for their useful recommendations to elaborate the protocol.
Footnotes
Source of Support: None
Conflict of Interest: The authors report no conflict of interest.
REFERENCES
- 1.Afshari R, Majdzadeh R, Balali-Mood M. Pattern of acute poisonings in Mashhad, Iran 1993-2000. J Toxicol Clin Toxicol. 2004;42:965–75. doi: 10.1081/clt-200042550. [DOI] [PubMed] [Google Scholar]
- 2.Tehran: Publications of Ministry of Health and Medical Education; 2010. Iran Ministry of Health and Medical Education. National Report of Envenomation. In Persian. [Google Scholar]
- 3.Dadpour B, Shafahi A, Monzavi SM, Zavar A, Afshari R, Khoshdel AR. Snakebite prognostic factors: Leading factors of weak therapeutic response following snakebite envenomation. Asia Pac J Med Toxicol. 2012;1:27–33. [Google Scholar]
- 4.Firouz E. London: I.B. Tauris; 2005. The complete fauna of Iran. [Google Scholar]
- 5.Latifi M. Tehran: Publications of Iran Department of Environment; 1991. Snakes of Iran. In Persian. [Google Scholar]
- 6.Rezaie-Atagholipour M, Riyahi-Bakhtiari A, Rajabizadeh M, Ghezellou P. Status of the Annulated Sea Snake, Hydrophis cyanocinctus, in the Hara Protected Area of the Persian Gulf. Zool Middle East. 2012;57:53–60. doi: 10.1016/j.marpolbul.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Afshari R, Khadem-Rezaiyan M, Balali-Mood M. Spider bite (latrodectism) in Mashhad, Iran. Hum Exp Toxicol. 2009;28:697–702. doi: 10.1177/0960327109350668. [DOI] [PubMed] [Google Scholar]
- 8.Afshari R. Mashhad: Mashhad University of Medical Sciences; 1995. Spiderbite and Snakebite in northeast Iran. [Google Scholar]
- 9.Warrell DA. Snake bite. Lancet. 2010;375:77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO). Snakebite. 2009. [Last accessed on 2013 May 10]. Available from: http://www.who.int/neglected_diseases/diseases/snakebites/en/index.html .
- 11.Chippaux JP, Williams V, White J. Snake venom variability: Methods of study, results and interpretation. Toxicon. 1991;29:1279–303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- 12.Theakston RD, Warrell DA. Antivenoms: A list of hyperimmune sera currently available for the treatment of envenoming by bites and stings. Toxicon. 1991;29:1419–70. doi: 10.1016/0041-0101(91)90002-9. [DOI] [PubMed] [Google Scholar]
- 13.Afshari R, Monzavi SM. Venomous animals and arthropods envenomation. In: Afshari R, Monzavi SM, editors. Afshari's Clinical Toxicology and Poisoning Emergency Care. 2nd ed. Mashhad: Mashhad University of Medical Sciences Publication; 2012. pp. 221–41. [Google Scholar]
- 14.Pizon AF, Riley BD, Ruha AM. Antidotes in Depth: Antivenom (Crotaline) In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, editors. Goldfrank's Toxicologic Emergencies. 9th ed. New York: McGraw-Hill; 2011. pp. 1611–4. [Google Scholar]
- 15.Walter FG, Chase PB, Fernández MC, McNally J, Borron SW. Venomous snakes. In: Shannon MW, Borron SW, Burns MJ, editors. Haddad and Winchester's clinical management of poisoning and drug overdose. 4th ed. Philadelphia: Saunders; 2007. pp. 399–432. [Google Scholar]
- 16.Cribari C. Chicago: American College of Surgeons Committee on Trauma; 2004. [Last accessed on 2011 Dec 20]. Management of Poisonous Snakebites. Available from: http://www.facs.org/trauma/publications/snakebite.pdf . [Google Scholar]
- 17.de Haro L. Management of snakebites in France. Toxicon. 2012;60:712–8. doi: 10.1016/j.toxicon.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Otero-Patiño R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009;54:998–1011. doi: 10.1016/j.toxicon.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Burgess JL, Dart RC. Snake venom coagulopathy: Use and abuse of blood products in the treatment of pit viper envenomation. Ann Emerg Med. 1991;20:795–801. doi: 10.1016/s0196-0644(05)80845-5. [DOI] [PubMed] [Google Scholar]
- 20.Weant KA, Bowers RC, Reed J, Braun KA, Dodd DM, Baker SN. Safety and cost-effectiveness of a clinical protocol implemented to standardize the use of Crotalidae polyvalent immune Fab antivenom at an academic medical center. Pharmacotherapy. 2012;32:433–40. doi: 10.1002/j.1875-9114.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- 21.Lavonas EJ, Ruha AM, Banner W, Bebarta V, Bernstein JN, Bush SP, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: Results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2. doi: 10.1186/1471-227X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO/SEARO Guidelines for the clinical management of snake bites in the Southeast Asian region. Southeast Asian J Trop Med Public Health. 1999;30(Suppl 1):1–85. [PubMed] [Google Scholar]
- 23.Warrell DA. 2nd ed. New Delhi: WHO Press; 2010. Guidelines for the management of snake-bites. [Google Scholar]
- 24.White J. Adelaide: Department of Health; 2006. Snakebite and spider bite Management Guidelines South Australia. [Google Scholar]
- 25.Ghosh S, Maisnam I, Murmu BK, Mitra PK, Roy A, Simpson ID. A locally developed snakebite management protocol significantly reduces overall anti snake venom utilization in West Bengal, India. Wilderness Environ Med. 2008;19:267–74. doi: 10.1580/08-WEME-OR-219.1. [DOI] [PubMed] [Google Scholar]
- 26.Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347–56. doi: 10.1056/NEJMra013477. [DOI] [PubMed] [Google Scholar]
- 27.White J. Snake venoms and coagulopathy. Toxicon. 2005;45:951–67. doi: 10.1016/j.toxicon.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Sano-Martins IS, Fan HW, Castro SC, Tomy SC, Franca FO, Jorge MT, et al. Reliability of the simple 20 minute whole blood clotting test (WBCT20) as an indicator of low plasma fibrinogen concentration in patients envenomed by Bothrops snakes. Butantan Institute Antivenom Study Group. Toxicon. 1994;32:1045–50. doi: 10.1016/0041-0101(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 29.Isbister GK, Duffull SB, Brown SG ASP Investigators. Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM. 2009;102:563–8. doi: 10.1093/qjmed/hcp081. [DOI] [PubMed] [Google Scholar]
- 30.Brown SG, Caruso N, Borland ML, McCoubrie DL, Celenza A, Isbister GK. Clotting factor replacement and recovery from snake venom-induced consumptive coagulopathy. Intensive Care Med. 2009;35:1532–8. doi: 10.1007/s00134-009-1556-7. [DOI] [PubMed] [Google Scholar]
- 31.Bucaretchi F, Hyslop S, Vieira RJ, Toledo AS, Madureira PR, de Capitani EM. Bites by coral snakes (Micrurus spp.) in Campinas, State of São Paulo, Southeastern Brazil. Rev Inst Med Trop Sao Paulo. 2006;48:141–5. doi: 10.1590/s0036-46652006000300005. [DOI] [PubMed] [Google Scholar]
- 32.Gold BS. Neostigmine for the treatment of neurotoxicity following envenomation by the Asiatic cobra. Ann Emerg Med. 1996;28:87–9. doi: 10.1016/s0196-0644(96)70142-7. [DOI] [PubMed] [Google Scholar]
- 33.Del Brutto OH, Del Brutto VJ. Neurological complications of venomous snake bites: A review. Acta Neurol Scand. 2012;125:363–72. doi: 10.1111/j.1600-0404.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- 34.Bolla KI, Cadet JL. Exogenous acquired metabolic disorders of the nervous system: Toxins and Illicit Drugs. In: Goetz CG, editor. Textbook of Clinical Neurology. 3rd ed. Chicago: Saunders; 2007. pp. 865–96. [Google Scholar]
- 35.Kularatne SA, Kumarasiri PV, Pushpakumara SK, Dissanayaka WP, Ariyasena H, Gawarammana IB, et al. Routine antibiotic therapy in the management of the local inflammatory swelling in venomous snakebites: Results of a placebo-controlled study. Ceylon Med J. 2005;50:151–5. doi: 10.4038/cmj.v50i4.1405. [DOI] [PubMed] [Google Scholar]
- 36.Boels D, Hamel JF, Bretaudeau Deguigne M, Harry P. European viper envenomings: Assessment of Viperfav™ and other symptomatic treatments. Clin Toxicol (Phila) 2012;50:189–96. doi: 10.3109/15563650.2012.660695. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein EJC. Bites. In: Mandel GL, Bennet JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed. New York: Churchill Livingstone; 2010. pp. 3911–15. [Google Scholar]
- 38.Leversedge FJ, Moore TJ, Peterson BC, Seiler JG., 3rd Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544–59. doi: 10.1016/j.jhsa.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Bucaretchi F, de Capitani EM, Hyslop S, Mello SM, Madureira PR, Zanardi V, et al. Compartment syndrome after Bothrops jararaca snakebite: Monitoring, treatment, and outcome. Clin Toxicol (Phila) 2010;48:57–60. doi: 10.3109/15563650903356201. [DOI] [PubMed] [Google Scholar]
- 40.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 41.Sitprija V. Snakebite nephropathy. Nephrology (Carlton) 2006;11:442–8. doi: 10.1111/j.1440-1797.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 42.Phillips CM. Sea snake envenomation. Dermatol Ther. 2002;15:58–61. [Google Scholar]
- 43.Boyer LV, Seifert SA, Cain JS. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann Emerg Med. 2001;37:196–201. doi: 10.1067/mem.2001.113134. [DOI] [PubMed] [Google Scholar]
- 44.Ireland G, Brown SG, Buckley NA, Stormer J, Currie BJ, White J, et al. Changes in serial laboratory test results in snakebite patients: When can we safely exclude envenoming? Med J Aust. 2010;193:285–90. doi: 10.5694/j.1326-5377.2010.tb03909.x. [DOI] [PubMed] [Google Scholar]
- 45.Dadpour B, Monzavi SM, Afshari R. Coagulopathic Disorders Following Snakebite: A 5-Year Study of 108 Patients in Khorasan Region. Abstracts of the 11th Scientific Congress of Asia Pacific Association of Medical Toxicology, Hong Kong, 29 November-1 December 2012. Hong Kong J Emerg Med. 2012;19:443. [Google Scholar]
- 46.Balali-Mood M, Afshari R. Tehran, Iran: Shahid Beheshti University of Medical Sciences; 1995. Comparison of spider and snake bites in Mashhad. Paper presented at: 4 th Congress of the Iranian Society of Toxicology. [Google Scholar]
- 47.Richardson WH, Goto CS, Gutglass DJ, Williams SR, Clark RF. Rattlesnake envenomation with neurotoxicity refractory to treatment with crotaline Fab antivenom. Clin Toxicol (Phila) 2007;45:472–5. doi: 10.1080/15563650701338187. [DOI] [PubMed] [Google Scholar]
- 48.Isbister GK, Brown SG, MacDonald E, White J, Currie BJ Australian Snakebite Project Investigators. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Med J Aust. 2008;188:473–6. doi: 10.5694/j.1326-5377.2008.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 49.de Silva HA, Pathmeswaran A, Ranasinha CD, Jayamanne S, Samarakoon SB, Hittharage A, et al. Low-dose adrenaline, promethazine, and hydrocortisone in the prevention of acute adverse reactions to antivenom following snakebite: A randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8:e1000435. doi: 10.1371/journal.pmed.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DJ, Jensen SD, Nimorakiotakis B, Müller R, Winkel KD. Antivenom use, premedication and early adverse reactions in the management of snake bites in rural Papua New Guinea. Toxicon. 2007;49:780–92. doi: 10.1016/j.toxicon.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 51.Premawardhena AP, de Silva CE, Fonseka MM, Gunatilake SB, de Silva HJ. Low dose subcutaneous adrenaline to prevent acute adverse reactions to antivenom serum in people bitten by snakes: Randomised, placebo controlled trial. BMJ. 1999;318:1041–3. doi: 10.1136/bmj.318.7190.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dart RC, Seifert SA, Boyer LV, Clark RF, Hall E, McKinney P, et al. A randomized multicenter trial of crotalinae polyvalent immune Fab (ovine) antivenom for the treatment for crotaline snakebite in the United States. Arch Intern Med. 2001;161:2030–6. doi: 10.1001/archinte.161.16.2030. [DOI] [PubMed] [Google Scholar]
- 53.Tehran: Razi Vaccine and Serum Research Institute (RVSRI); 2002. Razi Vaccine and Serum Research Institute (RVSRI). Razi™ Polyvalent snake antivenin prescribing information. [Google Scholar]
- 54.Yin S, Kokko J, Lavonas E, Mlynarchek S, Bogdan G, Schaeffer T. Factors associated with difficulty achieving initial control with crotalidae polyvalent immune fab antivenom in snakebite patients. Acad Emerg Med. 2011;18:46–52. doi: 10.1111/j.1553-2712.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- 55.Lalloo DG, Theakston RD. Snake antivenoms. J Toxicol Clin Toxicol. 2003;41:277. doi: 10.1081/clt-120021113. [DOI] [PubMed] [Google Scholar]
- 56.Isbister GK. Snakebite doesn’t cause disseminated intravascular coagulation: Coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost. 2010;36:444–51. doi: 10.1055/s-0030-1254053. [DOI] [PubMed] [Google Scholar]
- 57.Levi M, Seligsohn U. Disseminated Intravascular Coagulation. In: Kaushansky K, Lichtman M, Beutler E, Kipps T, Prchal J, Seligsohn U, editors. Williams Hematology. 8th ed. New York: McGraw-Hill; 2010. pp. 2101–20. [Google Scholar]
- 58.Lindquist S, Stangel M. Update on treatment options for Lambert-Eaton myasthenic syndrome: Focus on use of amifampridine. Neuropsychiatr Dis Treat. 2011;7:341–9. doi: 10.2147/NDT.S10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Ismaili Z, Piccioni M, Zappitelli M. Rhabdomyolysis: Pathogenesis of renal injury and management. Pediatr Nephrol. 2011;26:1781–8. doi: 10.1007/s00467-010-1727-3. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt JM. Antivenom therapy for snakebites in children: Is there evidence? Curr Opin Pediatr. 2005;17:234–8. doi: 10.1097/01.mop.0000152621.67049.f2. [DOI] [PubMed] [Google Scholar]
- 61.Offerman SR, Bush SP, Moynihan JA, Clark RF. Crotaline Fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics. 2002;110:968–71. doi: 10.1542/peds.110.5.968. [DOI] [PubMed] [Google Scholar]
- 62.Sebe A, Satar S, Acikalin A. Snakebite during pregnancy. Hum Exp Toxicol. 2005;24:341–5. doi: 10.1191/0960327105ht535oa. [DOI] [PubMed] [Google Scholar]
- 63.Brown SA, Seifert SA, Rayburn WF. Management of envenomations during pregnancy. Clin Toxicol (Phila) 2013;51:3–15. doi: 10.3109/15563650.2012.760127. [DOI] [PubMed] [Google Scholar]


