Abstract
It is well-known that deficiency or over exposure to various elements has noticeable effects on human health. The effect of an element is determined by several characteristics, including absorption, metabolism, and degree of interaction with physiological processes. Iron is an essential element for almost all living organisms as it participates in a wide variety of metabolic processes, including oxygen transport, deoxyribonucleic acid (DNA) synthesis, and electron transport. However, as iron can form free radicals, its concentration in body tissues must be tightly regulated because in excessive amounts, it can lead to tissue damage. Disorders of iron metabolism are among the most common diseases of humans and encompass a broad spectrum of diseases with diverse clinical manifestations, ranging from anemia to iron overload, and possibly to neurodegenerative diseases. In this review, we discuss the latest progress in studies of iron metabolism and bioavailability, and our current understanding of human iron requirement and consequences and causes of iron deficiency. Finally, we discuss strategies for prevention of iron deficiency.
Keywords: Anemia, human iron requirement, iron bioavailability, iron deficiency, iron metabolism
INTRODUCTION
From ancient times, man has recognized the special role of iron in health and disease.[1] Iron had early medicinal uses by Egyptians, Hindus, Greeks, and Romans.[2,3] During the 17th century, iron was used to treat chlorosis (green disease), a condition often resulting from the iron deficiency.[4] However, it was not until 1932 that the importance of iron was finally settled by the convincing proof that inorganic iron was needed for hemoglobin synthesis.[5] For many years, nutritional interest in iron focused on its role in hemoglobin formation and oxygen transport.[6] Nowadays, although low iron intake and/or bioavailability are responsible for most anemia in industrialized countries, they account for only about half of the anemia in developing countries,[7] where infectious and inflammatory diseases (especially malaria), blood loss from parasitic infections, and other nutrient deficiencies (vitamin A, riboflavin, folic acid, and vitamin B12) are also important causes.[8]
Biochemistry and physiology
In contrast to zinc, iron is an abundant element on earth[2,9] and is a biologically essential component of every living organism.[10,11] However, despite its geologic abundance, iron is often a growth limiting factor in the environment.[9] This apparent paradox is due to the fact that in contact with oxygen iron forms oxides, which are highly insoluble, and thus is not readily available for uptake by organisms.[2] In response, various cellular mechanisms have evolved to capture iron from the environment in biologically useful forms. Examples are siderophores secreted by microbes to capture iron in a highly specific complex[12] or mechanisms to reduce iron from the insoluble ferric iron (Fe+3) to the soluble ferrous form (Fe+2) as in yeasts.[13] Many of the mechanisms found in lower organisms, have analogous counterparts in higher organisms, including humans. In the human body, iron mainly exists in complex forms bound to protein (hemoprotein) as heme compounds (hemoglobin or myoglobin), heme enzymes, or nonheme compounds (flavin-iron enzymes, transferring, and ferritin).[3] The body requires iron for the synthesis of its oxygen transport proteins, in particular hemoglobin and myoglobin, and for the formation of heme enzymes and other iron-containing enzymes involved in electron transfer and oxidation-reductions.[14,3] Almost two-thirds of the body iron is found in the hemoglobin present in circulating erythrocytes, 25% is contained in a readily mobilizable iron store, and the remaining 15% is bound to myoglobin in muscle tissue and in a variety of enzymes involved in the oxidative metabolism and many other cell functions.[15]
Iron is recycled and thus conserved by the body. Figure 1 shows a schematic diagram of iron cycle in the body. Iron is delivered to tissues by circulating transferrin, a transporter that captures iron released into the plasma mainly from intestinal enterocytes or reticuloendothelial macrophages. The binding of iron-laden transferrin to the cell-surface transferrin receptor (TfR) 1 results in endocytosis and uptake of the metal cargo. Internalized iron is transported to mitochondria for the synthesis of heme or iron-sulfur clusters, which are integral parts of several metalloproteins, and excess iron is stored and detoxified in cytosolic ferritin.
Figure 1.
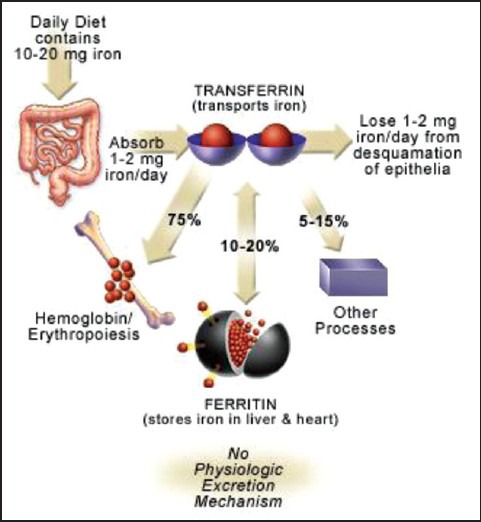
Iron is bound and transported in the body via transferrin and stored in ferritin molecules. Once iron is absorbed, there is no physiologic mechanism for excretion of excess iron from the body other than blood loss, that is, pregnancy, menstruation, or other bleeding
METABOLISM
Absorption
The fraction of iron absorbed from the amount ingested is typically low, but may range from 5% to 35% depending on circumstances and type of iron.[3]
Iron absorption occurs by the enterocytes by divalent metal transporter 1, a member of the solute carrier group of membrane transport proteins. This takes place predominantly in the duodenum and upper jejunum.[16] It is then transferred across the duodenal mucosa into the blood, where it is transported by transferrin to the cells or the bone marrow for erythropoiesis [producing red blood cells (RBCs)].[14,17,18] A feedback mechanism exists that enhances iron absorption in people who are iron deficient. In contrast, people with iron overload dampen iron absorption via hepcidin. It is now generally accepted that iron absorption is controlled by ferroportin which allows or does not allow iron from the mucosal cell into the plasma.
The physical state of iron entering the duodenum greatly influences its absorption. At physiological pH, ferrous iron (Fe+2) is rapidly oxidized to the insoluble ferric (Fe+3) form. Gastric acid lowers the pH in the proximal duodenum reducing Fe+3 in the intestinal lumen by ferric reductases, thus allowing the subsequent transport of Fe+2 across the apical membrane of enterocytes. This enhances the solubility and uptake of ferric iron. When gastric acid production is impaired (for instance by acid pump inhibitors such as the drug, prilosec), iron absorption is reduced substantially.
Dietary heme can also be transported across the apical membrane by a yet unknown mechanism and subsequently metabolized in the enterocytes by heme oxygenase 1 (HO-1) to liberate (Fe+2).[19] This process is more efficient than the absorption of inorganic iron and is independent of duodenal pH. It is thus not influenced by inhibitors such as phytate and polyphenols. Consequently, red meats high in hemoglobin are excellent nutrient sources of iron. Directly internalized Fe+2 is processed by the enterocytes and eventually (or not) exported across the basolateral membrane into the bloodstream via Fe+2 transporter ferroportin. The ferroportin-mediated efflux of Fe+2 is coupled by its reoxidation to Fe+2, catalyzed by the membrane-bound ferroxidase hephaestin that physically interacts with ferroportin[20] and possibly also by its plasma homologue ceruloplasmin. Exported iron is scavenged by transferrin, which maintains Fe+3 in a redox-inert state and delivers it into tissues. The total iron content of transferrin (≈3 mg) corresponds to less than 0.1% of body iron, but it is highly dynamic and undergoes more than 10 times daily turnover to sustain erythropoiesis. The transferrin iron pool is replenished mostly by iron recycled from effete RBCs and, to a lesser extent, by newly absorbed dietary iron. Senescent RBCs are cleared by reticuloendothelial macrophages, which metabolize hemoglobin and heme, and release iron into the bloodstream. By analogy to intestinal enterocytes, macrophages export Fe+2 from their plasma membrane via ferroportin, in a process coupled by reoxidation of Fe+2 to Fe+3 by ceruloplasmin and followed by the loading of Fe+3 to transferrin.[21]
Theil et al.,[21] recently reported that an independent mechanism also exists for the absorption of plant ferritins mostly present in legumes. However, the relevance of the ferritin transporter is unclear as most ferritin seems to be degraded during food processing and digestion, thereby releasing inorganic iron from the ferritin shell for absorption by the normal mechanism.[22] As one ferritin molecule contains 1000 or more iron atoms, and should also be unaffected by iron absorption inhibitors, such a mechanism would provide an important source of iron in the developing world where legumes are commonly consumed.
Regulation of iron homeostasis
Since iron is required for a number of diverse cellular functions, a constant balance between iron uptake, transport, storage, and utilization is required to maintain iron homeostasis.[11] As the body lacks a defined mechanism for the active excretion of iron, iron balance is mainly regulated at the point of absorption.[23,24]
Hepcidin is a circulating peptide hormone secreted by the liver that plays a central role in the regulation of iron homeostasis. It is the master regulator of systemic iron homeostasis, coordinating the use and storage of iron with iron acquisition.[25] This hormone is primarily produced by hepatocytes and is a negative regulator of iron entry into plasma [Figure 2]. Hepcidin acts by binding to ferroportin, an iron transporter present on cells of the intestinal duodenum, macrophages, and cells of the placenta. Binding of hepcidin induces ferroportin internalization and degradation.[26] The loss of ferroportin from the cell surface prevents iron entry into plasma [Figure 2a]. Decreased iron entry into plasma results in low transferrin saturation and less iron is delivered to the developing erythroblast. Conversely, decreased expression of hepcidin leads to increased cell surface ferroportin and increased iron absorption[27] [Figure 2c]. In all species, the concentration of iron in biological fluids is tightly regulated to provide iron as needed and to avoid toxicity, because iron excess can lead to the generation of reactive oxygen species.[28] Iron homeostasis in mammals is regulated at the level of intestinal absorption, as there is no excretory pathway for iron.
Figure 2.
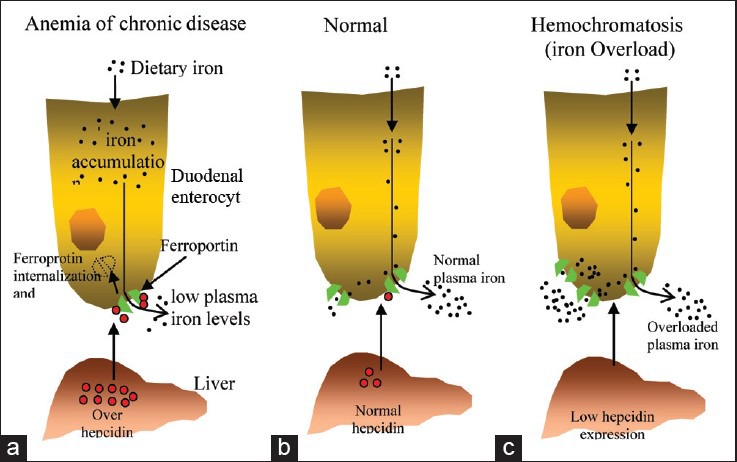
Hepcidin-mediated regulation of iron homeostasis. (a) Increased hepcidin expression by the liver results from inflammatory stimuli. High levels of hepcidin in the bloodstream result in the internalization and degradation of the iron exporter ferroportin. Loss of cell surface ferroportin results in macrophage iron loading, low plasma iron levels, and decreased erythropoiesis due to decreased transferrin-bound iron. The decreased erythropoiesis gives rise to the anemia of chronic disease. (b) Normal hepcidin levels, in response to iron demand, regulate the level of iron import into plasma, normal transferrin saturation, and normal levels of erythropoiesis. (c) Hemochromatosis, or iron overload, results from insufficient hepcidin levels, causing increased iron import into plasma, high transferrin saturation, and excess iron deposition in the liver. Source: De Domenico, et al.[27]
Plasma hepcidin levels are regulated by different stimuli, including cytokines, plasma iron, anemia, and hypoxia. Dysregulation of hepcidin expression results in iron disorders. Overexpression of hepcidin leads to the anemia of chronic disease, while low hepcidin production results in hereditary hemochromatosis (HFE) with consequent iron accumulation in vital organs [Figure 2]. Most hereditary iron disorders result from inadequate hepcidin production relative to the degree of tissue iron accumulation. Impaired hepcidin expression has been shown to result from mutations in any of 4 different genes: TfR2, HFE, hemochromatosis type 2 (HFE2), and hepcidin antimicrobial peptide (HAMP). Mutations in HAMP, the gene that encodes hepcidin, result in iron overload disease, as the absence of hepcidin permits constitutively high iron absorption. The role for other genes (TFR2, HFE, and HFE2) in the regulation of hepcidin production has been unclear.[27]
Storage
Ferritin concentration together with that of hemosiderin reflects the body iron stores. They store iron in an insoluble form and are present primarily in the liver, spleen, and bone marrow.[2] The majority of iron is bound to the ubiquitous and highly conserved iron-binding protein, ferritin.[18] Hemosiderin is an iron storage complex that less readily releases iron for body needs. Under steady state conditions, serum ferritin concentrations correlate well with total body iron stores.[29] Thus, serum ferritin is the most convenient laboratory test to estimate iron stores.
Excretion
Apart from iron losses due to menstruation, other bleeding or pregnancy, iron is highly conserved and not readily lost from the body.[30] There are some obligatory loss of iron from the body that results from the physiologic exfoliation of cells from epithelial surfaces,[30] including the skin, genitourinary tract, and gastrointestinal tract.[3] However, these losses are estimated to be very limited (≈1 mg/day).[31] Iron losses through bleeding can be substantial and excessive menstrual blood loss is the most common cause of iron deficiency in women.
BIOAVAILABILITY
Dietary iron occurs in two forms: heme and nonheme.[23] The primary sources of heme iron are hemoglobin and myoglobin from consumption of meat, poultry, and fish, whereas nonheme iron is obtained from cereals, pulses, legumes, fruits, and vegetables.[32] Heme iron is highly bioavailable (15%-35%) and dietary factors have little effect on its absorption, whereas nonheme iron absorption is much lower (2%-20%) and strongly influenced by the presence of other food components.[23] On the contrary, the quantity of nonheme iron in the diet is manyfold greater than that of heme-iron in most meals. Thus despite its lower bioavailability, nonheme iron generally contributes more to iron nutrition than heme-iron.[33] Major inhibitors of iron absorption are phytic acid, polyphenols, calcium, and peptides from partially digested proteins.[23] Enhancers are ascorbic acid and muscle tissue which may reduce ferric iron to ferrous iron and bind it in soluble complexes which are available for absorption[23]
Factors enhancing iron absorption
A number of dietary factors influence iron absorption. Ascorbate and citrate increase iron uptake in part by acting as weak chelators to help to solubilize the metal in the duodenum [Table 1].[34] Iron is readily transferred from these compounds into the mucosal lining cells. The dose-dependent enhancing effect of native or added ascorbic acid on iron absorption has been shown by researchers.[34] The enhancing effect is largely due to its ability to reduce ferric to ferrous iron but is also due to its potential to chelate iron.[35] Ascorbic acid will overcome the negative effect on iron absorption of all inhibitors, which include phytate,[36] polyphenols,[37] and the calcium and proteins in milk products,[38] and will increase the absorption of both native and fortification iron. In fruit and vegetables, the enhancing effect of ascorbic acid is often cancelled out by the inhibiting effect of polyphenols.[39] Ascorbic acid is the only absorption enhancer in vegetarian diets, and iron absorption from vegetarian and vegan meals can be best optimized by the inclusion of ascorbic acid-containing vegetables.[40] Cooking, industrial processing, and storage degrade ascorbic acid and remove its enhancing effect on iron absorption.[41]
Table 1.
Factors that could influence iron absorption
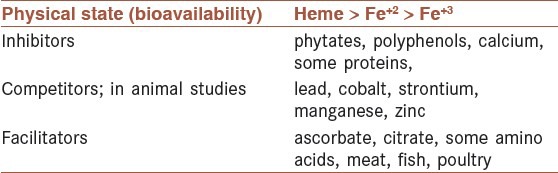
The enhancing effect of meat, fish, or poultry on iron absorption from vegetarian meals has been shown,[42] and 30 g muscle tissue is considered equivalent to 25 mg ascorbic acid.[33] Bjorn-Rasmussen and Hallberg[43] reported that the addition of chicken, beef, or fish to a maize meal increased nonheme iron absorption 2-3-fold with no influence of the same quantity of protein added as egg albumin. As with ascorbic acid, it has been somewhat more difficult to demonstrate the enhancing effect of meat in multiple meals and complete diet studies. Reddy et al.,[44] reported only a marginal improvement in iron absorption (35%) in self-selected diets over 5 days when daily muscle tissue intake was increased to 300 g/day, although, in a similar 5-day study, 60 g pork meat added to a vegetarian diet increased iron absorption by 50%.[45]
Factors inhibiting iron absorption
In plant-based diets, phytate (myo-inositol hexakisphosphate) is the main inhibitor of iron absorption.[23] The negative effect of phytate on iron absorption has been shown to be dose dependent and starts at very low concentrations of 2-10 mg/meal.[37,46] The molar ratio of phytate to iron can be used to estimate the effect on absorption. The ratio should be 1:1 or preferably, 0.4:1 to significantly improve iron absorption in plain cereal or legume-based meals that do not contain any enhancers of iron absorption, or, 6:1 in composite meals with certain vegetables that contain ascorbic acid and meat as enhancers.[47]
Polyphenols occur in various amounts in plant foods and beverages, such as vegetables, fruit, some cereals and legumes, tea, coffee, and wine. The inhibiting effect of polyphenols on iron absorption has been shown with black tea and to a lesser extent with herbal teas.[48,49] In cereals and legumes, polyphenols add to the inhibitory effect of phytate, as was shown in a study that compared high and low polyphenol sorghum.[23]
Calcium has been shown to have negative effects on nonheme and heme iron absorption, which makes it different from other inhibitors that affect nonheme iron absorption only.[50] Dose-dependent inhibitory effects were shown at doses of 75-300 mg when calcium was added to bread rolls and at doses of 165 mg calcium from milk products.[51] It is proposed that single-meal studies show negative effects of calcium on iron absorption, whereas multiple-meal studies, with a wide variety of foods and various concentrations of other inhibitors and enhancers, indicate that calcium has only a limited effect on iron absorption.[52]
Animal proteins such as milk proteins, egg proteins, and albumin, have been shown to inhibit iron absorption.[53] The two major bovine milk protein fractions, casein and whey, and egg white were shown to inhibit iron absorption in humans.[54] Proteins from soybean also decrease iron absorption.[55]
Competition with iron
Competition studies suggest that several other heavy metals may share the iron intestinal absorption pathway. These include lead, manganese, cobalt, and zinc Table 1. As iron deficiency often coexists with lead intoxication, this interaction can produce particularly serious medical complications in children.[56]
Lead is a particularly pernicious element to iron metabolism.[57] Lead is taken up by the iron absorption machinery (DTM1), and secondarily blocks iron through competitive inhibition. Further, lead interferes with a number of important iron-dependent metabolic steps such as heme biosynthesis. This multifaceted influence has particularly dire consequences in children, were lead not only produces anemia, but can impair cognitive development. Lead exists naturally at high levels in ground water and soil in some regions, and can clandestinely attack children's health. For this reason, most pediatricians in the U.S. routinely test for lead at an early age through a simple blood test.
HUMAN REQUIREMENTS
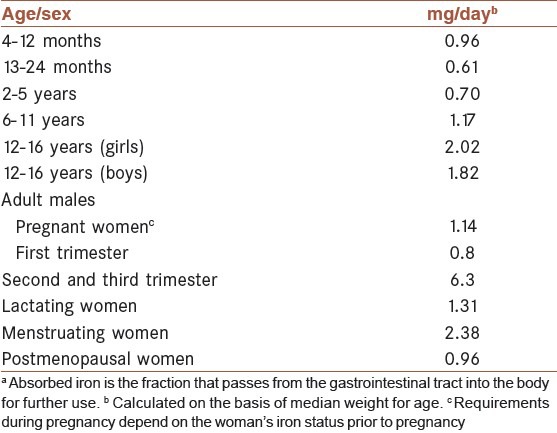
During early infancy, iron requirements are met by the little iron contained in the human milk.[58] The need for iron rises markedly 4-6 months after birth and amounts to about 0.7-0.9 mg/day during the remaining part of the first year.[58] Between 1 and 6 years of age, the body iron content is again doubled.[58] Iron requirements are also very high in adolescents, particularly during the period of growth spurt. Girls usually have their growth spurt before menarche, but growth is not finished at that time. In boys there is a marked increase in hemoglobin mass and concentration during puberty. In this stage, iron requirements increase to a level above the average iron requirements in menstruating women[58] [see Table 2].
Table 2.
Iron requirements of 97.5% of individuals in terms of absorbed irona, by age group and sex (World Health Organization, 1989)
The average adult stores about 1-3 g of iron in his or her body. A fine balance between dietary uptake and loss maintains this balance. About 1 mg of iron is lost each day through sloughing of cells from skin and mucosal surfaces, including the lining of the gastrointestinal tract.[59] Menstruation increases the average daily iron loss to about 2 mg per day in premenopausal female adults.[60] The augmentation of body mass during neonatal and childhood growth spurts transiently boosts iron requirements.[61]
A dietary intake of iron is needed to replace iron lost in the stools and urine as well as through the skin. These basal losses represent approximately 0.9 mg of iron for an adult male and 0.8 mg for an adult female.[62] The iron lost in menstrual blood must be taken into consideration for women of reproductive age [Table 2].
GROUPS AT HIGH RISK
The highest probability of suffering iron deficiency is found in those parts of a population that have inadequate access to foods rich in absorbable iron during stages of high iron demand. These groups correspond to children, adolescents, and women of reproductive age, in particular during pregnancy.[63,58]
In the case of infants and adolescents, the increased iron demand is the result of rapid growth. For women of reproductive age the principle reason is the excessive blood loss during menstruation. During pregnancy, there is a significant increase in iron requirement due to the rapid growth of the placenta and the fetus and the expansion of the globular mass.[63] In contrast, adult men and postmenopausal women are at low risk of iron deficiency and the amount of iron in a normal diet is usually sufficient to cover their physiological requirements.[63]
CONSEQUENCES AND CAUSES OF IRON DEFICIENCY
Consequences of iron deficiency
Iron deficiency is defined as a condition in which there are no mobilizable iron stores and in which signs of a compromised supply of iron to tissues, including the erythron, are noted.[64] Iron deficiency can exist with or without anemia. Some functional changes may occur in the absence of anemia, but the most functional deficits appear to occur with the development of anemia.[2] Even mild and moderate forms of iron deficiency anemia can be associated with functional impairments affecting cognitive development,[65] immunity mechanisms,[66] and work capacity.[67] Iron deficiency during pregnancy is associated with a variety of adverse outcomes for both mother and infant, including increased risk of sepsis, maternal mortality, perinatal mortality, and low birth weight.[68] Iron deficiency and anemia also reduce learning ability and are associated with increased rates of morbidity.[68]
Causes of iron deficiency
Iron deficiency results from depletion of iron stores and occurs when iron absorption cannot keep pace over an extended period with the metabolic demands for iron to sustain growth and to replenish iron loss, which is primarily related to blood loss.[2] The primary causes of iron deficiency include low intake of bioavailable iron, increased iron requirements as a result of rapid growth, pregnancy, menstruation, and excess blood loss caused by pathologic infections, such as hook worm and whipworm causing gastrointestinal blood loss[69,70,71,72] and impaired absorption of iron.[73] The frequency of iron deficiency rises in female adolescents because menstrual iron losses are superimposed with needs for rapid growth.[74] Other risk factors for iron deficiency in young women are high parity, use of an intrauterine device, and vegetarian diets.[75]
Nutritional iron deficiency arises when physiological requirements cannot be met by iron absorption from the diet.[72] Dietary iron bioavailability is low in populations consuming monotonous plant-based diets with little meat.[72] In many developing countries, plant-based weaning-foods are rarely fortified with iron, and the frequency of anemia exceeds 50% in children younger than 4 years.[64]
When iron stores are depleted and insufficient iron is available for erythropoiesis, hemoglobin synthesis in erythrocyte precursors become impaired and hematologic signs of iron deficiency anemia appear.
EVALUATION OF IRON STATUS
Iron deficiency and eventually anemia develop in stages and can be assessed by measuring various biochemical indices. Although some iron enzymes are sensitive to iron deficiency,[63] their activity has not been used as a successful routine measure of iron status.[2]
Laboratory measurements are essential for a proper diagnosis of iron deficiency. They are most informative when multiple measures of iron status are examined and evaluated in the context of nutritional and medical history.
The plasma or serum pool of iron is the fraction of all iron in the body that circulates bound primarily to transferrin. Three ways of estimating the level of iron in the plasma or serum include 1) measuring the total iron content per unit volume in μg/dL; 2) measuring the total number of binding sites for iron atoms on transferrin, known as total iron-binding capacity in μg/dL2; and 3) estimating the percentage of the two bindings sites on all transferrin molecules that are occupied called the percentage transferrin saturation.[76] However, marked biologic variation can occur in these values as a result of diurnal variation, the presence of infection or inflammatory conditions and recent dietary iron intake.[76]
Zinc protoporphyrin reflects the shortage of iron supply in the last stages of hemoglobin synthesis so that zinc is inserted into the protoporphyrin molecule in the place of iron. Zinc protoporphyrin can be detected in RBCs by fluorimetry and is a measure of the severity of iron deficiency.[76]
Serum ferritin is a good indicator of body iron stores under most circumstances. When the concentration of serum ferritin is ≥15 μg/L iron stores are present; higher concentrations reflect the size of the iron store; when the concentration is low (<12 μg/L for <5 years of age and <15 μg/L for >5 years of age) iron stores are depleted.[76] However, ferritin is an acute phase reactant protein and its serum concentrations can be elevated, irrespective of a change in iron stores, by infection or inflammation.[76,2] This means that it might be difficult to interpret the concentration of ferritin where infectious diseases are common.
Another indicator of iron status is the concentration of TfR in serum. Since TfR is mostly derived from developing RBCs, it reflects the intensity of erythropoiesis and the demand for iron. As iron stores are exhausted, the concentration rises in iron deficiency anemia indicating sever iron insufficiency. This is provided that there are no other causes of abnormal erythropoiesis.[76] Clinical studies indicate that the serum TfR is less affected by inflammation than serum ferritin.[77] The major advantage of TfR as an indicator is the possibility of estimating the magnitude of the functional iron deficit once iron stores are depleted.[78]
The ratio of TfR to ferritin (TfR/ferritin) was designed to evaluate changes in both stored iron and functional iron and was thought to be more useful than either TfR or ferritin alone.[79] TfR/ferritin has been used to estimate body iron stores in both children and adults.[80] However, the high cost and the lack of standardization of the TfR assay so far have limited the applicability of the method.[81]
Low hemoglobin concentration is a measure of anemia, the end stage of iron deficiency.[76,2]
ANEMIA AND ITS CAUSES
Anemia describes the condition in which the number of RBCs in the blood is low, or the blood cells have less than the normal amount of hemoglobin. A person who has anemia is called anemic. The purpose of the RBC is to deliver oxygen from the lungs to other parts of the body. The hemoglobin molecule is the functional unit of the RBCs and is a complex protein structure that is inside the RBCs. Even though the RBCs are made within the bone marrow, many other factors are involved in their production. For example, iron is a very important component of the hemoglobin molecule; erythropoietin, a molecule secreted by the kidneys, promotes the formation of RBCs in the bone marrow.
Having the correct number of RBCs and prevention of anemia requires cooperation among the kidneys, the bone marrow, and nutrients within the body. If the kidneys or bone marrow are not functioning, or the body is poorly nourished, then normal RBC count and functions may be difficult to maintain.
Anemia is actually a sign of a disease process rather than a disease itself. It is usually classified as either chronic or acute. Chronic anemia occurs over a long period of time. Acute anemia occurs quickly. Determining whether anemia has been present for a long time or whether it is something new, assists doctors in finding the cause. This also helps predict how severe the symptoms of anemia may be. In chronic anemia, symptoms typically begin slowly and progress gradually; whereas in acute anemia symptoms can be abrupt and more distressing.
RBCs live about 100 days, so the body is constantly trying to replace them. In adults, RBC production occurs in the bone marrow. Doctors try to determine if a low RBC count is caused by increased blood loss of RBCs or from decreased production of them in the bone marrow. Knowing whether the number of white blood cells and/or platelets has changed also helps determine the cause of anemia.
World Health Organization (WHO) estimates that two billion people are anemic worldwide and attribute approximately 50% of all anemia to iron deficiency.[64] It occurs at all stages of the life cycle but is more prevalent in pregnant women and young children.[82] Anemia is the result of a wide variety of causes that can be isolated, but more often coexist. Some of these causes include the following:
Iron deficiency anemia
The most significant and common cause of anemia is iron deficiency.[82] If iron intake is limited or inadequate due to poor dietary intake, anemia may occur as a result. This is called iron deficiency anemia. Iron deficiency anemia can also occur when there are stomach ulcers or other sources of slow, chronic bleeding (colon cancer, uterine cancer, intestinal polyps, hemorrhoids, etc).[83]
Anemia of chronic disease
Any long-term medical condition can lead to anemia. This type of anemia is the second most prevalent after anemia caused by iron deficiency and develops in patients with acute or chronic systemic illness or inflammation.[84] The condition has thus been termed “anemia of inflammation” due to elevated hepcidin which blocks both the recycling of iron from the macrophages and iron absorption.[85]
Anemia from active bleeding
Loss of blood through heavy menstrual bleeding or wounds can cause anemia.[82] Gastrointestinal ulcers or cancers such as cancer of the colon may slowly lose blood and can also cause anemia.[86,87]
Anemia related to kidney disease
The kidneys releases a hormone called the erythropoietin that helps the bone marrow make RBCs. In people with chronic (long-standing) kidney disease, the production of this hormone is diminished, and this in turn diminishes the production of RBCs, causing anemia.[88] Although deficiency of erythropoietin is the primary cause of anemia in chronic renal failure, it is not the only cause. Therefore, a minimal workup is necessary to rule out iron deficiency and other cell-line abnormalities.[89]
Anemia related to pregnancy
A gain in plasma volume during pregnancy dilutes the RBCs and may be reflected as anemia.[90] Iron deficiency anemia accounts for 75% of all anemia in pregnancy.[90]
Anemia related to poor nutrition
Vitamins and minerals are required to make RBCs. In addition to iron, vitamin B12, viamin A, folate, riboflavin, and copper are required for the proper production of hemoglobin.[82] Deficiency in any of these micronutrients may cause anemia because of inadequate production of RBCs. Poor dietary intake is an important cause of low vitamin levels and therefore anemia.
Obesity and anemia
Obesity is characterized by chronic, low-grade, systemic inflammation, elevated hepcidin, which, in turn has been associated with anemia of chronic disease. Ausk and Ioannou[91] hypothesized that obesity may be associated with the features of anemia of chronic disease, including low hemoglobin concentration, low serum iron and transferrin saturation, and elevated serum ferritin. Overweight and obesity were associated with changes in serum iron, transferrin saturation, and ferritin that would be expected to occur in the setting of chronic, systemic inflammation. Obesity-related inflammation may increase hepcidin concentrations and reduce iron availability. Aeberli et al.,[92] compared iron status, dietary iron intake and bioavailability, as well as circulating levels of hepcidin, leptin, and interleukin-6 (IL-6), in overweight versus normal weight children. They indicated that there is reduced iron availability for erythropoiesis in overweight children and that this is likely due to hepcidin-mediated reduced iron absorption and/or increased iron sequestration rather than low dietary iron supply.
Alcoholism
Alcohol has numerous adverse effects on the various types of blood cells and their functions.[93] Alcoholics frequently have defective RBCs that are destroyed prematurely.[93,94] Alcohol itself may also be toxic to the bone marrow and may slow down the RBC production.[93,94] In addition, poor nutrition and deficiencies of vitamins and minerals are associated with alcoholism.[95] The combination of these factors may lead to anemia in alcoholics.
Sickle cell anemia
Sickle cell anemia is one of the most common inherited diseases.[96] It is a blood-related disorder that affects the hemoglobin molecule and causes the entire blood cell to change shape under stressed conditions.[97] In this condition, the hemoglobin problem is qualitative or functional. Abnormal hemoglobin molecules may cause problems in the integrity of the RBC structure and they may become crescent-shaped (sickle cells).[97] There are different types of sickle cell anemia with different severity levels. It is particularly common in African, Middle Eastern, and Mediterranean ancestry.[97]
Thalassemia
This is another group of hemoglobin-related causes of anemia, which involves the absence of or errors in genes responsible for production of hemoglobin.[97] A hemoglobin molecule has subunits commonly referred to as alpha and beta globin chains. A lack of a particular subunit determines the type of alpha or beta thalassemia.[97,98] There are many types of thalassemia, which vary in severity from mild (thalassemia minor) to severe (thalassemia major).[98] These are also hereditary, but they cause quantitative hemoglobin abnormalities, meaning an insufficient amount of the correct hemoglobin type molecules is made. The alpha and beta thalassemias are the most common-inherited single-gene disorders in the world with the highest prevalence in areas where malaria was or still is endemic.[97]
Aplastic anemia
Aplastic anemia is a disease in which the bone marrow is destructed and the production of blood cells is diminished.[99] This causes a deficiency of all three types of blood cells (pancytopenia) including RBCs (anemia), white blood cells (leukopenia), and platelets (thrombocytopenia).[100,101] Many common medications can occasionally cause this type of anemia as a side effect in some individuals.[99]
Hemolytic anemia
Hemolytic anemia is a type of anemia in which the RBCs rupture, known as hemolysis, and are destroyed faster than the bone marrow can replace them.[102] Hemolytic anemia could happen due to a variety of reasons and is often categorized as acquired or hereditary. Common acquired causes of hemolytic anemia are autoimmunity, microangiopathy, and infection. Disorders of RBC enzymes, membranes, and hemoglobin cause hereditary hemolytic anemia.[102]
PREVENTION OF IRON DEFICIENCY (INTERVENTION STRATEGIES)
The four principle strategies for correcting micronutrient efficiencies in populations can be used for correcting iron deficiency, either alone or in combination. These are education combined with dietary modification, to improve iron intake and bioavailability; iron supplementation (provision of iron, usually in higher doses, without food), iron fortification of foods and the new approach of biofortification. However, there are some difficulties in the application of some of these strategies when considering iron.
Food diversification
Dietary modifications for reducing Indian Dental Association involve increased intake of iron rich foods, especially flesh foods, increased consumption of fruits and vegetables rich in ascorbic acid to enhance nonheme iron absorption, and reduced intake of tea and coffee, which inhibit nonheme iron absorption.[103,58] Another strategy is to reduce antinutrient contents in order to make the iron supplied from their food sources more available. Iron bioavailability may be increased by techniques such as germination and fermentation, which promote enzymatic hydrolysis of phytic acid in whole grain cereals and legumes by enhancing the activity of endogenous or exogenous phytase enzymes.[104] Even the use of nonenzymatic methods, such as thermal processing, soaking, and milling, for reducing phytic acid content in plant-based staples has been successful in improving the bioavailability of iron (and zinc).[105,106]
Supplementation
For oral iron supplementation, ferrous iron salts (ferrous sulfate and ferrous gluconate) are preferred because of their low cost and high bioavailability.[72] Although iron absorption is higher when iron supplements are given on an empty stomach, nausea, and epigastric pain might develop due to the higher iron doses administered (usually 60 mg Fe/day). If such side-effects arise, lower doses between meals should be attempted or iron should be provided with meals, although food reduces absorption of medicinal iron by about two-thirds.[107] Iron supplementation during pregnancy is advisable in developing countries, where women often enter pregnancy with low iron stores.[108] Although the benefits of iron supplementation have generally been considered to outweigh the putative risks, there is some evidence to suggest that supplementation at levels recommended for otherwise healthy children carries the risk of increased severity of infectious disease in the presence of malaria.[109,110]
Fortification
Fortification of foods with iron is more difficult than fortification with nutrients, such as zinc in flour, iodine in salt, and vitamin A in cooking oil.[72] The most bioavailable iron compounds are soluble in water or diluted acid but often react with other food components to cause off-flavors, color changes or fat oxidation.[103] Thus, less soluble forms of iron, although less well absorbed, are often chosen for fortification to avoid unwanted sensory changes.[72] Fortification is usually made with much lower iron doses than supplementation. It is closer to the physiological environment and might be the safest intervention in malarious areas.[111] There is no concern over the safety of iron supplementation or iron fortification in nonmalarial endemic areas.[112]
Iron compounds recommended for food fortification by the[7] include ferrous sulfate, ferrous fumarate, ferric pyrophosphate, and electrolytic iron powder. Wheat flour is the most common iron fortified food and it is usually fortified with elemental iron powders which are not recommended by WHO.[7,113] Hurrell and Egli[23] reported that of the 78 national wheat flour programs only eight would be expected to improve iron status. These programs used recommended iron compounds at the recommended levels. The other countries used non recommended compounds or lower levels of iron relative to flour intake. Commercial infant foods, such as formulas and cereals, are also commonly fortified with iron.
Biofortification
Iron contents vary from 25 to 56 mg/kg in the different varieties of wheat and 7-23 mg/kg in rice grains. However, most of this iron is removed during the milling process. Iron absorption from cereals and legumes, many of which have high native iron content, is generally low because of their high contents of phytate and sometimes polyphenols.[48] Biofortification strategies include plant breeding and genetic engineering. Iron levels in common beans and millet have been successfully increased by plant breeding but other staple is more difficult or not possible (rice) due to insufficient natural genetic variation. Lucca et al.,[114] increased the iron content in rice endosperm to improve its absorption in the human intestine by means of genetic engineering. They introduced a ferritin gene from Phaseolus vulgaris into rice grains, increasing their iron content up to twofold. To increase iron bioavailability, they introduced a thermotolerant phytase from Aspergillus fumigatus into the rice endosperm. They indicated that this rice, with higher iron content and rich in phytase has a great potential to substantially improve iron nutrition in those populations where iron deficiency is so widely spread.[114] Unfortunately the phytase did not resist cooking. The importance of various minerals as zinc[115] and iron needs more attention at individual and public health levels.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Beard JL, Dawson HD. Iron. In: O’Dell BL, Sunde RA, editors. Handbook of Nutritionally Essential Mineral Elements. New York: CRC Press; 1997. pp. 275–334. [Google Scholar]
- 2.Wood RJ, Ronnenberg A. Iron. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health And Disease. 10th ed. Baltimore: Lippincott Williams & Wilkins; 2005. pp. 248–70. [Google Scholar]
- 3.McDowell LR. 2nd ed. Amsterdam: Elsevier Science; 2003. Minerals in Animal And Human Nutrition; p. 660. [Google Scholar]
- 4.Guggenheim KY. Chlorosis: The rise and disappearance of a nutritional disease. J Nutr. 1995;125:1822–5. doi: 10.1093/jn/125.7.1822. [DOI] [PubMed] [Google Scholar]
- 5.Yip R, Dallman PR. Iron. In: Ziegler EE, Filer LJ, editors. Present knowledge in nutrition. 7th ed. Washington DC: ILSI Press; 1996. pp. 278–92. [Google Scholar]
- 6.Underwood EJ, Suttle NF. 3rd ed. Wallingford: CABI International Publishing; 1999. The mineral nutrition of livestock; p. 614. [Google Scholar]
- 7.Allen L, de Benoist B, Dary O, Hurrell R, editors. Geneva: WHO and FAO; 2006. WHO. Guidelines on food fortification with micronutrients; p. 236. [Google Scholar]
- 8.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131:636–45S. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- 9.Quintero-Gutiérrez AG, González-Rosendo G, Sánchez-Muñoz J, Polo-Pozo J, Rodríguez-Jerez JJ. Bioavailability of heme iron in biscuit filling using piglets as an animal model for humans. Int J Biol Sci. 2008;4:58–62. doi: 10.7150/ijbs.4.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–59. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 11.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;2:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 12.Guerinot ML. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–72. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 13.Askwith C, Kaplan J. Iron and copper transport in yeast and its relevance to human disease. Trends Biochem Sci. 1998;23:135–8. doi: 10.1016/s0968-0004(98)01192-x. [DOI] [PubMed] [Google Scholar]
- 14.Hurrell RF. Bioavailability of iron. Eur J Clin Nutr. 1997;51:S4–8. [PubMed] [Google Scholar]
- 15.Washington, DC: National Academy Press; 2001. IOM. Institute of Medicine. iron. In: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; pp. 290–393. [PubMed] [Google Scholar]
- 16.Muir A, Hopfer U. Regional specificity of iron uptake by small intestinal brush-boarder membranes from normal and iron deficient mice. Am J Physiol. 1985;248:G376–9. doi: 10.1152/ajpgi.1985.248.3.G376. [DOI] [PubMed] [Google Scholar]
- 17.Frazer DM, Anderson GJ. Iron imports. I. Intestinal iron absorption and its regulation. Am J Physiol Gastrointest Liver Physiol. 2005;289:G631–5. doi: 10.1152/ajpgi.00220.2005. [DOI] [PubMed] [Google Scholar]
- 18.Nadadur SS, Srirama K, Mudipalli A. Iron transport and homeostasis mechanisms: Their role in health and disease. Indian J Med Res. 2008;128:533–44. [PubMed] [Google Scholar]
- 19.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434:365–81. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh KY, Yeh M, Mims L, Glass J. Iron feeding induces ferroportin 1 and hephaestin migration and interaction in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296:55–65. doi: 10.1152/ajpgi.90298.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theil EC, Chen H, Miranda C, Janser H, Elsenhans B, Núñez MT, et al. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J Nutr. 2012;142:478–83. doi: 10.3945/jn.111.145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoppler M, Schoenbaechler A, Meile L, Hurrell RF, Walczyk T. Ferritin-iron is released during boiling and in vitro gastric digestion. J Nutr. 2008;138:878–84. doi: 10.1093/jn/138.5.878. [DOI] [PubMed] [Google Scholar]
- 23.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461–7S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 24.Finberg KE. Unraveling mechanisms regulating systematic iron homeostasis. Am Soc Hematol. 2011;1:532–7. doi: 10.1182/asheducation-2011.1.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–42. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 26.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 27.De Domenico I, Ward DM, Kaplan J. Hepcidin regulation: Ironing out the detail. J Clin Invest. 2007;117:1755–8. doi: 10.1172/JCI32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun V, Killmann H. Bacterial solutions to the iron-supply problem. Trends Biochem Sci. 1999;24:104–9. doi: 10.1016/s0968-0004(99)01359-6. [DOI] [PubMed] [Google Scholar]
- 29.Hunt JR. How important is dietary iron bioavailability? Am J Clin Nutr. 2001;73:3–4. doi: 10.1093/ajcn/73.1.3. [DOI] [PubMed] [Google Scholar]
- 30.Hunt JR, Zito CA, Johnson LA. Body iron excretion by healthy men and women. Am J Clin Nutr. 2009;89:1–7. doi: 10.3945/ajcn.2009.27439. [DOI] [PubMed] [Google Scholar]
- 31.Fairbanks VF. Iron in medicine and nutrition. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10th ed. Baltimore: Lippincott Williams & Wilkins; 1999. pp. 193–221. [Google Scholar]
- 32.human vitamin and mineral requirements. Rome: FAO; 2001. FAO/WHO. Food based approaches to meeting vitamin and mineral needs; pp. 7–25. [Google Scholar]
- 33.Monsen ER, Hallberg L, Layrisse M, Hegsted DM, Cook JD, Mertz W, et al. Estimation of available dietary iron. Am J Clin Nutr. 1978;31:134–41. doi: 10.1093/ajcn/31.1.134. [DOI] [PubMed] [Google Scholar]
- 34.Conrad ME, Umbreit JN. A concise review: Iron absorption – the mucin-mobilferrin-integrin pathway. A competitive pathway for metal absorption. Am J Hematol. 1993;42:67–73. doi: 10.1002/ajh.2830420114. [DOI] [PubMed] [Google Scholar]
- 35.Conrad ME, Schade SG. Ascorbic acid chelates in iron absorption: A role for hydrochloric acid and bile. Gastroenterology. 1968;55:35–45. [PubMed] [Google Scholar]
- 36.Hallberg L, Brune M, Rossander L. Iron absorption in man: Ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr. 1989;49:140–4. doi: 10.1093/ajcn/49.1.140. [DOI] [PubMed] [Google Scholar]
- 37.Siegenberg D, Baynes RD, Bothwell TH, Macfarlane BJ, Lamparelli RD, Car NG, et al. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am J Clin Nutr. 1991;53:537–41. doi: 10.1093/ajcn/53.2.537. [DOI] [PubMed] [Google Scholar]
- 38.Stekel A, Olivares M, Pizarro F, Chadud P, Lopez I, Amar M. Absorption of fortification iron from milk formulas in infants. Am J Clin Nutr. 1986;43:917–22. doi: 10.1093/ajcn/43.6.917. [DOI] [PubMed] [Google Scholar]
- 39.Ballot D, Baynes RD, Bothwell TH, Gillooly M, MacFarlane BJ, MacPhail AP, et al. The effects of fruit juices and fruits on the absorption of iron from a rice meal. Br J Nutr. 1987;57:331–43. doi: 10.1079/bjn19870041. [DOI] [PubMed] [Google Scholar]
- 40.Lynch SR, Cook JD. Interaction of vitamin C and iron. Ann N Y Acad Sci. 1980;355:32–44. doi: 10.1111/j.1749-6632.1980.tb21325.x. [DOI] [PubMed] [Google Scholar]
- 41.Teucher B, Olivares M, Cori H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403–19. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- 42.Lynch SR, Hurrell RF, Dassenko SA, Cook JD. The effect of dietary proteins on iron bioavailability in man. Adv Exp Med Biol. 1989;249:117–32. doi: 10.1007/978-1-4684-9111-1_8. [DOI] [PubMed] [Google Scholar]
- 43.Bjorn-Rasmussen E, Hallberg L. Effect of animal proteins on the absorption of food iron in man. Nutr Metab. 1979;23:192–202. doi: 10.1159/000176256. [DOI] [PubMed] [Google Scholar]
- 44.Reddy MB, Hurrell RF, Cook JD. Consumption in a varied diet marginally influences nonheme iron absorption in normal individuals. J Nutr. 2006;136:576–81. doi: 10.1093/jn/136.3.576. [DOI] [PubMed] [Google Scholar]
- 45.Bach Kristensen M, Hels O, Morberg C, Marving J, Bugel S, Tetens I. Pork meat increases iron absorption from a 5-day fully controlled diet when compared to a vegetarian diet with similar vitamin C and phytic acid content. Br J Nutr. 2005;94:78–83. doi: 10.1079/bjn20051417. [DOI] [PubMed] [Google Scholar]
- 46.Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron-absorption in humans. Am J Clin Nutr. 1992;56:573–8. doi: 10.1093/ajcn/56.3.573. [DOI] [PubMed] [Google Scholar]
- 47.Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. 2004;74:445–52. doi: 10.1024/0300-9831.74.6.445. [DOI] [PubMed] [Google Scholar]
- 48.Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81:289–95. [PubMed] [Google Scholar]
- 49.Hallberg L, Rossander L. Effect of different drinks on the absorption of non-heme iron from composite meals. Hum Nutr Appl Nutr. 1982;36:116–23. [PubMed] [Google Scholar]
- 50.Hallberg L, Rossander-Hulthen L, Brune M, Gleerup A. Inhibition of haem-iron absorption in man by calcium. Br J Nutr. 1993;69:533–40. doi: 10.1079/bjn19930053. [DOI] [PubMed] [Google Scholar]
- 51.Hallberg L, Rossander-Hulthen L. Iron requirements in menstruating women. Am J Clin Nutr. 1991;54:1047–58. doi: 10.1093/ajcn/54.6.1047. [DOI] [PubMed] [Google Scholar]
- 52.Lynch SR. The effect of calcium on iron absorption. Nutr Res Rev. 2000;13:141–58. doi: 10.1079/095442200108729043. [DOI] [PubMed] [Google Scholar]
- 53.Cook JD, Monsen ER. Food iron absorption in human subjects. III. Comparison of the effect of animal proteins on nonheme iron absorption. Am J Clin Nutr. 1976;29:859–67. doi: 10.1093/ajcn/29.8.859. [DOI] [PubMed] [Google Scholar]
- 54.Hurrell RF, Lynch SR, Trinidad TP, Dassenko SA, Cook JD. Iron absorption in humans: Bovine serum albumin compared with beef muscle and egg white. Am J Clin Nutr. 1988;47:102–7. doi: 10.1093/ajcn/47.1.102. [DOI] [PubMed] [Google Scholar]
- 55.Lynch SR, Dassenko SA, Cook JD, Juillerat MA, Hurrell RF. Inhibitory effect of a soybean-protein-related moiety on iron absorption in humans. Am J Clin Nutr. 1994;60:567–72. doi: 10.1093/ajcn/60.4.567. [DOI] [PubMed] [Google Scholar]
- 56.Piomelli S, Seaman C, Kapoor S. Lead-induced abnormalities of porphyrin metabolism, the relationship with iron deficiency. Ann N Y Acad Sci. 1987;514:278–88. doi: 10.1111/j.1749-6632.1987.tb48783.x. [DOI] [PubMed] [Google Scholar]
- 57.Goyer RA. Lead toxicity: Current concerns. Environ Health Perspect. 1993;100:177–87. doi: 10.1289/ehp.93100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.2nd ed. Bangkok: 2004. FAO/WHO. Expert Consultation on Human Vitamin and Mineral Requirements, Vitamin and mineral requirements in human nutrition: Report of joint FAO/WHO expert consolation; p. 341. [Google Scholar]
- 59.Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood. 1986;68:726–31. [PubMed] [Google Scholar]
- 60.Bothwell TH, Charlton RW. A general approach of the problems of iron deficiency and iron overload in the population at large. Semin Hematol. 1982;19:54–67. [PubMed] [Google Scholar]
- 61.Gibson RS, MacDonald AC, Smit-Vanderkooy PD. Serum ferritin and dietary iron parameters in a sample of Canadian preschool children. J Can Dietetic Assoc. 1988;49:23–8. [Google Scholar]
- 62.DeMaeyer EM, Dallman P, Gurney JM, Hallberg L, Sood SK, Srikantia SG, editors. Geneva: World Health Organization; 1989. WHO. Preventing and controlling iron deficiency anaemia through primary health care: A guide for health administrators and programme managers; p. 58. [Google Scholar]
- 63.Dallman P. Iron. In: Brown ML, editor. Present Knowledge in Nutrition. 6th ed. Washington DC: Nutrition Foundation; 1990. pp. 241–50. [Google Scholar]
- 64.Geneva: Switzerland: World Health Organization; 2001. WHO/UNICEF/UNU. Iron Deficiency Anemia Assessment, Prevention, and Control; p. 114. [Google Scholar]
- 65.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 66.Failla ML. Trace elements and host defense: Recent advances and continuing challenges. J Nutr. 2003;133:S1443–7. doi: 10.1093/jn/133.5.1443S. [DOI] [PubMed] [Google Scholar]
- 67.Viteri FE, Torun B. Anemia and physical work capacity. In: Garby L, editor. Clinics in Hematology. Vol. 3. London: WB Saunders; 1974. pp. 609–26. [Google Scholar]
- 68.CDC. Breastfeeding Report Card, United states: Outcome Indicators (Publication, from Centers for Disease Control and Prevention, National Immunization Survey. 2010. [Last accessed on 11 May 2010]. http://www.cdc.gov/breastfeeding/data/index.htm .
- 69.Cooper ES, Bundy DA. Trichuriasis. Ballieres Clin Trop Med Commun Dis. 1987;2:629–43. [Google Scholar]
- 70.World Health Organization, Geneva; 1995. WHO. Report of the WHO informal consultation on hookworm infection and anaemia in girls and women; p. 46. [Google Scholar]
- 71.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–99. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 72.Larocque R, Casapia M, Gotuzzo E, Gyorkos TW. Relationship between intensity of soil-transmitted helminth infections and anemia during pregnancy. Am J Trop Med Hyg. 2005;73:783–9. [PubMed] [Google Scholar]
- 73.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:115–20. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 74.Harvey LJ, Armah CN, Dainty JR, Foxall RJ, John Lewis D, Langford NJ, et al. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br J Nutr. 2005;94:557–64. doi: 10.1079/bjn20051493. [DOI] [PubMed] [Google Scholar]
- 75.Beard JL. Iron requirement in adolescent females. Symposium: Improving adolescent iron status before childbearing. J Nutr. 2000;130:S440–2. [Google Scholar]
- 76.WHO; 2004. [last accessed on Dec 2013]. WHO/CDC. Expert consultation agrees on best indicators to assess iron deficiency, a major cause of anaemia. Available from: http://www.who.int/mediacentre/news/notes/2004/anaemia/en . [Google Scholar]
- 77.Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clinica Chimica Acta. 2003;329:9–22. doi: 10.1016/s0009-8981(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 78.Baynes RD. Assessment of iron status. Clin Biochem. 1996;29:209–15. doi: 10.1016/0009-9120(96)00010-k. [DOI] [PubMed] [Google Scholar]
- 79.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of bodyiron. Blood. 2003;101:3359–64. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 80.Cook JD, Boy E, Flowers C, Daroca Mdel C. The influence of high altitude living on body iron. Blood. 2005;106:1441–6. doi: 10.1182/blood-2004-12-4782. [DOI] [PubMed] [Google Scholar]
- 81.Yang Z, Dewey KG, Lonnerdal B, Hernell O, Chaparro C, Adu-Afarwuah S, et al. Comparison of plasma ferritinconcentration with the ratio of plasma transferrin receptor to ferritin inestimating body iron stores: Results of 4 intervention trials. Am J Clin Nutr. 2008;87:1892–8. doi: 10.1093/ajcn/87.6.1892. [DOI] [PubMed] [Google Scholar]
- 82.De Benoist B, McLean E, Egli I, Cogswell M, editors. Geneva: WHO Press, World Health Organization; 2008. WHO/CDC. Library Cataloguing-in-Publication Data. Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia; p. 40. [Google Scholar]
- 83.Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Ther Adv Gastroenterol. 2011;4:177–84. doi: 10.1177/1756283X11398736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zarychanski R, Houston DS. Anemia of chronic disease: A harmful disorder or an adaptive, beneficial response? Can Med Assoc J. 2008;179:333–7. doi: 10.1503/cmaj.071131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 86.2nd ed. Geneva: 2004. WHO/CDC. Report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level; p. 108. [Google Scholar]
- 87.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am J Med. 2004;116:11–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 88.O’Mara NB. Anemia patients with chronic kidney diseases. Diabetes Spectrum. 2008;21:12–9. [Google Scholar]
- 89.Nurko S. Anemia in chronic kidney disease: Causes, diagnosis, treatment. Cleve Clin J Med. 2006;73:289–97. doi: 10.3949/ccjm.73.3.289. [DOI] [PubMed] [Google Scholar]
- 90.Horowitz KM, Ingardia CJ, Borgida AF. 2013, Anemia in pregnancy. Clin Lab Med. 2013;33:281–91. doi: 10.1016/j.cll.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 91.Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity. 2008;16:2356–61. doi: 10.1038/oby.2008.353. [DOI] [PubMed] [Google Scholar]
- 92.Aeberli I, Hurrell RF, Zimmermann MB. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes. 2009;33:1111–7. doi: 10.1038/ijo.2009.146. [DOI] [PubMed] [Google Scholar]
- 93.Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21:42–52. [PMC free article] [PubMed] [Google Scholar]
- 94.Lewis G, Wise MP, Poynton C, Godkin A. A case of persistent anemia and alcohol abuse. Nat Clin Pract Gastroenterol Hepatol. 2007;4:521–6. doi: 10.1038/ncpgasthep0922. [DOI] [PubMed] [Google Scholar]
- 95.Lindenbaum J, Roman MJ. Nutritional anemia in alcoholism. Am J Clin Nutr. 1980;33:2727–35. doi: 10.1093/ajcn/33.12.2727. [DOI] [PubMed] [Google Scholar]
- 96.Cox SE, L’Esperance V, Makani J, Soka D, Prentice AM, Hill CM, et al. Sickle cell anemia: Iron availability and nocturnal oximetry. J Clin Sleep Med. 2012;8:541–5. doi: 10.5664/jcsm.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.World Health Organization; 2013. [Last cited on 2013 Nov 10]. WHO. Genomic resource centre, Resources for patients and the public [Internet] Available from: http://www.who.int/genomics/public/geneticdiseases/en/index2.html#SCA . [Google Scholar]
- 98.Muncie HL, Jr, Campbell J. Alpha and beta thalassemia. Am Fam Physician. 2009;80:339–44. [PubMed] [Google Scholar]
- 99.Segel GB, Lichtman MA. Aplastic anemia: acquired and inherited. In: Kaushansky K, Williams WJ, editors. Williams Hematology. 8th ed. New York: McGraw-Hill Medical; 2010. pp. 569–90. [Google Scholar]
- 100.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–19. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scheinberg P, Chen J. Aplastic Anemia: What have we learned from animal models and from the clinic. Semin Hematol. 2013;50:156–64. doi: 10.1053/j.seminhematol.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 102.Dhaliwal G, Cornett PA, Tierney LM., Jr Hemolytic anemia. Am Fam Physician. 2004;69:2599–606. [PubMed] [Google Scholar]
- 103.Hurrell RF. How to ensure adequate iron absorption from iron-fortified food. Nutr Rev. 2002;60:S7–15. doi: 10.1301/002966402320285137. [DOI] [PubMed] [Google Scholar]
- 104.Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319–32. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 105.Schlemmer U, Frølich W, Prieto RM, Grases F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53:S330–75. doi: 10.1002/mnfr.200900099. [DOI] [PubMed] [Google Scholar]
- 106.Liang J, Han BZ, Nout MJR, Hamer RJ. Effects of soaking, germination and fermentation on phytic acid, total and in vitro soluble zinc in brown rice. Food Chem. 2008;110:821–8. doi: 10.1016/j.foodchem.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 107.Cavalli-Sforza T, Berger J, Smitasiri S, Viteri F. Weekly iron-folic acid supplementation of women of reproductive age: Impact overview, lessons learned, expansion plans, and contributions toward achievement of the millennium development goals. Nutr Rev. 2005;63:S152–8. [PubMed] [Google Scholar]
- 108.CDC. Iron Deficiency. Centers for Disease Control and Prevention. [Last accessed on Dec 2013];MMWR Weekly. 2002 51:897–9. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5140a1.htm#tab1 . [Google Scholar]
- 109.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:S616–33. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 110.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 111.World Health Organization; 2007. [Last cited on 2012 Mar 13]. WHO. Iron supplementation of young children in regions where malaria transmission is intense and infectious disease highly prevalent [Internet] Available from: http://www.who.int/nutrition/publications/WHOStatement_%20iron%20suppl.pdf . [Google Scholar]
- 112.Hurrell RF. Iron fortification: Its efficiency and safety in relation to infections. Food Nutr Bull. 2007;28:585–94. doi: 10.1177/15648265070284S411. [DOI] [PubMed] [Google Scholar]
- 113.Geneva, Switzerland: World Health Organization; 2009. [Last accessed on Dec 2013]. WHO. Recommendations on wheat and maize flour fortification. Meeting Report: Interim Consensus Statement (WHO, FAO, UNICEF, GAIN, MI, FFI) http://www.who.int/nutrition/publications/micronutrients/wheat_maize_fort.pdf . [PubMed] [Google Scholar]
- 114.Lucca P, Hurrell R, Potrykus I. Fighting iron deficiency anemia with iron-rich rice. J Am Coll Nutr. 2002;21:184S–90. doi: 10.1080/07315724.2002.10719264. [DOI] [PubMed] [Google Scholar]
- 115.Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013;18:144–57. [PMC free article] [PubMed] [Google Scholar]


