Abstract
Background:
Statins have anti-inflammatory effects in patients with chronic obstructive pulmonary disease (COPD). This study designed to evaluate the effects of atorvastatin on serum highly sensitive C-reactive protein (hs-CRP) and pulmonary function in sulfur mustard exposed patients with COPD.
Materials and Methods:
Fifty patients with chronic obstructive pulmonary disease due to sulfur mustard and high serum hs-CRP entered in this study. Participants were randomized to receive 40 mg atorvastatin or placebo in a double-blind clinical trial. Forty-five patients completed the study (n = 23 atorvastatin and n = 22 placebo). Pulse oximetry (SpO2), pulmonary function test (PFT), and 6 min walk distance test (6MWD) was measured. COPD assessment test (CAT) and St. George's respiratory questionnaire (SGRQ) were also completed by patients at the beginning of trial and after 9 weeks of prescription of 40 mg/day atorvastatin or placebo. At fourth week, SpO2, PFT, and 6MWD were again measured. After 9 weeks serum hs-CRP was re-measured.
Results:
There was no significant difference between atorvastatin and the placebo group in SpO2, FEV1, and 6MWD after fourth week (P = 0.79, P = 0.12, P = 0.12, respectively). The difference between baseline and ninth week was calculated for two groups of trial and control in term of serum hs-CRP, SpO2, FEV1, and 6MWD. Significant improvement was not observed between two groups in above mentioned variables (P = 0.35, P = 0.28, P = 0.94, P = 0.43, respectively). However, the quality of life was improved by administration of atorvastatin using the CAT score (P < 0.001) and SGRQ total score (P = 0.004).
Conclusion:
Atorvastatin does not alter serum hs-CRP and lung functions but may improve quality of life in SM-injured patients with COPD.
Keywords: Atorvastatin, COPD, hs-CRP, sulfur mustard
INTRODUCTION
Sulfur mustard (SM) as an alkylating agent was used against Iranian veterans by Iraq in the 1983-1988 Iraq–Iran war. It is a vesicant chemical weapon, affecting mainly the respiratory system, skin, and eyes with late complications. Long-term SM-induced complications have been reported in the intoxicated patients even 16-20 years after exposure.[1]
The most common late complication of SM inhalation is respiratory tract injury. There are about 100,000 surviving victims who were exposed to chemical warfare agents in Iran, many with permanent pulmonary disabilities such as bronchiolitis obliterans.[2,3,4,5,6,7]
The mechanism of effect of SM on respiratory tract and effective medications are poorly understood. Interestingly, only 40% to 50% of SM exposed subjects seem to develop the pulmonary disease.[8] Chronic obstructive pulmonary disease (COPD) and Bronchiolitis obliterans (BO) are the main complications due to SM exposure.
SM-exposed patients (SMP) suffer by an immune-mediated airway disease associated with airway and systemic inflammation. Previous studies have shown that circulating markers of systemic inflammation, such as interleukin 6 (IL-6), C-reactive protein (CRP), and sputum eosinophilic cationic protein (ECP) are elevated in this patients.[9,10,11] CRP is an important component of the innate immune response, synthesized predominantly in the liver in response to IL-6, but it is also produced in the respiratory epithelium during infection.[12,13] Several studies have shown that high levels of CRP are associated with obstructive lung disease.[14]
SMP often suffer from symptoms such as dyspnea, cough, phlegm, chest tightness, exercise intolerance, and psychosocial abnormality. Common treatment and management guidance is largely based on the pulmonary function test (PFT) and forced expiratory volume in the first second (FEV1). FEV1 is weakly associated with various impact of disease on pulmonary and extra-pulmonary organs.
Inflammatory markers in SMP may increase susceptibility to exacerbation and mortality. Quality of life (QOL) is directly affected by inflammatory markers or there are some steps that can possibly play a role in this pathway? QOL improvement is an important goal in management of these patients. It has been postulated that the anti-inflammatory effects of statins may have relevance for the treatment of respiratory diseases.
Validated respiratory questionnaires such as COPD assessment test (CAT) and St. George's respiratory questionnaire (SGRQ) are the preferred questionnaires for the assessment of QOL in these patients.
In a previous study, high serum level of hs-CRP was found in patients injured with SM inhalation and respiratory complications.[9] Some investigators have shown that statins may be beneficial in patients with ischemic heart disease and in COPD patients.[15,16]
Statins are inhibitors of 3-hydroxymethyl-3-glutaryl coenzyme A reductase (HMG CoA reductase); a rate-limiting step in cholesterol synthesis. In addition to cholesterol lowering properties, statins also have anti-inflammatory effects. Statin treatment therefore has the potential to modify immune-driven diseases.[17] Some investigators have shown that statins may be beneficial in patients with ischemic heart disease and in COPD patients.[15,16]
However, relevant data in SMP with airflow limitation are lacking. It has been postulated that the anti-inflammatory effects of statins may have benefit in the treatment of inflammatory airway disease such as SMP with late pulmonary complications.
A recent trial of atorvastatin showed no improvement in controlling asthma but reduces sputum inflammatory cells in atopic asthma.[17] Another study showed improvement in clinical outcome measures associated with reduction in blood inflammatory biomarkers such as CRP and IL-6 in rheumatoid arthritis.[18] To the best of our knowledge, this study is the first study that assessed the effect of atorvastatin in the SMP in a double blind clinical trial.
The aim of this study was to evaluate efficacy of adding atorvastatin to bronchodilator and corticosteroid inhalers on lung function improvement, airway inflammation indicators such as serum level of hs-CRP and QOL in SMP with obstructive pulmonary disease.
MATERIALS AND METHODS
We designed a randomized double-blind controlled trial to investigate the effect of oral atorvastatin (40 mg daily for 9 weeks) on airway inflammation (serum hs-CRP), saturation of hemoglobin with O2 by pulse oximetry (SpO2), PFT, 6 min walk distance (6MWD) test and QOL measured by CAT and SGRQ test in SMP with chronic obstructive pulmonary disease. Although the CAT is easy to use, SGRQ is time consuming and requires complicated spreadsheets to calculate the scores. The CAT questionnaire was translated to Persian and validated.[19]
Patients with obstructive pulmonary disease and documentation of SM exposure by military officials, the presence of respiratory complications, aged between 35 and 85 years and elevated serum hs-CRP were recruited from hospital clinics in Ardabil during 11 months (4th February 2011 to 6th December 2011).
All subjects should not have a history of exacerbation during last 8 weeks prior to randomization and cigarette smoking. Patients were encouraged to take their medications as usual stages of GOLD.
Patients were excluded from study in the case of chronic inflammatory diseases, active liver and kidney diseases, cancer, cardiac diseases (MI and unstable angina in previous 6 months), current smokers, ex-smokers, opium addiction, receiving statins or having an allergic history to statins, active respiratory tract infection, bronchiectasis, myositis, myopathy, hypercholesterolemia, asthma, and elevated serum CPK. This study was approved by the ethics committee of Ardabil University of Medical Sciences (Ref: 902/6) and all participants signed an informed consent letter.
Fifty males with confirmed history of exposure to SM gas during the Iraq–Iran war who suffered from persistent respiratory complaints; shortness of breath and cough with chronic pulmonary airway obstruction and high serum level of hs-CRP accepted to participate. Subjects randomly (using an envelope containing allocated group) allocated in two groups of trial (oral atorvastatin 40 mg daily) and control (placebo) based on CONSORT flow diagram) [see Diagram 1]. Information on SpO2, PFT, 6MWD, serum hs-CRP, and quality of life were collected from subjects. Each treatment was administered for 9 weeks period.
Diagram 1.
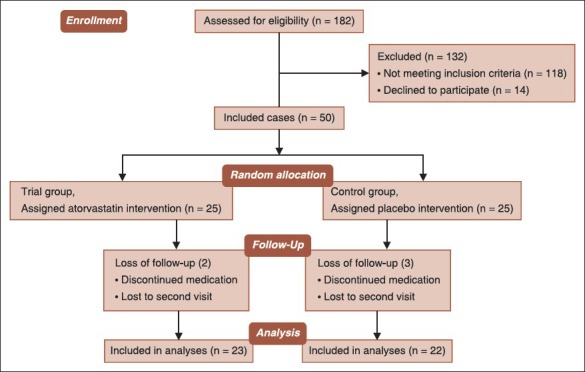
Atorvastatin on mustard-gas-exposed patients study flow diagram
At the first visit, age, respiratory symptoms, duration of symptoms, and drug history were asked. Meanwhile, height and weight, SpO2 and 6MWD were measured along with baseline lung function using a spirometer (HI-801 Chest Spirometer, Tokyo, Japan). These respiratory tests (SpO2, PFT, and 6MWD) were repeated in week 4 and 9. In addition, patients were asked to fill CAT and SGRQ which were repeated after 9 weeks.
Three component scores of SGRQ are calculated: symptoms, activity, impacts, and a total score. Blood sample was drawn for hs-CRP assay at the end of ninth week. Symptoms that were considered potential adverse effects of atorvastatin were acquired by self-administered questionnaire at second and third visits. 45 patients completed all three visits. Five patients did not complete the study due to incompliance to medicine (trial 2 and control 3).
The primary outcome was serum hs-CRP. Secondary outcomes included SpO2, PFT, 6MWD, CAT, and SGRQ scores. Some researchers reported that 8 weeks of atorvastatin was long enough to show a clinical effect, as serum cholesterol levels fall within 6 weeks of statin treatment. Therefore 9 weeks atorvastatin was chosen for trial period. Subjects were allocated in two groups of trial and control consequently.
Sulfur mustard exposure
Exposure to sulfur mustard was determined by documentation of military officials, pulmonary complication, and three pulmonologists documentation.
Serum hs-CRP
Hs-CRP test was done by means of cobas Integra 400, using particle enhanced turbimetric assay method, the agglutinated particles produced in 552 nanometer lenge wave was measured by turbimetric assay. The normal range of hs-CRP was considered (0-5 mg/l), by this kit.
PFT
All participants underwent spirometry (HI-801 Chest Spirometer, Tokyo, Japan) at the first visit, after 4 weeks of intervention and at the end of 9th weeks. The spirometer was calibrated using the device provided by the manufacturer. Forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio were measured to assess pulmonary function.
SpO2 and 6 min walk distance test
O2 saturation of hemoglobin was measured by a pulse oximeter. Exercise capacity was done by 6MWD test between 8:00 a.m. and 14:00 p.m.
Statistical analysis
the data were analyzed using SPSS software version 16. Normally distributed data were expressed as mean and standard deviation (SD). Continuous variables were compared with the T-test and repeated measurement ANOVA. Bivariate correlations between different parameters were assessed using Pearson's correlation coefficients. A value of P < 0.05 was considered as statistically significant.
RESULTS
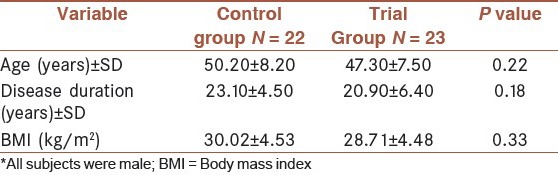
A total of 50 SMP with obstructive pulmonary disease from Ardabil university hospital clinics were invited to participate in the study. They were followed for 9 weeks with 10% loss of follow-up. The patients were all male. There were not significant differences at baseline between two groups in demographic characteristics, anthropometrics and history of disease [Table 1]. There were not differences between two groups in terms of age, BMI, and hs-CRP at the beginning of study (P = 0.22, P = 0.33, P = 0.32, respectively).
Table 1.
Distribution of basic information in two groups
There were no significant baseline hs-CRP differences between the placebo and trial groups. Atorvastatin did not reduce serum level of hs-CRP compared to placebo [Table 2].
Table 2.
Distribution of hs-CRP levels at baseline and week 9

Despite a significant decrease in serum hs-CRP which was found within both groups of control and trial after study (P < 0.01 and P = 0.01, respectively) [Figure 1], a significant difference between two groups was not observed at 9th week. (Mean difference = 2.93; 95% CI: -9.25 – 3.38) (P = 0.35)).
Figure 1.
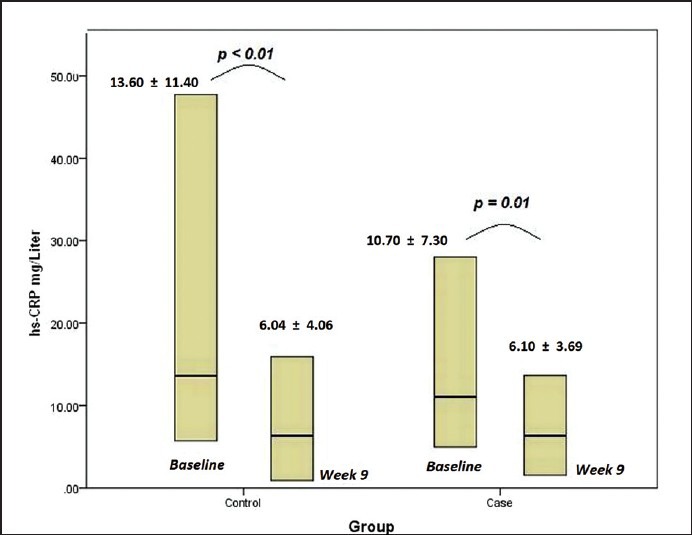
Serum hs-CRP within both groups of control and trial at beginning and after study
Table 3 demonstrates of FEV1, FEF 25–75%, SpO2, 6MWD, CAT score, and SGRQ total score in both groups at baseline and after 4 and 9 weeks.
Table 3.
Parameters of subjects
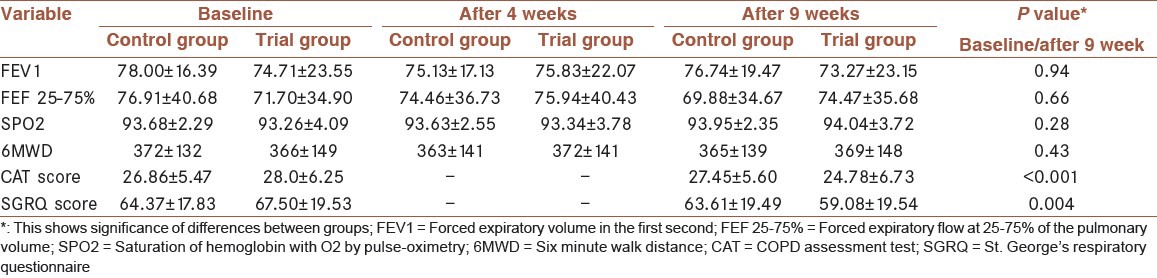
There was no significant difference in FEV1 between atorvastatin and placebo group in 9 weeks (P = 0.94) [Figure 2]. SpO2 and 6MWD test did not also improve by atorvastatin (P = 0.28 and P = 0.43, respectively).
Figure 2.
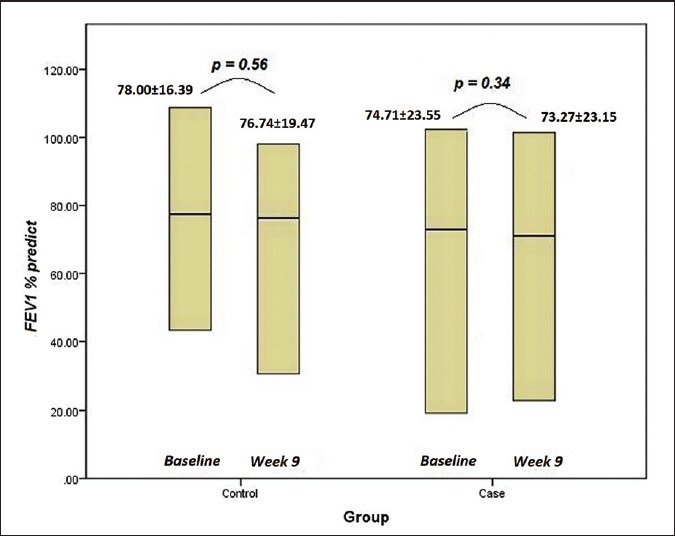
FEV1 in both groups of control and trial at baseline and 9th week
There was statically significant improvement with atorvastatin in quality of life with CAT score, and SGRQ total score compared with placebo (P < 0.001 and P = 0.004, respectively).
It is found that severity of airflow limitation increase CAT and SGRQ total scores significantly (P < 0.001 and P < 0.001, respectively). The SGRQ score and CAT scores show a positive linear correlation between two different tests assessing quality of life (P < 0.001, r = 0.97). Drug adverse event rates were similar in in two groups. Atorvastatin well tolerated by all patients none had any significant subjective side effects.
Patients under atorvastatin had a better quality of life measured by CAT and SGRQ test compared to the control group [Figures 3 and 4].
Figure 3.
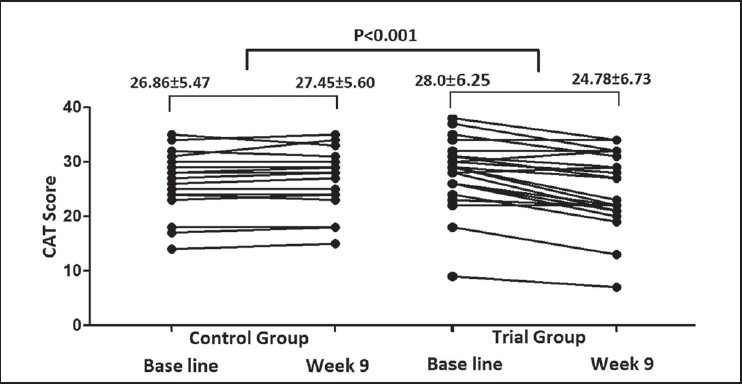
CAT score in control and trial groups at baseline and 9th week
Figure 4.
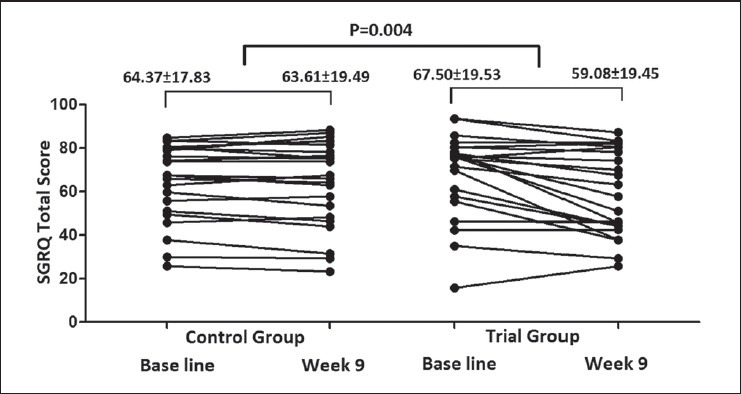
SGRQ total score in control and trial groups at baseline and 9th week
DISCUSSION
In this study, we observed a significant improvement on QOL measured with CAT and SGRQ by atorvastatin in the management of SMP with obstructive pulmonary disease while it was worse in placebo groups. On the other hand, despite QOL improvement in this study there were no statically difference in hs-CRP and FEV1 between atorvastatin and placebo group. This suggests that perhaps these patients may benefit from long-term treatment with atorvastatin. Although a significant decrease of hs-CRP was observed, it is unclear and may happen due to seasonal variation.
Previous studies have shown that statins may be beneficial in patients with systemic inflammation such as coronary artery disease and cause a significant decrease in hs-CRP over the course of the study in COPD patients.[15] Study by Soyseth et al. showed mortality reduction with atorvastatin in COPD patients.[16] In some studies short-term treatment with atorvastatin does not alter lung function but improve quality of life in smokers with mild to moderate asthma.[20] Hothersall et al. reported a significant reduction of sputum inflammatory cells in asthma patients after the addition of atorvastatin to inhaled corticosteroids but did not improve asthma control in short term.[17]
Study by Ghanei et al. found a significant improvement of severity of pulmonary symptoms and ventilator parameters after 4 months treatment with N-acetyl cysteine, their conclude that the beneficial effect of N-acetyl cysteine may be mediated by its antioxidative properties.[21]
Some studies suggest that tissue remodeling is involved in SM-exposed lung damage patients.[22] Numerous studies have revealed that this disorder is strongly associated with systemic inflammation and oxidative stress and decreased levels of endogenous antioxidants.[23,24,25]
Several mechanisms, either alone or together, might explain these finding. Firstly, statins may improve cardiovascular function; it is likely that some of the SMP had unrecognized IHD. Secondly, the lower score of CAT and SGRQ in patients treated with atorvastatin may be due to extra-pulmonary effect of statins and finally, statins may decrease the sputum inflammatory cells, and may reduce release of cytokines and inflammatory marker other than hs-CRP including IL-6 and TNFa from monocytes and endothelial cells.[17] Thus, the beneficial effect of atorvastatin may be mediated by an anti-inflammatory effect in the airway.
Pathophysiology of SM respiratory complication has not been clearly understood but these complications are induced by airway disease with systemic inflammation. SM can cause chronic injuries to a wide range of physiological systems. In particular, complications occur on the respiratory tract, skin, eyes, and immune system.[26]
Our study demonstrated that airway obstruction and ventilator parameters did not improved after 9 weeks administration of 40 mg atorvastatin. Atorvastatin is widely used as anti-inflammatory agents in cardiovascular and pulmonary disease (especially in COPD) does not show a significant benefit in pulmonary function in obstructive pulmonary complication due to sulfur mustard gas.
Lee et al. reported exercise capacity improvement and serum hs-CRP reduction with statins after 6 months intervention in COPD patients.[15] However, the finding of our study did not support this result. In this study, we did not find significantly improvement of FEV1 by atorvastatin in SMP. The possible reason for lack of association is that SMP-like subjects with COPD have a persistent and irreversible airflow limitation as was suggested by previous studies. Therefore, it can be assumed that FEV1 and PFT are not a reliable test for evaluating multiple consequences of systemic inflammation in pulmonary diseases.[27,28] Although PFT is a valuable diagnostic instrument for evaluation of severity of airflow obstruction this test cannot be used for evaluation of treatment efficacy on extra-pulmonary consequence of SM injured patients during regular follow-up.
Some investigators have reported a significant association between CAT and disease severity obtained by lung function in SMP with airflow obstruction.[29] The CAT and SGRQ were designed to measure QOL in COPD patients in clinical practice. These tests were used in SMP with airflow limitation. We observed that both CAT and SGRQ exhibit significant correlation with airflow limitation and can be used as a reliable tools to assess quality of life in SMP. The agreement between the two questionnaire scores is high. CAT is simple, shorter, and easily can be used compared to SGRQ. Patients with severe airflow limitation reported worse quality of life, measured with both CAT and SGRQ similarly to other studies in COPD patients.[30]
In addition, we noticed that atorvastatin do not have beneficial effect on SM patients exercise capacity measured by 6MWD test and SpO2.
This study has some limitations. First, the time of intervention and follow-up was not long, that may alter the study finding. Second, the serum level of other inflammatory markers such as IL-6 and TNFa has not been measured. Third, treatment with inhalation corticosteroids and bronchodilator may have affected atorvastatin metabolism. Fourth, all subjects were male. And finally the sample size was relatively small. Therefore, a complementary study with large sample size and long-term intervention with atorvastatin is appreciated.
CONCLUSION
In conclusion, we have shown that atorvastatin in SMP who suffered from chronic respiratory complications did not alter serum hs-CRP, PFT, and 6MWD and did not increase SpO2 at air room but associated with QOL improvement using CAT and SGRQ.
In summary, improvement in QOL suggests that atorvastatin could be used in addition to the conventional therapies in these patients.
ACKNOWLEDGMENTS
The authors would like to thank Mr. lacurach from Ardabil Bonyad Shahid for help in recruiting SMP. This paper has been derived from a specialty thesis in Ardabil University of Medical Sciences, Ardabil, Iran (ID: 010).
This trial has been registered in the Iranian Registry of Clinical Trials (ID: IRCT201101095579N1).
Footnotes
Source of Support: This research was funded by a grant from the Ardabil University of Medical sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Balali-mood M, Hefazi M. The clinical toxicology of sulfur mustard. Arch Iran Med. 2005;8:162–79. [Google Scholar]
- 2.Veress LA, O’Neill HC, Hendry-Hofer TB, Loader JE, Rancourt RC, White CW. Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am J Respir Crit Care Med. 2010;182:1352–61. doi: 10.1164/rccm.200910-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.New York, NY, USA: United Nations Security Council; 1986. United Nations Security Council, “Report of the specialist appointed by the secretary general to investigate allegation by the Islamic Republic of Iran concerning the use of chemical weapons,” Tech. Rep. 3/16433. [PubMed] [Google Scholar]
- 4.Khateri S, Ghanei M, Keshavarz S, Soroush M, Haines D. Incidence of Lung, Eye, and Skin Lesions as Late Complications in 34,000 Iranians with Wartime Exposure to Mustard Agent. J Occup Environ Med. 2003;45:1136–43. doi: 10.1097/01.jom.0000094993.20914.d1. [DOI] [PubMed] [Google Scholar]
- 5.Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: A review. Inhal Toxicol. 2007;19:451–6. doi: 10.1080/08958370601174990. [DOI] [PubMed] [Google Scholar]
- 6.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Ghanei M, Adibi I. Clinical Review of Mustard Lung. Iran J Med Sci. 2007;32:58–65. [Google Scholar]
- 8.Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: A review. Inhal Toxicol. 2007;19:451–6. doi: 10.1080/08958370601174990. [DOI] [PubMed] [Google Scholar]
- 9.Attaran D, Lari SM, Khajehdaluee M, Ayatollahi H, Towhidi M, Asnaashari A, et al. Highly sensitive C-reactive protein levels in Iranian patients with pulmonary complication of sulfur mustard poisoning and its correlation with severity of airway diseases. Hum Exp Toxicol. 2009;28:739–45. doi: 10.1177/0960327109354311. [DOI] [PubMed] [Google Scholar]
- 10.Mirsadraee M, Ghobadi H, Khakzad MR, Lari SM, Attaran D, Towhidi M, et al. Level of eosinophil cationic protein in sputum of chemical warfare victims. Iran J Basic Med Sci. 2011;14:249–55. [Google Scholar]
- 11.Attaran D, Lari SM, Towhidi M, Ghobadi-Marallu H, Ayatollahi H, Khajehdaluee M, et al. Interleukin-6 and airflow limitation in chemical warfare patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;5:335–40. doi: 10.2147/COPD.S12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould JM, Weiser JN. Expression of C-reactive protein in the human respiratory tract. Infect Immun. 2001;69:1747–54. doi: 10.1128/IAI.69.3.1747-1754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 15.Lee TM, Lin MS, Chang NC. Usefulness of C-reactive protein and Interleukin-6 as predictors of outcomes in patients with chronic obstructive pulmonary disease receiving pravastatin. Am J Cardiol. 2008;101:530–5. doi: 10.1016/j.amjcard.2007.09.102. [DOI] [PubMed] [Google Scholar]
- 16.Søyseth V, Brekke PH, Smith P, Omland T. Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007;29:279–83. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 17.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63:1070–5. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 18.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): Double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 19.CAT-Iran/Farsi, Version of Sep 10-Mapi Research Institute, GlaxoSmithKline) [Last accessed on 2013/08]. Available from: http://www.catestonline.org/english/index_Farsi.htm .
- 20.Braganza G, Chaudhuri R, McSharry C, Weir CJ, Donnelly I, Jolly L, et al. Effects of short-term treatment with atorvastatin in smokers with asthma--a randomized controlled trial. BMC Pulm Med. 2011;7:11–6. doi: 10.1186/1471-2466-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghanei M, Shohrati M, Jafari M, Ghaderi S, Alaeddini F, Aslani J. N-acetyl cysteine improves the clinical conditions of mustard gas-exposed patients with normal pulmonary function test. Basic Clin Pharmacol Toxicol. 2008;103:428–32. doi: 10.1111/j.1742-7843.2008.00318.x. [DOI] [PubMed] [Google Scholar]
- 22.Yazdani S, Karimfar MH, Imani Fooladi AA, Mirbagheri L, Ebrahimi M, Ghanei M, et al. Nuclear factor_B1/RelA mediates the inflammation and/or survival of human airway exposed to sulfur mustard. J Recept Signal Transduct Res. 2011;31:367–73. doi: 10.3109/10799893.2011.602415. [DOI] [PubMed] [Google Scholar]
- 23.Nourani MR, Ebrahimi M, Roudkenar MH, Vahedi E, Ghanei M, Imani Fooladi AA. Sulfur mustard induces expression of metallothionein-1A in human airway epithelial cells. Int J Gen Med. 2011;4:413–9. doi: 10.2147/IJGM.S17916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jafari M, Ghanei M. Evaluation of plasma, erythrocytes, and bronchoalveolar lavage fluid anti-oxidant defense system in sulfur mustard-injured patients. Clin Toxicol (Phila) 2010;48:184–92. doi: 10.3109/15563651003623297. [DOI] [PubMed] [Google Scholar]
- 25.Shohrati M, Ghanei M, Shamspour N, Babaei F, Abadi MN, Jafari M, et al. Glutathione and malondialdehyde levels in late pulmonary complications of sulfur mustard intoxication. Lung. 2010;188:77–83. doi: 10.1007/s00408-009-9178-y. [DOI] [PubMed] [Google Scholar]
- 26.Ghabili K, Agutter PS, Ghanei M, Ansarin K, Shoja MM. Mustard gas toxicity: The acute and chronic pathological effects. J Appl Toxicol. 2010;30:627–43. doi: 10.1002/jat.1581. [DOI] [PubMed] [Google Scholar]
- 27.Ghanei M, Sheyacy M, Abbasi MA, Ani A, Aslani J. Correlation between the degree of air trapping in chest HRCT and cardiopulmonary exercise test parameters: Could HRCT be a predictor of disease severity? Arch Iran Med. 2011;14:86–90. [PubMed] [Google Scholar]
- 28.Ghobadi H, Sadeghieh-Ahari S, Kameli A, Lari SM. The relationship between COPD Assessment Test (CAT) Scores and Severity of Airflow Obstruction in Stable COPD Patients. Tanaffos. 2012;11:22–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Fadaii A, Sohrabpour H, Taherkhanchi B, Bagheri B. Association between COPD Assessment Test (CAT) and Disease Severity Based on Reduction of Respiratory Volumes in Chemical Warfare Victims. Tanaffos. 2011;10:38–42. [PMC free article] [PubMed] [Google Scholar]
- 30.Tsiligianni IG, van der Molen T, Moraitaki D, Lopez I, Kocks JW, Karagiannis K, et al. Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ) BMC Pulm Med. 2012;20:12–20. doi: 10.1186/1471-2466-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


