Abstract
Renin expressing cells appear early in the embryo and are distributed broadly throughout the body as organogenesis ensues. Their appearance in the metanephric kidney is a relatively late event in comparison with other organs such as the fetal adrenal gland. The functions of renin cells in extra renal tissues remain to be investigated. In the kidney, they participate locally in the assembly and branching of the renal arterial tree and later in the endocrine control of blood pressure and fluid-electrolyte homeostasis. Interestingly, this endocrine function is accomplished by the remarkable plasticity of renin cell descendants along the kidney arterioles and glomeruli which are capable of reacquiring the renin phenotype in response to physiological demands, increasing circulating renin and maintaining homeostasis. Given that renin cells are sensors of the status of the extracellular fluid and perfusion pressure, several signaling mechanisms (B-adrenergic receptors, Notch pathway, gap junctions and the renal baroreceptor) must be coordinated to ensure the maintenance of renin phenotype -and ultimately the availability of renin- during basal conditions and in response to homeostatic threats. Notably, key transcriptional (Creb/CBP/p300, RBP-J) and posttranscriptional (miR-330, miR125b-5p) effectors of those signaling pathways are prominent in the regulation of renin cell identity. The next challenge, it seems, would be to understand how those factors coordinate their efforts to control the endocrine and contractile phenotypes of the myoepithelioid granulated renin-expressing cell.
Keywords: Juxtaglomerular cell, cell identity, cyclic AMP, microRNAs, cell-cell communication, gap junctions, RBP-
Introduction
Renin cells are necessary for the maintenance of blood pressure and fluid/electrolyte homeostasis. Perhaps because they synthesize a hormone, their rarity (they constitute 0.01% of the total kidney cell number), and their restricted juxtaglomerular (JG) location, these cells have been considered as terminally differentiated. However, such notion is not supported by experimental data. To start with their localization it must be stated that during early development, the location of renin cells is not restricted even to the kidneys. In fact, renin cells make their first appearance before organogenesis has been initiated and continue to emerge throughout the body as development progresses in multiple tissues and organs. Their presence in the kidney is a relatively late event in the developmental history of these cells. Within the developing murine metanephric kidney renin precursors make their first appearance around embryonic day 14 in the stromal compartment, well before arteriolar development is discernible (1). We found by lineage analysis that these renin precursors derive from FoxD1 positive stromal cells (2). Thereafter they participate in the assembling and branching of the kidney vasculature where they are, as a consequence, distributed broadly along large intrarenal arteries, and in newly appearing arteriolar branches and inside the glomeruli in what later will become the glomerular mesangium. Thus, throughout fetal and postnatal life there is an ever changing although extensive distribution of renin throughout the renal vasculature and it is not until arteriolar development is completed that these cells occupy their “classical” JG localization as usually seen in the adult animal. This pattern of development, it must be added, resembles the phylogenetic changes in renin distribution from the time these cells first emergence in cartilaginous fish (3). This brief review discusses the main factors that regulate the identity, fate and plasticity of renin cells during development and in response to homeostatic threats.
Lineage and fate of the juxtaglomerular cell
The JG cell is a highly specialized cell situated in the afferent arteriole at the entrance to the glomerulus (4;5). JG cells have granules containing renin (4;6). They also contain peroxisomes, small electron dense vesicles, myofilaments and few mitochondria (4). The cells are round, plump and epithelioid in nature. They also have numerous gap junctions that couple them to smooth muscle cells, mesangial cells as well as other JG cells (4). Because they contain myofilaments, it has been postulated that they derive from smooth muscle cells (4). However, using single cell nested RT-PCR and triple labeling studies with various phenotypic cell markers, we showed that renin cell progenitors express renin early and acquire the capacity to express smooth muscle later in fetal life, at the time of arteriolar assembly (1). Those studies challenged the dogma that renin cells derive from smooth muscle cells (4;7) and suggested instead that renin cells were precursors for vascular smooth muscle and other cell types in the kidney. To address this question, we generated mice having cre recombinase under control of the renin locus (Ren1d-cre and Ren1c-cre mice) and crossed them with R26R reporter mice. After cre-mediated recombination, mice permanently express β-gal in renin-expressing cells and its descendants, even if renin expression subsequently ceases, thus marking the renin cell lineage.
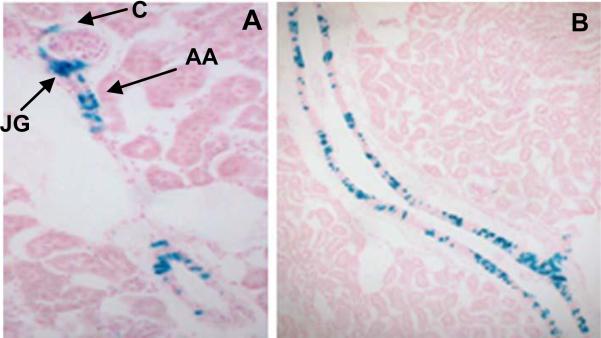
The experiments showed that renin cell progenitors are indeed capable of giving rise to JG cells, renal arteriolar smooth muscle cells (VSMCs), interstitial pericytes, and glomerular mesangial cells (Fig.1 and (8)).
Fig. 1.
Marking the renin cell lineage. A Ren-1c-cre transgenic mouse was bred to the ROSA loxP reporter line to label the renin cell lineage. A. Blue staining is seen in this adult kidney in the JGA (JG), in Bowman's capsule (C), along the afferent arteriole (AA) and B. in a large vessel in the same pattern as that revealed by the targeted Ren1d-cre mouse.
The phenomenon of recruitment: increasing the number of renin cells, a fundamental mechanism to maintain homeostasis
The issue of renin cell fate is closely related to a phenomenon of central importance to the regulation of blood pressure and body fluid homeostasis. If an adult animal is subjected to manipulations that threaten homeostasis such as hypotension, dehydration, hemorrhage or sodium depletion, circulating renin increases primarily due to an increase in the number of renin cells along the preglomerular arterioles (9-11). The increased circulating renin eventually reestablishes blood pressure and body fluid homeostasis. However, if the disequilibrium persists and the need for renin continues, as in mice deficient in angiotensinogen (12) or treated with hypotensive agents, additional smooth muscle-like cells (interstitial pericytes and glomerular mesangial cells) undergo transformation and are thus “recruited” to synthesize renin in a pattern resembling that of the embryo (13-15). Although this phenomenon has been called recruitment (9) and sometimes JG cell hyperplasia, it should be noted that it does not involve migration or replication of cells, but rather a transformation of preexisting cells. When this transformation occurs, the cells seem to dedifferentiate: they change morphology, become epithelioid, make granules that contain renin and express a set of genes characteristic of the renin phenotype, including Akr1b7 (aldo-keto-reductase 1B7) an enzyme we recently identified as characteristic of the renin endocrine phenotype and an independent marker for the renin cell (16). Akr1b7 and renin expression overlap at all developmental stages studied. Akr1b7 belongs to the aldo-keto reductase superfamily of enzymes which catalyze the reduction to alcohol of harmful aldehydes and ketones generated by hormone synthesizing cells (16). The detoxifying function of Akr1b7 seems crucial to protect renin cells from those harmful compounds and promote cell survival. Overall, the aforementioned findings indicate that adult kidney cells retain the plasticity to reenact the renin cell phenotype. Further, it is the renin cell descendants (as opposed to any other kidney cell) that re-express the genetic program of the renin cell when homeostasis is threatened (8). These results suggest that developmental decisions made in embryonic life affect physiological responses in adult life and the repertoire of physiological responses may be limited by the developmental history of our cells.
MicroRNAs and renin cell fate
Endogenous miRNAs are small non-coding RNAs that regulate gene expression at the post-transcriptional level. They are highly conserved and exhibit spatial, temporal, and tissue/cell specificity suggesting their involvement in cell differentiation and morphogenesis (17-20). The generation of miRNAs is a multi step process whereby a primary miRNA of about 100-1000 nucleotides is cleaved by the enzymatic complex Drosha/DGCR8 into a stem loop precursor (pre-miRNA, about 70 nt long) and exported to the cytoplasm where it is processed to a 22 nt long mature miRNA by Dicer, another RNase III endonuclease. To define whether miRNAs were important in renin cell specification, we crossed our Ren1d-cre mice with floxed Dicer mice to produce a conditional deletion of Dicer, the miRNA processing enzyme, specifically in renin-expressing cells. By 2 months of age, renin cells in mutant mice had virtually disappeared and the few remaining renin positive cells were thinner and smaller. The marked reduction in the number of renin positive cells was accompanied a marked decrease in circulating renin and hypotension. Kidneys were smaller and had characteristic striped fibrosis and sclerotic glomeruli clumped together around abnormally formed or missing blood vessels. It is likely that this peculiar type of scarring was due to the combination of abnormally formed vessels and hypotension leading to regional ischemia. Using microRNA microarrays, in situ hybridization and functional assays, we identified two microRNAs miR-330 and miR-125b-5p that mark the JG cells and seem to balance their endocrine-smooth muscle phenotype (21). We proposed a model whereby under basal conditions, miR-125b-5p is expressed in arteriolar smooth muscle cells and JG cells to ensure their contractility. When homeostasis is challenged, miR-125b-5p decreases along the arteriole allowing them to regain the renin phenotype but stays in JG cells to ensure their contractile function. miR-330 is then expressed in JG cells and inhibits contractility favoring their endocrine character. Thus these two microRNAs with opposite actions balance the myo-endocrine phenotype of the renin cells. There are potential miR-330 binding sites in the 3'UTR of Acta2, Myh11, Smtn, Cnn1 that could explain a direct inhibitory effect of miR-330 on smooth muscle gene expression. The stimulatory effect of miR-125b-5p on smooth muscle phenotype, on the other hand, is likely to be mediated by targeting smooth muscle inhibitory gene or genes. Potential candidates are for example NFKb and Elk1 which inhibit myocardin and are predicted targets of miR-125b-5p (21).
Regulation of renin by cAMP
cAMP plays an important role in the regulation of renin synthesis and release: it controls renin mRNA synthesis and stability and renin exocytosis. The constant physiological demands on renin secretion in response to changes in posture, renal perfusion pressure, sodium balance and other factors are met by rapid regulation of renin release. cAMP mediates the effects of numerous signals (β-adrenoreceptor activation, adenosine, prostaglandins, low calcium) that affect renin secretion (22). The constant demand on renin secretion requires stimulation of renin synthesis to replace renin stores and cAMP regulates expression of the renin gene and augments renin mRNA levels. Newborn kidney microvessels and isolated single renal microvascular cells release renin in response to adenylate cyclase stimulation and increase renin mRNA levels in response to forskolin treatment. Interestingly, isolated single microvascular cells respond to forskolin administration by increasing the number of renin-secreting and renin-expressing cells without changes in the amount of renin secreted by individual cells. The increase in renin release is therefore due to recruitment of microvascular cells secreting renin (23). More recently, using cells harboring yellow fluorescent protein (YFP) driven by the renin promoter; we demonstrated an increase in the number of renin-expressing cells in response to manipulations that increase cAMP levels. These results in vitro corroborate many reports in whole animals (9;10;13;14) indicating that the control of hormone availability is achieved by regulating the number of cells that express renin.
The renin promoter contains a cAMP responsive element (CRE) which is critical in the basal expression of the renin gene (24;25) as well as stimulation in response to cAMP administration (26). The CRE confers activation of the renin gene in reporter assays and it was shown that Creb1 competes for binding at this site in EMSA assays (24). Creb1 and its associated coactivators CBP/p300 may be the final common point that integrates multiple signals controlling renin synthesis/release. We hypothesized that CREB and its associated histone acetyl transferases play a crucial role in renin cell identity. Because knockout embryos (CBP and p300) die before kidney organogenesis starts (~E11) or just at birth (Creb1) there was no data on JG cell development in these mice. Therefore to test the hypothesis that histone acetyl transferases (CBP and p300) are important in the determination of the renin phenotype we used a conditional crelox system to delete both transferases specifically in renin cells in mice. The results showed a marked reduction in the number of JG cells accompanied by diminished renin expression and abnormal renal vascular development indicating that CBP and p300 are necessary for the maintenance of renin cell identity and nephrovascular integrity. Further, mice with conditional deletion of CBP and p300 cannot respond to a homeostatic challenge to recruit renin-expressing cells from the cells of the renin lineage or other cell types indicating that CBP and p300 are necessary for the re-acquisition of the renin phenotype in response to a challenge to homeostasis. The inability of these mice to recruit renin cells is accompanied functionally by an inability to increase circulating renin.
Given the crucial importance of the cAMP pathway for renin expression it was interesting to test whether more proximal components of the cAMP generation cascade were necessary for the maintenance or acquisition of the renin cell identity. Gsα is responsible for the generation of endogenous cAMP through activation of adenylyl cyclase. Deletion of Gsα in the renin cell lineage, causes a remarkable reduction in the endowment of renin cells from embryonic life, accompanied by terminal branching defects of the renal arterioles and renal failure(27;28). These results underscore the crucial role of the cAMP pathway in regulating renin cell fate and plasticity.
Cell to Cell Communication
Proper functioning and maintenance of the renin cell phenotype requires appropriate cell- to-cell communication. Notch receptors are known to regulate intercellular communication and cell fate. Given that Notch receptors, their ligands, and their final transcriptional effector, RBP-J, are all expressed in renin cells we hypothesized that Notch/RBP-J may be involved in the acquisition and/or maintenance of the renin phenotype. To test this hypothesis we deleted RBP-J in renin cells (29). Mice with conditional deletion of RBP-J had a severe reduction in the number of renin cells, low circulating renin and decreased blood pressure. Further, mutant mice were unable to elicit a recruitment of renin cells in response to a homeostatic challenge indicating that RBP-J is necessary to maintain the memory of the renin phenotype. Although some cells attempted to increase renin expression those cells were unusually thin, had few granules and barely detectable amounts of immunoreactive renin (29). As a consequence, the cells were incapable of fully adopting the endocrine phenotype of a renin cell. Those experiments indicated that RBP-J is required to maintain basal renin expression and the ability of smooth muscle cells along the kidney vasculature to regain the renin phenotype, a fundamental mechanism to preserve homeostasis.
Gap junctions allow cells to inform each other about their physiological status and coordinate the activity of functional units such as the juxtaglomerular apparatus (JGA) composed of the afferent and efferent arterioles, the macula densa and the extra glomerular mesangium. JG cells possess abundant gap junctions that connect them with all the component cells of the JGA and allow them to sense rapid changes in perfusion pressure and extracellular fluid composition and respond accordingly with appropriate changes in renin secretion. Gap junctions are formed when two adjacent cells contribute a hemichannel, a conexon, composed of six membrane connexins. The most abundant connexin in JG cells is Cx40 (16). Deletion of Cx40 results in the expression of renin by periglomerular cells, instead of JG cells (30). As a result, JG cells seem to lose their sensor capabilities and “misinterpret” physiological signals. JG cells operate as if they are exposed to a continuous low perfusion pressure even though animals are hypertensive. In addition, Cx40 null mice are less sensitive to angiotensin II and less able to propagate vasodilator responses. The result is that Cx40 null animals have severe malignant hypertension. Overall, these studies illustrate the fundamental importance of cell to cell communication and spatial information in the operation of the JGA.
Renin expression and renin cells are necessary for nephrovascular development
Deletion of the single Ren1c gene has severe consequences for kidney development (31). Although the kidneys of the KO mice seem normal at birth they subsequently develop hydronephrosis. The kidneys have a thin medulla with an atrophic/hypoplastic papilla and a dilated renal pelvis. The KO kidneys also show interstitial fibrosis, focal glomerulosclerosis, and perivascular infiltration of mononuclear cells. There is medial thickening of the small arteries in the kidney but not of the vessels outside the kidney. This phenotype has been described by others in renin-deficient mice (32) as well as in mice deficient of Agt (33-35), Ace (36-38), Agtr1a (39), and Agtr1a/1b (40;41). Mice in which all cells that express or have previously expressed renin have been ablated with diphtheria toxin do not show this concentric hypertrophy of arterioles (42) suggesting that renin-producing cells per se may contribute to the vessel thickening, possibly by synthesizing some factor(s) other than renin that stimulates concentric vessel proliferation. This hypothesis is currently being tested in our laboratory.
In summary, renin expressing cells appear early in the embryo and are distributed broadly throughout the body. Their functions in sites beyond the kidney remain for the most part to be investigated. In the kidney, they participate, locally in the growth of the kidney vasculature. Systemically, renin cells control blood pressure and fluid-electrolyte equilibrium by varying the number of renin synthesizing cells and thus renin availability. More important, renin cell number does not depend on proliferation or migration but is achieved by the plasticity of renin cell descendants - such as smooth muscle cells- that turn on and off the renin gene as part of a whole genetic program, which determines the myo-endocrine phenotype of the cell and in turn whole body homeostasis.
Reference List
- 1.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol. 2001;281:F345–F356. doi: 10.1152/ajprenal.2001.281.2.F345. [DOI] [PubMed] [Google Scholar]
- 2.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura H, Ogawa M, Sawyer WH. Renin-angiotensin system in primitive bony fishes and a holocephalian. Am J Physiol. 1973;224:950–956. doi: 10.1152/ajplegacy.1973.224.4.950. [DOI] [PubMed] [Google Scholar]
- 4.Taugner R, Hackenthal E. The Juxtaglomerular Apparatus: structure and function. Springer Verlag; Heidelberg: 1989. pp. 104–126. [Google Scholar]
- 5.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol. 1989;257:F850–F858. doi: 10.1152/ajprenal.1989.257.5.F850. [DOI] [PubMed] [Google Scholar]
- 6.Ice KS, Geary KM, Gomez RA, Johns DW, Peach MJ, Carey RM. Cell and molecular studies of renin secretion. Clin Exp Hypertens A. 1988;10:1169–1187. doi: 10.1080/07300077.1988.11878809. [DOI] [PubMed] [Google Scholar]
- 7.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 8.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 9.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 10.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 11.Gomez RA, Norwood VF. Developmental consequences of the renin-angiotensin system. Am J Kidney Dis. 1995;26:409–431. doi: 10.1016/0272-6386(95)90487-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210–14217. doi: 10.1074/jbc.274.20.14210. [DOI] [PubMed] [Google Scholar]
- 13.Tufro-McReddie A, Arrizurieta EE, Brocca S, Gomez RA. Dietary protein modulates intrarenal distribution of renin and its mRNA during development. Am J Physiol. 1992;263:F427–F435. doi: 10.1152/ajprenal.1992.263.3.F427. [DOI] [PubMed] [Google Scholar]
- 14.Tufro-McReddie A, Chevalier RL, Everett AD, Gomez RA. Decreased perfusion pressure modulates renin and ANG II type 1 receptor gene expression in the rat kidney. Am J Physiol. 1993;264:R696–R702. doi: 10.1152/ajpregu.1993.264.4.R696. [DOI] [PubMed] [Google Scholar]
- 15.Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez MLS. Pericytes Synthesize Renin. World J Nephrol. 2013;2:11–16. doi: 10.5527/wjn.v2.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol. 2011;22:2213–2225. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, Tuan RS. MicroRNAs and cell differentiation in mammalian development. Birth Defects Res C Embryo Today. 2006;78:140–149. doi: 10.1002/bdrc.20070. [DOI] [PubMed] [Google Scholar]
- 18.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 19.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 20.Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medrano S, Monteagudo MC, Sequeira-Lopez MLS, Pentz ES, Gomez RA. Two microRNAs -miR-330 and miR-125b-5p-mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am J Physiol Renal Physiol. 2011;302(1):F29–37. doi: 10.1152/ajprenal.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson PB, Skalweit A, Mrowka R, Thiele BJ. Control of renin synthesis. Am J Physiol Regul Integr Comp Physiol. 2003;285:R491–R497. doi: 10.1152/ajpregu.00101.2003. [DOI] [PubMed] [Google Scholar]
- 23.Everett AD, Carey RM, Chevalier RL, Peach MJ, Gomez RA. Renin release and gene expression in intact rat kidney microvessels and single cells. J Clin Invest. 1990;86:169–175. doi: 10.1172/JCI114680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, Kane C, Sigmund CD, Gross KW. Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem. 2001;276:45530–45538. doi: 10.1074/jbc.M103010200. [DOI] [PubMed] [Google Scholar]
- 25.Todorov VT, Volkl S, Friedrich J, Kunz-Schughart LA, Hehlgans T, Vermeulen L, Haegeman G, Schmitz ML, Kurtz A. Role of CREB1 and NF{kappa}B-p65 in the down-regulation of renin gene expression by tumor necrosis factor {alpha}. J Biol Chem. 2005;280:24356–24362. doi: 10.1074/jbc.M502968200. [DOI] [PubMed] [Google Scholar]
- 26.Klar J, Sandner P, Muller MW, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch. 2002;444:335–344. doi: 10.1007/s00424-002-0818-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–F37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Faulhaber-Walter R, Wen Y, Huang Y, Mizel D, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Renal failure in mice with Gsalpha deletion in juxtaglomerular cells. Am J Nephrol. 2010;32:83–94. doi: 10.1159/000314635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez ML, Gomez RA. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics. 2011;43:1021–1028. doi: 10.1152/physiolgenomics.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de WC, Kurtz A. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int. 2010;78:762–768. doi: 10.1038/ki.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi N, Lopez ML, Cowhig JE, Jr., Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 32.Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kihara M, Umemura S, Sumida Y, Yokoyama N, Yabana M, Nyui N, Tamura K, Murakami K, Fukamizu A, Ishii M. Genetic deficiency of angiotensinogen produces an impaired urine concentrating ability in mice. Kidney Int. 1998;53:548–555. doi: 10.1046/j.1523-1755.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 35.Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA. Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension. 1997;29:216–221. doi: 10.1161/01.hyp.29.1.216. [DOI] [PubMed] [Google Scholar]
- 37.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 38.Esther CR, Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 39.Inokuchi S, Kimura K, Sugaya T, Inokuchi K, Murakami K, Sakai T. Hyperplastic vascular smooth muscle cells of the intrarenal arteries in angiotensin II type 1a receptor null mutant mice. Kidney Int. 2001;60:722–731. doi: 10.1046/j.1523-1755.2001.060002722.x. [DOI] [PubMed] [Google Scholar]
- 40.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;286:R474–R483. doi: 10.1152/ajpregu.00426.2003. [DOI] [PubMed] [Google Scholar]