Abstract
Tumor lysis syndrome (TLS) is a potentially deadly complication of tumors or their treatment. This syndrome consists of a constellation of laboratory parameters such as hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia and clinical complications such as seizures, acute renal insult, cardiac dysrhythmias and death. TLS is especially common in patients with hematological malignancies with rapid cellular turnover rates such as acute lymphocytic leukemia and Burkitt lymphoma, but is very rare in patients with solid tumors. However, it is essential to keep in mind that solid tumors can also lead to TLS. We present a case of a 66-year-old African American male with metastatic cholangiocarcinoma complicated by the development of spontaneous TLS. TLS has never been reported in a patient with cholangiocarcinoma.
Keywords: Cholangiocarcinoma, tumor lysis syndrome (TLS), acute renal failure
Background
Tumor lysis syndrome (TLS) is one of the major oncological emergencies commonly seen with rapidly proliferating hematological malignancies. TLS comprises a clinicolaboratory derangement of cellular metabolism which can lead to acute renal impairment, cardiac arrhythmias, seizures and patient demise (1). Cellular damage mediated by cancer targeted therapy or spontaneous cellular death in rapidly dividing tumors (which is known as spontaneous TLS) leads to efflux of material rich in potassium, phosphorus, and uric acid. On the other hand, serum calcium is typically decreased in patients with TLS because of its binding to phosphorus. These biochemical derangements lead to renal dysfunction, cardiac arrhythmogenicity, central nervous system toxicity, and eventually death. The most widely used diagnostic criteria were proposed by Cairo and Bishop in 2004 (1). According to their classification, TLS can be defined as laboratory TLS, when TLS is clinically silent, as well as clinical TLS, when laboratory evidence of TLS is complicated by clinical manifestations such as arrhythmias, renal insult, seizures and ultimately death. The diagnostic criteria proposed by Cairo and Bishop are presented in Tables 1 and 2. It is important to mention that laboratory TLS is defined as the presence of at least two or more biochemical variables within three days before chemotherapy or seven days after chemotherapy in the face of adequate hydration and use of uric acid lowering agent. Clinical TLS is defined as the presence of at least one clinical criterion that is not believed to be attributable to chemotherapy agent (1). However, this definition is not perfect since other treatments (such as radiation therapy) can also cause to TLS as well as TLS be a spontaneous event without obvious precipitant.
Table 1. Cairo-Bishop definition of laboratory TLS for adults [adapted from reference (2)].
| Variable | Value | Change from baseline value |
|---|---|---|
| Uric acid | ≥8 mg/dL (476 micromol/L) | 25% increase |
| Potassium | ≥6.0 mEq/L (or 6 mmol/l) | 25% increase |
| Phosphorus | ≥4.5 mg/dL (1.45 mmol/L) for adults and ≥2.1 mmol/L (6.5 mg/dL) for children | 25% increase |
| Calcium | ≤7 mg/dL (1.75 mmol/L) | 25% decrease |
TLS, tumor lysis syndrome.
Table 2. Cairo-Bishop grading of clinical TLS for adults [adapted from reference (2)].
| Variable | Grade 0 | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|---|---|---|---|---|---|
| Creatinine | None | 1.5 times upper limits of normal (ULN) | >1.5-3.0 times ULN | >3.0-6.0 times ULN | >6.0 times ULN | Death |
| Cardiac arrhythmia | None | Intervention not indicated | Nonurgent medical intervention indicated | Symptomatic and incompletely controlled medically or controlled with device (e.g., defibrillator) | Life-threatening (e.g., arrhythmia associated with HF, hypotension, syncope, shock) | Death |
| Seizures | None | – | One brief, generalized seizure; seizure(s) well controlled by anticonvulsants or infrequent focal motor seizures not interfering with ADL | Seizure in which consciousness is altered; poorly controlled seizure disorder; with breakthrough generalized seizures despite medical intervention | Seizure of any kind which are prolonged, repetitive or difficult to control (e.g., status epilepticus, intractable epilepsy) | Death |
Comprehensive discussion of TLS pathophysiology, clinical presentation and management is outside the scope of this manuscript. The interested reader is referred to well written review articles on this topic (1-5).
As noted above hematological malignancies comprise the vast majority of TLS which is believed to be secondary to sensitivity to treatment and rapid proliferative rates. Nevertheless, TLS can occur in patients with solid cancers as a result of therapy or even spontaneously as will be discussed later in the text. Below we will present a case of spontaneous TLS in a patient with metastatic cholangiocarcinoma.
Case presentation
A 66-year-old African American male with past history of hypertension, smoking (20 pack years), and diabetes mellitus was admitted to the hospital because of worsening right upper quadrant abdominal pain which started 3 weeks ago (negative colonoscopy and esophagogastroduodenoscopy 1.5 years prior). Abdominal ultracsound showed evidence of cholelithiasis and gallbladder wall thickening. The patient was jaundiced and computed tomography (CT) scan of the abdomen and pelvis with contrast was done to rule out malignancy. Indeed, his CT scan showed scattered multiple liver metastases, evidence of ascites and normal appearing pancreas (please see Figure 1). Vital signs and physical examination was unremarkable, except for jaundice, hepatomegaly and ascites. Laboratory values on admission showed elevated liver function tests (AST 227 IU/L, ALT 163 IU/L, alkaline phosphatase 336 IU/L, total bilirubin 6.7 mg/dL), elevated LDH (899 IU/L), elevated INR (3.4) and elevated uric acid (9.9 mg/dL) normal creatinine (0.91 mg/dL), normal potassium (4.8 mg/dL), normal phosphorus (3.8 mg/dL) and normal calcium (8.7 mg/dL). Creatine kinase was within normal limits. Tumor markers were checked: elevated CEA (690.3 ng/mL), elevated CA 19-9 (666.5 U/mL) and normal AFP (0.9 ng/mL). Viral hepatitis panel was negative.
Figure 1.
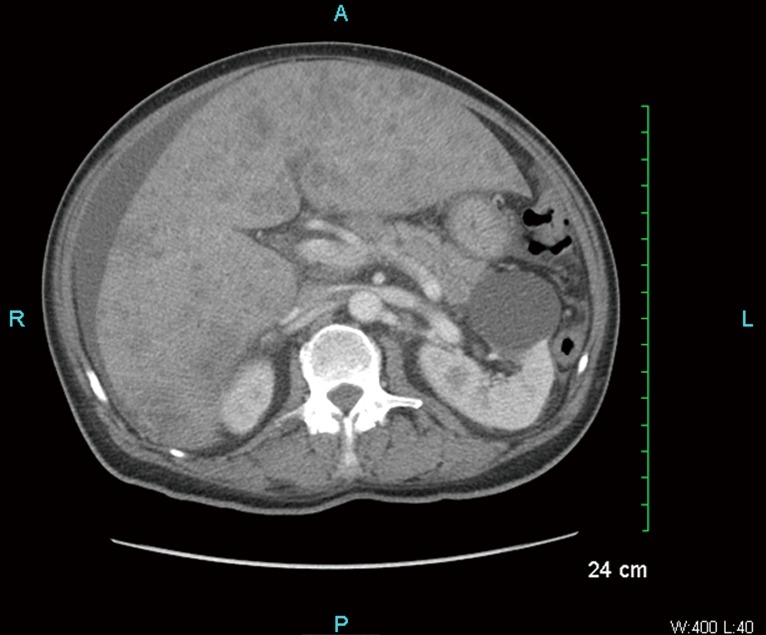
Multiple tiny ill-defined lesions scattered throughout the liver and ascites.
The patient was started on intravenous hydration with normal saline and allopurinol was started (300 mg three times a day). CT chest was negative for any malignancy. However, on the next day the patient started developing increase in creatinine (1.76 mg/dL), potassium (5.8 mg/dL) and phosphorus (8.1 mg/dL) as well as decrease in calcium (7.1 mg/dL). No chemotherapy, radiation therapy or even biopsy was undertaken. CT guided liver biopsy was performed the next day after development of spontaneous TLS. Liver specimen was reviewed by the pathologist with a preliminary diagnosis of poorly differentiated adenocarcinoma. Immunohistochemistry stains were positive for cytokeratin 7, cytokeratin 20, CDX2 and negative for HEP PAR 1, TTF 1, chromogranin, synaptophysin and PSAP. Based on these results, hepatocellular cancer (based on negativity for HEP PAR 1), colorectal carcinoma (based on positivity for cytokeratin 7), and lung cancers (based on negative chromogranin and synaptophysin) were considered to be unlikely. Further staining for cytokeratin 19 (please see Figure 2) and CA 19-9 was done. Tumor was strongly positive for cytokeratin 19 and minimally positive for CA 19-9. Based on the clinical picture, imaging studies and immunohistochemistry, cholangiocarcinoma was deemed to be the primary tumor (6,7). Unfortunately, the patient clinical course was complicated by the development of liver failure and ultimately death two days after liver biopsy. Family refused autopsy.
Figure 2.
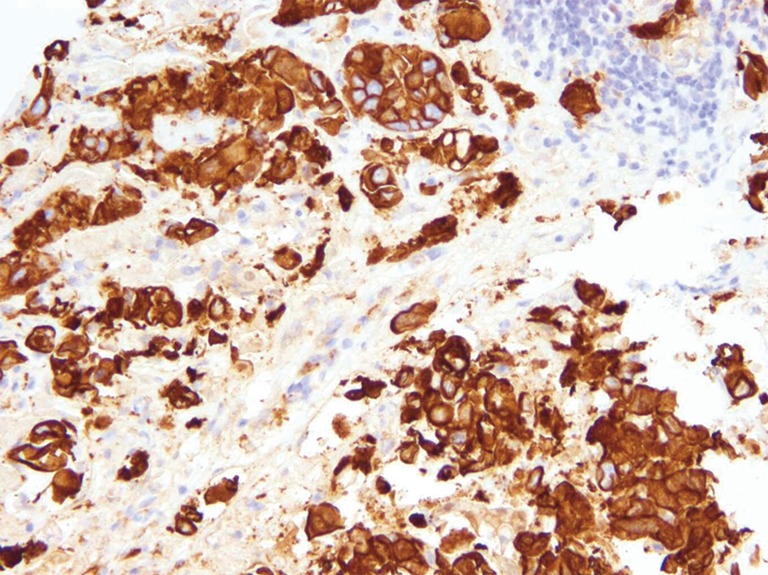
Strongly positive immunostain for cytokeratin 19 (IHC 20×).
Discussion
TLS is a true oncological emergency comprised of laboratory derangement of cellular metabolism, which can lead to acute renal impairment, cardiac rhythm disturbances, seizures and death (1). Laboratory manifestations of TLS include hyperkalemia (>6.0 mEq/L), hyperphosphatemia (>4.5 mg/dL), hyperuricemia (>8.0 mg/dL) and hypocalcemia (<7.0 mg/dL). TLS can be either spontaneous (without cancer targeted treatment) or therapy related (chemotherapy or radiation therapy). TLS is common in patients with rapidly proliferating hematological malignancies such as acute lymphocytic leukemia, Burkitt lymphoma and diffuse large B cell lymphoma (2,3). The predilection of TLS to hematological malignancies can be explained by their sensitivity to therapy and proliferative rates (3).
The treatment consists of aggressive hydration, correction of electrolyte disturbances and uric acid lowering therapy (2,4). TLS is a rare occurrence in patients with solid tumors, which can be explained by differences in proliferation rates and sensitivity to chemotherapy and/or radiation therapy (8).
Furthermore, spontaneous TLS is even rarer event in patients with solid malignancies (8). Nevertheless, clinicians should keep in mind that patients with solid tumors may develop this potentially deadly syndrome. Based on the literature review it seems that patients with advanced and metastatic tumors may be at risk for TLS (8). Other potential risk factors might be the presence of elevated baseline creatinine and decreased renal function, elevated LDH, elevated phosphorus, elevated potassium and elevated uric acid. It is unclear whether liver metastasis represents an individual risk factor for the development of TLS or is a simply marker of advanced disease. To our best knowledge this is the first case of TLS in a patient with cholangiocarcinoma.
Conclusions
This is the first reported case of TLS in a patient with cholangiocarcinoma. TLS in patients with solid malignancies may be more common than expected.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004;127:3-11 [DOI] [PubMed] [Google Scholar]
- 2.Wilson FP, Berns JS. Onco-nephrology: tumor lysis syndrome. Clin J Am Soc Nephrol 2012;7:1730-9 [DOI] [PubMed] [Google Scholar]
- 3.Will A, Tholouli E.The clinical management of tumour lysis syndrome in haematological malignancies. Br J Haematol 2011;154:3-13 [DOI] [PubMed] [Google Scholar]
- 4.Mika D, Ahmad S, Guruvayoorappan C.Tumour lysis syndrome: implications for cancer therapy. Asian Pac J Cancer Prev 2012;13:3555-60 [DOI] [PubMed] [Google Scholar]
- 5.McBride A, Westervelt P.Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies. J Hematol Oncol 2012;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oien KA, Dennis JL. Diagnostic work-up of carcinoma of unknown primary: from immunohistochemistry to molecular profiling. Ann Oncol 2012;23Suppl 10:x271-7 [DOI] [PubMed] [Google Scholar]
- 7.Wong HH, Chu P. Immunohistochemical features of the gastrointestinal tract tumors. J Gastrointest Oncol 2012;3:262-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gemici C.Tumour lysis syndrome in solid tumours. Clin Oncol (R Coll Radiol) 2006;18:773-80 [DOI] [PubMed] [Google Scholar]


