Abstract
Background
The incidence of gastric cancer varies in different parts of the world and among various ethnic groups. It remains the fifth most common cancer among males and seventh most common cancer among females in India.
Materials and methods
We conducted a retrospective study using the data base of 158 patients of primary gastric cancer diagnosed in the Department of Surgery at Regional Cancer Centre, RIMS, Manipur, India from July 2009 to June 2013.
Results
Our study revealed a male to female ratio of 2.16:1, distribution of age varied from 28 to 91 years. Majority of the men were in the age group of more than 60 years (45.37%) and majority of females were of 51-60 years (44%). Nearly 7.6% patients had a positive family history. Dietary history of intake salted, fermented fish was present in 67.7% of patients, whereas history of consumption of smoked meat was found in 77.8% of patients. Only 27.8% of patients in our study had history of regular consumption of fresh fruits. About 35.4% of the patients had poor drinking water source. Nearly, 67.6% of males and 44% of females had smoking history. Combined consumption of alcohol and smoking was present in 33.5% of patients. Vague abdominal discomfort was the most common presenting symptom in 61.4% of patients. The most common site of gastric cancer in our study was antrum (50.6%) followed by cardia (17.1%). The most common histological type was adenocarcinoma (95.6%). Most of our patients presented in locally advanced stage (62.7%).
Conclusions
Our analysis suggests that poor dietary habits such as smoked mead, dried fish and excessive use of tobacco are associated with high occurrence of gastric cancer in this part of the India. Increasing the awareness regarding the aetiology and varied clinical presentation among general population and health providers is needed for prevention and early detection.
Keywords: Gastric cancer, North-eastern India, dried fish, smoked meat
Introduction
Cancer is a biggest burden of modern society. This is the second most common disease after cardiovascular disorders for maximum deaths in the world (1). Carcinoma of the stomach is a second leading cause of cancer death worldwide. The incidence of gastric cancer varies in different parts of the world and among various ethnic groups. It remains the fifth most common cancer among males and seventh most common cancer among females in India (2). However, the overall incidence of gastric cancer in India is less compared to the worldwide incidence and India falls under the low incidence region category for gastric cancer. Incidence of gastric cancer varies widely among the various regions within India due diverse culture and related food habits. Reports from the National Cancer Registry Programme (NCRP) 2010, suggested that the mean age-adjusted rate (AAR) of gastric cancer among urban registries in India varied from 3.0 to 13.2, with the highest rate being recorded in Chennai registry (3-5). However, the prevalence was found to be much higher in the north eastern region of India. Currently, the north eastern state of Mizoram occupies the first position among Indian states and fifth position globally with AAR of 46.3 to 70.2 (6).
The prevalence of gastric cancer is also high in the state of Manipur. Based on our Hospital Based Cancer Registry (HBCR) 2012 gastric cancer is the second most common cancer among males comprising 6.1% of all the cancers and represents 2% in females. The aetiology of gastric cancer is multi-factorial and various dietary and environmental factors have been attributed. Diet is believed to play a major role in the development of gastric cancer. It is very well known that salt rich, smoked or poorly preserved foods, nitrates, nitrites have been associated with an increase in gastric cancer. Conversely, diets high in raw vegetables, fresh fruits (containing vitamin C, antioxidants) are associated with decreased risk (7-9). Helicobacter pylori infection is associated with an approximately two-fold increased risk of developing gastric cancer (10-12). Pylori H have been categorized as a “Group-1 human carcinogen” by the International Agency for Research on Cancer (13). The role of tobacco in the occurrence of gastric cancers cannot be undermined (6).
The state of Manipur, located in the north eastern region of India bordering Myanmar, has different customs, food habits, life-style, diverse ethnic groups, and the pattern of tobacco use as compared to the rest of the country. Majority of the people here consume dried salted fish, fermented, smoked and pickled meat and the use of tobacco is also widely prevalent. We undertook this study to analyse the demographic pattern, clinical presentations, pathological characteristics and stage at presentation of stomach cancer at Regional Cancer centre, Regional Institute of Medical Sciences (RIMS), situated in Imphal, Manipur state, is the biggest referral centre for the neighbouring North eastern states in India and bordering Myanmar.
Materials and methods
We conducted a retrospective study using the data base of 158 patients of primary gastric cancer diagnosed in the Department of Surgery at Regional Cancer Centre, RIMS, Manipur, India from July 2009 to June 2013. All these patients were diagnosed on clinical, radiological and endoscopic examination. The diagnosis was confirmed pathologically after the histopathological examination of either the resected specimen or the endoscopic biopsy specimen. All the patients with a confirmed gastric carcinoma were included in the study. The cases with primary gastric lymphoma, gastro intestinal stromal tumours (GIST) and gastric melanoma were excluded. Restaging was performed according to AJCC staging system (7th edition) based on the available clinical and radiological findings. The compiled data included demographic data, medical history of chronic gastritis, peptic ulcer disease, family history of gastric cancer, dietary habits (intake of fermented, smoked meat, red meat), drinking water source, smoking habits, consumption of alcohol, chief presenting complaints, histological grade, TNM staging and the site of metastasis.
Descriptive statistics were used for analysing the data using SPSS version 20 and results were presented in percentage and simple frequency.
Results
This study included 158 patients with male to female ratio of 2.16:1, distribution of age varied from 28 to 91 years old. Majority of the men were in the age group of more than 60 years old (45.37%), followed by 51-60-year age group (31.4%) whereas majority of females were of 51-60-year-old category (44%), followed by more than 60-year-old group (36%). All in all, 93.5% males and 96% of females were more than 50 years old. Out of 158 patients in this study, 7.6% patients had a positive family history. Dietary history of intake salted, fermented fish was present in 67.7% of patients, whereas history of consumption of smoked meat was found in 77.8% of patients. Only 27.8% of patients in our study had history of regular consumption of fresh fruits. About 35.4% of the patients had poor drinking water source. Nearly, 67.6% of males and 44% of females had smoking history. Male to female ratio of smoking was 3.3:1. History of alcohol consumption was present in 55.5% of male and 10% of female cases. Combined consumption of alcohol and smoking was present in 33.5% of patients. Vague abdominal discomfort was the most common presenting symptom in 61.4% of patients followed by weight loss (59.5%), nausea (39.9%), early satiety and poor appetite (34.8%), vomiting (20.9%), dysphagia (18.4%) and melaena (15.8%) (Figure 1). About 25.3% of patients presented with abdominal lump, 55.5% of patients with tumour at cardia had history of dyphagia, 62.3% of patients with tumour in antro-pyloric region had history of weight loss, and 84.2% of patients had multiple presenting symptoms (Table 1). Pallor was noted in 48.7% of patients at presentation, 53/108 males (49.07%) and 24/50 females (48%). Most common site of gastric cancer in our study was antrum (50.6%) followed by cardia (17.1%), body (13.9%), pylorus (13.3%) and fundus (2.5%). The most common site of tumour in both males and females was antrum, 57.4% and 36% respectively. The second most common site was cardia (17.6%) in males and body of the stomach (22%) in females. The most common histological type was adenocarcinoma (95.6%) followed by squamous cell carcinoma (3.2%). About 44.3% of the tumours were poorly differentiated, 35.8% moderately differentiated and 19.6% well differentiated (Table 2). Majority of the patients were of T3 stage (53.2%) at presentation followed by T2 (23.4%), T4 (15.8%) and T1 (7.6%). Likewise, N2 nodal staging was leading with 35.4% followed by N0 (27.8%), N1 (20.3%) and N3 (16.5%). Overall 37.3% of patients had distant metastasis at the time of presentation. Liver was the most common site of metastasis found in 17.1% patients followed by left supraclavicular lymph node (7.6%), peritoneal meatastais (7%) and multiple metastases (5.1%). Majority of the patients in our study were found to have locoregional disease at presentation (62.7%); of these early gastric cancers was found in 7.6% patients (Figure 2).
Figure 1.
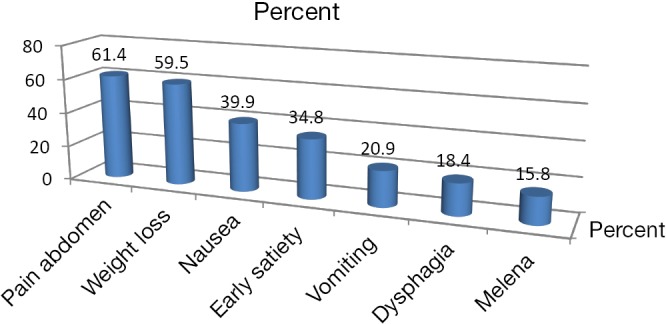
Common symptoms in gastric cancer patients.
Table 1. Symptoms with respect to location of tumour.
| Symptoms | Cardia | Fundus | Body | Antrum | Pylorus | Nos | Total [%] |
|---|---|---|---|---|---|---|---|
| Weight loss | 16 | 3 | 10 | 50 | 13 | 2 | 94 [59.5] |
| Pain abdomen | 9 | 3 | 14 | 55 | 12 | 4 | 97 [61.4] |
| Nausea | 10 | 3 | 9 | 32 | 7 | 2 | 63 [39.9] |
| Vomiting | 6 | 1 | 4 | 17 | 4 | 1 | 33 [20.9] |
| Early satiety | 7 | 2 | 7 | 33 | 6 | 0 | 55 [34.8] |
| Dysphagia | 15 | 1 | 2 | 9 | 2 | 0 | 29 [18.4] |
| Melena | 3 | 2 | 5 | 11 | 4 | 0 | 25 [15.8] |
| Anemia | 13 | 3 | 10 | 39 | 10 | 2 | 77 [48.7] |
| Mass abdomen | 4 | 2 | 5 | 22 | 6 | 1 | 40 [25.3] |
Table 2. Demographic and Clinico-pathologic characteristics of patients with gastric cancer.
| Variable | Subgroup | N [%] |
|---|---|---|
| Age at diagnosis [years] [n=158] | <40 | 9 [5.7] |
| 41-50 | 26 [16.5] | |
| 51-60 | 56 [35.4] | |
| >60 | 67 [42.4] | |
| Sex | Male | 108 [68.4] |
| Female | 50 [31.6] | |
| Family history | Present | 12 [7.6] |
| Dietary history | Dried, fermented fish | 107 [67.7] |
| Fresh fruits | 44 [27.8] | |
| Smoking | 95 [60.12] | |
| Alcohol | 65 [41.13] | |
| Tumour site | Antrum | 80 [50.6] |
| Cardia | 27 [17.1] | |
| Pylorus | 21 [13.3] | |
| Body | 22 [13.9] | |
| Others | 8 [5] | |
| T stage | T1 | 12 [7.6] |
| T2 | 37 [23.4] | |
| T3 | 84 [53.2] | |
| T4 | 25 [15.8] | |
| N stage | N0 | 44 [27.8] |
| N1 | 32 [20.3] | |
| N2 | 56 [35.4] | |
| N3 | 26 [16.5] | |
| M stage | M0 | 99 [62.7] |
| M1 | 59 [37.3] | |
| Tumour grade* | Well differentiated | 31 [19.6] |
| Moderately | 55 [34.8] | |
| Poory | 70 [44.3] | |
| Tumour stage | Early gastric cancer | 12 [7.6] |
| Advanced Gastric cancer | 87 [55.1] | |
| Systemic disease | 59 [37.3] |
*, tumour grade information not available for two cases.
Figure 2.
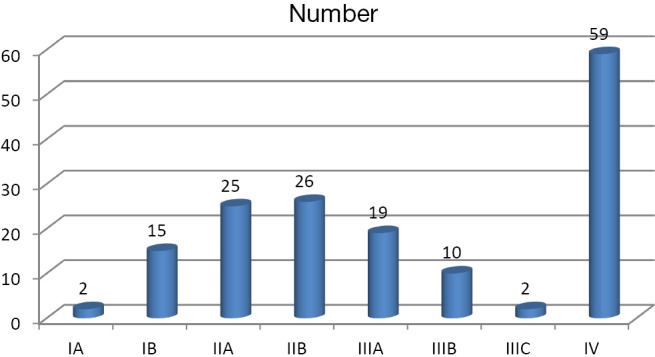
Frequency of overall staging.
Discussion
There is worldwide variation regarding the incidence and patterns of gastric cancer. Countries of Southeast Asia, Japan, South Korea and China have noted a high incidence of gastric cancer (14,15). The overall incidence of gastric cancer in India is less compared to rest of the world (4-6). However, certain regions of India have recorded a high incidence, especially the north eastern states like Mizoram (6). In North-East region very high incidence of all sites of cancers in general and tobacco related cancers in particular have been reported. Pattern of tobacco use is noted to be different in North-East region. The genetic susceptibility of cancer due to ethnic variation related to polymorphism and mutation in autosomal recessive genes has been suspected. Certain dietary and tobacco related carcinogens are known to act as co-factors to bring out genetic changes (16). A high incidence of gastric cancer has also been reported in the state of Manipur, where it constitutes the second most common malignancy among males. There is lack of clinic-pathological information about gastric cancer from Manipur.
In our study, the peak incidence of gastric cancer was in age group older than 60 years old (42.4%). Also male predominance was noted with male to female ratio of 2.16:1, which are comparable with other studies (17-21). Presumably, this male preponderance could be attributed to the high incidence of smoking (67.6%) found among the males, with male to female smoking ratio of 3.3:1 in our study. About 7.6% of patients in our study had a positive family history which was similar to another study (17). However, many other studies have reported a positive family history of 17% of patients (22). Our low estimate of family history could have been because of poor reporting by patient attendees. An overwhelming majority of patients (77.8%) in our study had a history of consumption of smoked meat, and 67.7% of patients had history of consumption of dried, fermented fish. Whereas, only 27.8% of the patients had a history of regular consumption of fresh fruits. Consumption of dried fish has found to increase the risk of gastric cancer (23). It is also well known that high consumption of smoked meat and decreased consumption of fresh fruits increases the risk of gastric cancer (8,9). The most common presenting symptoms in our study abdominal pain (61.4%) and weight loss (59.5%), which were similar to other studies (17,24). Our findings revealed that most common site of tumour was antrum (57.45%) followed by cardia (17.1%) which are consistent with many other studies (25-28). However, increased incidence of tumour occurrence in gastro-esophageal junction has been noted in many western studies (27).
Considering the histological type, majority (95.6%) were found to be adenocarcinoma consistent with other studies (17,29). Majority of the tumours (44.3%) in our study were poorly differentiated, similar to other studies (17,30). Studies have shown that elder patients were more likely to have well or moderately differentiated tumours and young patients were more likely to have poorly-differentiated tumours [Nakamura et al., (31)]. Similarly in our study six out of nine patients with <40 years old of age had poorly differentiated tumours. Early gastric cancer was present in 7.6% cases and majority (62.7%) had locally advanced gastric cancers at the time of presentation in our study. This figure is less compared 9-17% seen in western countries and far less compared to the prevalence of Japan where mass screening programmes for gastric cancer are in place (32). This highlights the need for aggressive endoscopy and biopsy for minimally symptomatic patients to improve the survival.
There is evidence to implicate chronic Pylori H infection as a major risk factor for the development of intestinal type of gastric cancer (9,11,12). However, we had no information regarding the infection status of patients in our study.
Conclusions
Our analysis suggests that poor dietary habits such as smoked meat, dried fish and excessive use of tobacco are associated with high occurrence of gastric cancer in this part of the India. Symptoms of weight loss and abdominal pain in elderly population should alert the healthcare providers about the possibility of gastric cancer. Increasing the awareness regarding the aetiology and varied clinical presentation among general population and health providers is needed for prevention and early detection. High risk subset may be undertaken for screening the disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66 [DOI] [PubMed] [Google Scholar]
- 2.Rao DN, Ganesh B. Estimate of cancer incidence in India in 1991. Indian J Cancer 1998;35:10-8 [PubMed] [Google Scholar]
- 3.Yeole BB. Trends in cancer incidence in esophagus, stomach, colon, rectum and liver in males in India. Asian Pac J Cancer Prev 2008;9:97-100 [PubMed] [Google Scholar]
- 4.Satyanarayana L, Asthana S.Life time risk for development of ten major cancers in India and its trends over the years 1982 to 2000. Indian J Med Sci 2008;62:35-44 [PubMed] [Google Scholar]
- 5.Rastogi T, Devesa S, Mangtani P, et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol 2008;37:147-60 [DOI] [PubMed] [Google Scholar]
- 6.Sahasrabudhe MR, Lakshminarayan Rao MV. The influence of dietary protein on the cystine and methionine contents of liver protein. Curr Sci 1950;19:285-6 [PubMed] [Google Scholar]
- 7.Barker DJ, Coggon D, Osmond C, et al. Poor housing in childhood and high rates of stomach cancer in England and Wales. Br J Cancer 1990;61:575-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirohata T, Kono S.Diet/nutrition and stomach cancer in Japan. Int J Cancer 1997;Suppl 10:34-6 [DOI] [PubMed] [Google Scholar]
- 9.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881-8 [DOI] [PubMed] [Google Scholar]
- 10.Eslick GD. Helicobacter pylori infection causes gastric cancer? A review of the epidemiological, meta-analytic, and experimental evidence. World J Gastroenterol 2006;12:2991-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784-9 [DOI] [PubMed] [Google Scholar]
- 12.Huang JQ, Sridhar S, Chen Y, et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998;114:1169-79 [DOI] [PubMed] [Google Scholar]
- 13.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 1994;61:1-241 [PMC free article] [PubMed] [Google Scholar]
- 14.Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999;83:18-29 [DOI] [PubMed] [Google Scholar]
- 15.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol 2003;14Suppl 2:ii31-6 [DOI] [PubMed] [Google Scholar]
- 16.Das BC. Cancers in North-East Regions of India. An ICMR-Taskforce multicentric collaborative study. 2005. Available online: http://www.icmr.nic.in/annual/2004-05/icpo/cancer_neregion.pdf
- 17.Safaee A, Moghimi-Dehkordi B, Fatemi SR, et al. Clinicopathological Features of Gastric Cancer: A Study Based on Cancer Registry Data. IJCP 2009;2:67-70 [Google Scholar]
- 18.Sasagawa T, Solano H, Mena F.Gastric cancer in Costa Rica. Gastrointest Endosc 1999;50:594-5; discussion 595-6 [PubMed] [Google Scholar]
- 19.Yao JC, Tseng JF, Worah S, et al. Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution’s experience over 15 years. J Clin Oncol 2005;23:3094-103 [DOI] [PubMed] [Google Scholar]
- 20.Sadjadi A, Malekzadeh R, Derakhshan MH, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer 2003;107:113-8 [DOI] [PubMed] [Google Scholar]
- 21.Kong SH, Park DJ, Lee HJ, et al. Clinicopathologic features of asymptomatic gastric adenocarcinoma patients in Korea. Jpn J Clin Oncol 2004;34:1-7 [DOI] [PubMed] [Google Scholar]
- 22.Medina-Franco H, Heslin MJ, Cortes-Gonzalez R. Clinicopathological characteristics of gastric carcinoma in young and elderly patients: a comparative study. Ann Surg Oncol 2000;7:515-9 [DOI] [PubMed] [Google Scholar]
- 23.Rao DN, Ganesh B, Dinshaw KA, et al. A case-control study of stomach cancer in Mumbai, India. Int J Cancer 2002;99:727-31 [DOI] [PubMed] [Google Scholar]
- 24.Eskandar H, Hossein SS, Rahim M, et al. Clinical profile of gastric cancer in Khuzestan, southwest of Iran. World J Gastroenterol 2006;12:4832-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plummer JM, Gibson TN, McFarlane ME, et al. Clinicopathologic profile of gastric carcinomas at the University Hospital of the West Indies. West Indian Med J 2005;54:364-8 [DOI] [PubMed] [Google Scholar]
- 26.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003;56:1-9 [DOI] [PubMed] [Google Scholar]
- 27.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991;265:1287-9 [PubMed] [Google Scholar]
- 28.Inoue M, Tsugane S.Epidemiology of gastric cancer in Japan. Postgrad Med J 2005;81:419-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambasivaiah K, Ibrarullah M, Reddy MK, et al. Clinical profile of carcinoma stomach at a tertiary care hospital in south India. Trop Gastroenterol 2004;25:21-6 [PubMed] [Google Scholar]
- 30.Kim DY, Ryu SY, Kim YJ, et al. Clinicopathological characteristics of gastric carcinoma in young patients. Langenbecks Arch Surg 2003;388:245-9 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Yao T, Niho Y, et al. A clinicopathological study in young patients with gastric carcinoma. J Surg Oncol 1999;71:214-9 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Ueyama T, Yao T, et al. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer 1992;70:1030-7 [DOI] [PubMed] [Google Scholar]


