Abstract
Background
Patients with locally advanced esophageal cancer who require neoadjuvant therapy have significant dysphagia and may severely impair nutritional status. We conducted a meta-analysis to assess the efficacy of self-expandable metal stents prior to neoadjuvant therapy.
Methods
A systematic search was conducted using MEDLINE, PubMed, EMBASE, Current Contents Connect, Cochrane library, Google Scholar, Science Direct, and Web of Science. Original data was abstracted from each study and used to calculate a pooled odd ratio (OR) and 95% confidence interval (95% CI).
Results
Only nine studies comprising of 180 patients were included for analysis. The overall procedural success rate was 95% (95% CI, 0.895-0.977). There was a substantial decrease in the dysphagia scores standard difference in means (SDM) –0.81 [standard error (SE) 0.15, 95% CI, –1.1 to –0.51], similar increase in weight SDM 0.591 (SE 0.434, 95% CI, –0.261 to 1.442) and serum albumin SDM 0.35 (SE 0.271, 95% CI, –0.181 to 0.881). The incidence of major adverse events included stent migration 32% (95% CI, 0.258-0.395) and chest discomfort 51.4% (95% CI, 0.206-0.812).
Conclusions
Placement of stents in patients with locally advanced esophageal cancer significantly improves dysphagia and allows for oral nutrition during neoadjuvant therapy. Stents appear to be effective for palliating dysphagia. Stent migration was a common occurrence; however, migration may be a sign of tumor response to neoadjuvant therapy.
Keywords: Neoadjuvant therapy, self-expandable metal stents, esophageal cancer
Introduction
The incidence and mortality from cancer of all types in the United States has decreased during the 1991-2006 timeframe (1). However, the opposite is true for esophageal cancer. Its incidence and mortality continue to rise. In 2010, estimated new cases of esophageal cancer number 16,640 in the United States, while deaths total 14,500 (1). The United States has seen an average increase of 20.6% per year in the incidence of adenocarcinoma of the esophagus since that time (2). Esophageal cancer is a highly lethal disease in which only one-third of patients present with resectable disease. Of this select group, the average 5-year survival is only 35-45% (3). The overwhelming majority of patients have a fatal outcome, but advances in multimodality therapy appear to be improving the long term survival outcome for patients with locally advanced disease.
Most patients with advanced esophageal cancer have significant dysphagia, which contributes to weight loss and malnourishment. The majority of patients with esophageal cancer present with signs of malnutrition at the time of diagnosis as a result of both dysphagia and tumor-induced cachexia (4). Additionally, patients undergoing multimodal therapy have been shown to have significantly worse nutritional parameters than those only undergoing resection (5). Radiation-induced esophagitis develops in 15-28% of treated patients’ further aggravating dysphagia (6,7). Also, the side effects of 5-fluorouracil and cisplatin, the most common chemotherapy regimen employed to treat esophageal cancer, include nausea, vomiting, and diarrhea. Malnutrition reduces the potential response of the malignancy to chemoradiotherapy and impairs the patient’s ability to tolerate the full course of treatment (8). In addition, the importance of adequate nutritional status prior to a major operation is well recognized (9).
Evidence clearly indicates that malnourished patients who undergo major operations are predisposed to infectious complications and worse postoperative outcomes (9-11). Nutritional deficiencies may also contribute to the trend of amplified perioperative morbidity and mortality among esophageal cancer patients receiving multimodal therapy compared with patients undergoing resection alone (12,13).
We hypothesized that patients treated with neoadjuvant therapy and who received removable stents would have better nutrition-related outcomes compared with those who were not stented. The objective of this study was to evaluate of the effectiveness of stents for improving the nutritional status of patients undergoing neoadjuvant therapy for esophageal cancer.
Methods
Study protocol
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses PRISMA guidelines where possible in performing our systematic review (14). We performed a systematic search through MEDLINE (from 1950), PubMed (from 1946), EMBASE (from 1949), Current Contents Connect (from 1998), Cochrane library, Google scholar, Science Direct, and Web of Science to May 2013. The search terms included “esophageal cancer”, “neoadjuvant therapy” and “stents”, which were searched as text word and as exploded medical subject headings where possible. No language restrictions were used in either the search or study selection. The reference lists of relevant articles were also searched for appropriate studies. A search for unpublished literature was not performed.
Study selection
We included studies that met the following inclusion criteria:
• Studies identifying the population of patients with esophageal cancer undergoing stent implantation prior or during neoadjuvant therapy.
Data extraction
We performed the data extraction using a standardized data extraction form, collecting information on the publication year, study design, number of cases, total sample size, population type, country, continent, mean age and clinical data. The event rate and confidence intervals (CIs) were calculated.
Statistical analysis
Pooled event rate and 95% CI were using a random effects model (15). We tested heterogeneity with Cochran’s Q statistic, with P<0.10 indicating heterogeneity, and quantified the degree of heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I2 values of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity respectively (16). The quantified publication bias using the Egger’s regression model (17), with the effect of bias assessed using the fail-safe number method. The fail-safe number was the number of studies that we would need to have missed for our observed result to be nullified to statistical non-significance at the P<0.05 level. Publication bias is generally regarded as a concern if the fail-safe number is less than 5n+10, with n being the number of studies included in the meta-analysis (18). All analyses were performed with Comprehensive Meta-analysis (version 2.0), Biostat, Englwood, NJ, USA [2005].
Results
The original search strategy 418 retrieved studies (Figure 1). The abstracts were reviewed and after applying the inclusion and exclusion criteria, articles were selected for full-text evaluation. Of the articles selected, only nine studies (180 patients) met full criteria for analysis and are summarised in Table 1. The years of publication ranged from 2007 to 2012.
Figure 1.
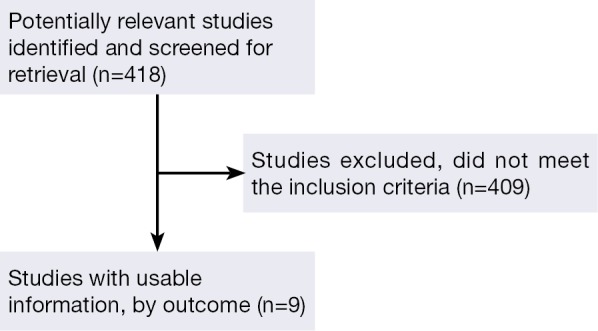
Flow of included studies.
Table 1. Characteristics of the studies included in the systematic review and meta-analysis.
| Author | Country | Year | Patients | Stent |
|---|---|---|---|---|
| Siddiqui et al. (19) | USA | 2009 | 12 | Polyflex stent |
| Adler et al. (20) | USA | 2009 | 13 | Polyflex stent |
| Siddiqui et al. (21) | USA | 2007 | 6 | Polyflex stent |
| Bower et al. (22) | USA | 2009 | 25 | Polyflex stent |
| Langer et al. (23) | Austria | 2010 | 38 | Self-expanding, plastic stents, covered metal stents |
| Pellen et al. (24) | UK | 2012 | 16 | Self-expanding removable metal stents |
| Siddiqui et al. (25) | USA | 2012 | 55 | ALIMAXX-E stent, WallFlex stent |
| Lopes et al. (26) | USA | 2010 | 11 | ALIMAXX-E, stent |
| Martin et al. (27) | USA | 2009 | 5 | Polyflex stent |
The overall procedural success rate was 95% (95% CI, 0.895-0.977). There was a substantial decrease in the dysphagia scores standard difference in means (SDM) –0.81 [standard error (SE) 0.15, 95% CI, –1.1 to –0.51] (Figure 2), similar increase in weight SDM 0.591 (SE 0.434, 95% CI, –0.261 to 1.442) and serum albumin SDM 0.35 (SE 0.271, 95% CI, –0.181 to 0.881). The incidence of major adverse events included stent migration 32% (95% CI, 0.258-0.395) and chest discomfort 51.4% (95% CI, 0.206-0.812) (Figure 3).
Figure 2.
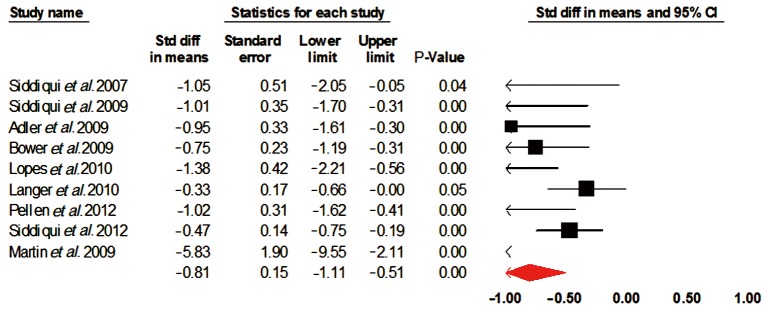
Dysphagia scores. CI, confidence interval.
Figure 3.
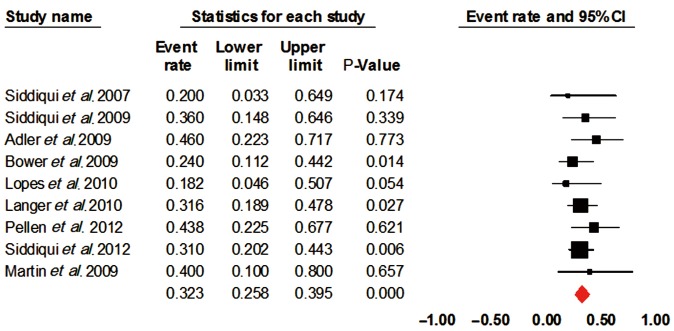
Stent migration. CI, confidence interval.
Heterogeneity and publication bias
The heterogeneity of outcomes has been summarized in Tables 2 and 3. The reason for significant heterogeneity may be attributed to different population groups. No publication bias was detected using the Egger’s regression model.
Table 2. Overall odds ratio and 95% CI for patient outcomes.
| Outcome | Event rate | 95% CI | I2 | P value |
|---|---|---|---|---|
| Successful placement | 0.95 | 0.895-0.977 | 0 | 0.76 |
| Stent migration | 0.32 | 0.258-0.395 | 0 | 0.82 |
| Chest discomfort | 0.514 | 0.206-0.812 | 82.16 | <0.0001 |
CI, confidence interval.
Table 3. Standard difference in means and 95% CI for patient outcomes.
| Outcome | Standard difference in means | Standard error | 95% CI | I2 | P value |
|---|---|---|---|---|---|
| Dysphagia scores | –0.81 | 0.15 | –1.1 to –0.51 | 59.72 | 0.01 |
| Increase in weight | 0.591 | 0.434 | –0.261 to 1.442 | 86.98 | <0.001 |
| Increase in serum albumin | 0.35 | 0.271 | –0.181 to 0.881 | 70.68 | 0.01 |
Discussion
The current standard of care is to offer neoadjuvant therapy to patients with locally advanced esophageal cancer (28). These patients receive three to six weeks of therapy before surgery (29,30). During oncologic therapy, dysphagia often increases due to mucositis and esophagitis induced by chemotherapy and radiotherapy. Others factors contributing to this include obstruction to sufficient dietary intake by luminal narrowing, anorexia and tumor cachexia. Improved baseline nutritional status independently predicts superior response to definitive chemoradiotherapy (albumin >35 g/L) and survival (BMI >18 kg/m2) in locally advanced esophageal cancer receiving nonsurgical treatment with curative intent (31). Therefore, the need for nutritional support is increased.
Options for nutritional supplementation during neoadjuvant therapy include parenteral nutrition or enteral nutrition given via a feeding tube. Parenteral nutrition is generally avoided because of increased costs, higher rates of infectious complications, and less efficacious reversal of malnutrition (32-36). Enteral supplementation requires feeding tube placement by either an open, laparoscopic or percutaneous technique. In fact, some centers advocate routine feeding tube placement in all patients undergoing multimodal therapy (37,38). Nasogastric feeding can be poorly tolerated and unsightly for the patient. It is associated with blockage, displacement, reflux and aspiration risks, and do not palliate dysphagia.
Percutaneous endoscopic gastrostomy (PEG) mandates that the tumor be negotiable with an endoscope and even if traversable, the pull-through technique may traumatize or transfer disease from the primary tumor. In the case of PEG tube placement, the potential exists for injury to the gastroepiploic artery rendering the stomach unusable as a replacement conduit for the esophagus (39). Besides procedure-related morbidity, tube placement delays chemotherapy by 1-2 weeks to allow for resolution of local inflammation and contamination that develops at the insertion site.
Jejunostomies arguably represent the mainstay of perioperative nutritional supplementation in esophagectomy patients and may be performed radiologically or surgically. However, both pre- and postoperative jejunostomies are associated with morbidity including displacement, obstruction, tube-site infection and peritonitis (40,41).
Preoperative esophageal stenting provides a possible alternative to address the nutritional status of patients receiving multimodal therapy. Removable self-expanding silicone stents can be placed prior to neoadjuvant therapy and later removed endoscopically or at the time of surgery (27). The overall procedural success rate was good according to our analysis.
Complications
The overall incidence of stent migration was 32%. However, the majority of them did not require stent replacement because the stent migration probably was a result of tumor shrinkage from neoadjuvant therapy (25). Additionally, all the migrations were of stents that were deployed across the gastroesophageal junction and hence were at increased risk for migration. Stent migration correlated with restoration of an esophageal lumen that allowed for adequate oral nutritional intake (25). Another advantage of preoperative esophageal stenting not all patients with locally advanced esophageal cancer will have a curative resection. Patients who do not proceed to surgery can have their stents left in place as a palliative measure.
Quality of life (QoL)
The primary aim of treatment in patients with inoperable EC is to relieve dysphagia with minimal morbidity and mortality, and thus improve their QoL. Implantation of a SEMS has become established as a treatment modality for the palliation of malignant dysphagia. SEMS relieves dysphagia rapidly and improves the nutritional status. However, in most studies, relief of dysphagia is the only aspect of health-related quality of life (HRQoL) being measured, although physical, mental and social functioning and other EC-specific aspects of HRQoL are additional important outcome measures.
A randomized clinical trial comparing SEMS with plastic endoprostheses published in 2002 by University of Glasgow and Edinburgh (42) included 50 patients suffering from dysphagia due to an inoperable EC, and measured QoL using EORTC QLQ-30, a multi-dimensional cancer-specific QoL questionnaire and an EC specific questionnaire (EORTC OES-24), allowing QoL to be measured over 26 components relating to cancer in general and EC in particular. Although the authors found no statistical significance in any of the 26 components, 21 of the 26 components showed a trend towards the metal group, five were neutral and none favored plastic stents.
Shenfine et al. (43) in a randomized controlled trial regarding the cost-effectiveness of palliative therapies for patients with inoperable EC studied QoL in detail using four different questionnaires including Spitzer QoL index, Karnowsky performance scale, Euroqol EQ-5D and EORTC QLQ-30. They also used proxy and self-administered questionnaires. These authors reported differences in the baseline QoL index favoring the non-SEMS group and went on to report one and six wk QoL data for the different treatment groups. Mean QoL index for the SEMS group at six wk was significantly lower than for the QoL index at baseline for the same group. The authors concluded that decreased QoL in the SEMS group at six wk, although not statistically significant, reflected the presence of pain following the intervention; the effect of pain on QoL may have significant implications for treatment with SEMS.
Sahlgrenska University Hospital (44) in their randomized controlled clinical trial published in 2005, compared endoluminal brachytherapy with endoscopic stent placement for newly diagnosed patients with advanced EC or gastroesophageal junction cancer, with a primary outcome being the detailed evaluation of HRQoL. Sixty-five patients eligible for the study were enrolled; 34 were randomized to stent treatment and 31 to brachytherapy. The authors assessed dysphagia improvement as a part of disease-specific HRQoL questionnaire EORTC OES-23 and found a statistically significant improvement in dysphagia grade, ability to swallow saliva, choking and coughing compared to baseline scores. There was no improvement in these outcomes for patients treated with brachytherapy. In an interim inter-group analysis at one mo a significant improvement in dysphagia scale favored the SEMS group. At three mo, some of the dysphagia-related parameters continued to show clinical improvement in the SEMS group but these did not achieve statistical significance. In the brachytherapy group, clinically significant improvements were noted in some of the parameters related to dysphagia at three mo and these were maintained at six mo. However, these data did not achieve statistical significance. General health QoL was measured using the EORTC QLQ-30 scale. In the stent group all functional scales and single symptom scales deteriorated compared to mean scores at inclusion. The largest deterioration was found for social function, followed by pain, role function and insomnia. In the brachytherapy group, a clinically relevant deterioration was found for most variables on the function and single symptom scales with physical function, global QoL and pain scales reaching statistical significance.
Madhusudhan et al. (45) in their prospective study assessed the QoL using EORTC QLQ-C30 (version 3) and EORTC QLQ-OES 18 questionnaires before stenting, and at one, four and eight wk following placement of the stent. The results showed significant improvement following stenting. The general health scale and function scores increased significantly. Most symptom scores, except pain, showed improvement. The pain score deteriorated at one wk, as initial expansion of SEMS following its placement led to an increase in pain sensation. Over a period of two mo, the pain scores decreased to baseline values. The financial strain scores also showed a significant improvement. The studies did not specifically address the influence of stents on patient QoL; although anecdotally we have extrapolated that improved swallowing will result in improved QoL. Improvement of dysphagia is likely a result of stent placement along with decreased tumor burden from neoadjuvant therapy. A generous decrease in the dysphagia scores SDM –0.81 was observed in our investigation.
Other applications of stent implantation in perioperative and postoperative care of the carcinoma of the esophagus
Removable self-expanding silicone stents have previously demonstrated utility for relieving dysphagia from benign strictures and from both resectable and unresectable malignant disease (27,46-49). University Medical Centre Utrecht (50) performed a pooled analysis regarding placement of fully covered and partially covered SEMS (FSEMS and PSEMS) and SEPS for treating benign esophageal ruptures and anastomotic leaks. Twenty-five studies, including 267 patients with complete follow-up on outcome, were identified. Clinical success was achieved in 85% of patients and was not different between stent types (SEPS 84%, FSEMS 85% and PSEMS 86%, P=0.97). Time of stent placement was longest for SEPS (eight weeks) followed by FSEMS and PSEMS (both six weeks). In total, 65 (34%) patients had a stent-related complication. Stent migration occurred more often with SEPS [n=47 (31%)] and FSEMS [n=7 (26%)] than with PSEMS [n=2 (12%), P≤0.001], whereas there was no significant difference in tissue in- and overgrowth between PSEMS [12% vs. 7% (FSEMS) and 3% (SEPS), P=0.68].
Martin et al. (51) compared early esophageal stenting vs. repeated dilation in esophagectomy strictures. The median number of dilatations were 2 (range, 1 to 3) for the 18 stent patients, with all stents placed for three months’ duration, and 4 dilations (range, 2 to 12 dilations) in 24 patients treated solely with dilatation. An evaluation of median, high and low total charges, net revenue, and direct margin demonstrated that the use of a removable stent after one failed dilation was more cost-efficient than repeated dilations.
In conclusion, self-expanding stents are a safe and effective method for endoscopic improvement of dysphagia in patients with malignant esophageal strictures receiving neoadjuvant therapy. The stents represent a new, alternative and cost-effective therapy for maintaining adequate oral nutrition. The QoL benefits gained by restoring the patient’s ability to eat and enjoy food is admirable.
Acknowledgements
Guarantor of the article: Guy D. Eslick. Specific author contributions: study concept and design: Vinayak Nagaraja, Michael R. Cox, Guy D. Eslick; acquisition of data: Vinayak Nagaraja; analysis and interpretation of data: Vinayak Nagaraja, Michael R. Cox, Guy D. Eslick; drafting of the manuscript: Vinayak Nagaraja; critical revision of the manuscript for important intellectual content: Vinayak Nagaraja, Michael R. Cox, Guy D. Eslick; statistical analysis: Vinayak Nagaraja, Guy D. Eslick; study supervision: Michael R. Cox, Guy D. Eslick.
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300 [DOI] [PubMed] [Google Scholar]
- 2.Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001;92:549-55 [DOI] [PubMed] [Google Scholar]
- 3.Thompson SK, Ruszkiewicz AR, Jamieson GG, et al. Improving the accuracy of TNM staging in esophageal cancer: a pathological review of resected specimens. Ann Surg Oncol 2008;15:3447-58 [DOI] [PubMed] [Google Scholar]
- 4.Larrea J, Vega S, Martinez T, et al. The nutritional status and immunological situation of cancer patients. Nutricion hospitalaria: organo oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral 1992;7:178-84. [PubMed]
- 5.Han-Geurts IJ, Hop WC, Tran TC, et al. Nutritional status as a risk factor in esophageal surgery. Dig Surg 2006;23:159-63 [DOI] [PubMed] [Google Scholar]
- 6.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7 [DOI] [PubMed] [Google Scholar]
- 7.Jatoi A, Martenson JA, Foster NR, et al. Paclitaxel, carboplatin, 5-fluorouracil, and radiation for locally advanced esophageal cancer: phase II results of preliminary pharmacologic and molecular efforts to mitigate toxicity and predict outcomes: North Central Cancer Treatment Group (N0044). Am J Clin Oncol 2007;30:507-13 [DOI] [PubMed] [Google Scholar]
- 8.Daly JM, Weintraub FN, Shou J, et al. Enteral nutrition during multimodality therapy in upper gastrointestinal cancer patients. Ann Surg 1995;221:327-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Windsor A, Braga M, Martindale R, et al. Fit for surgery: an expert panel review on optmising patients prior to surgery, with a particular focus on nutrition. The surgeon: journal of the Royal Colleges of Surgeons of Edinburgh and Ireland 2004;2:315-9. [DOI] [PubMed]
- 10.Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg 1999;134:36-42 [DOI] [PubMed] [Google Scholar]
- 11.O’Gorman RB, Feliciano DV, Matthews KS, et al. Correlation of immunologic and nutritional status with infectious complications after major abdominal trauma. Surgery 1986;99:549-56 [PubMed] [Google Scholar]
- 12.Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg 2006;132:549-55 [DOI] [PubMed] [Google Scholar]
- 13.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2003;185:538-43 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Journal of clinical epidemiology 2009;62:1006-12 [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N.Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orwin R.A fail-safe N for effect size in meta-analysis. Journal of educational statistics 1983;8:157-9 [Google Scholar]
- 19.Siddiqui AA, Glynn C, Loren D, et al. Self-expanding plastic esophageal stents versus jejunostomy tubes for the maintenance of nutrition during neoadjuvant chemoradiation therapy in patients with esophageal cancer: a retrospective study. Dis Esophagus 2009;22:216-22 [DOI] [PubMed] [Google Scholar]
- 20.Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614-9 [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui AA, Loren D, Dudnick R, et al. Expandable polyester silicon-covered stent for malignant esophageal strictures before neoadjuvant chemoradiation: a pilot study. Dig Dis Sci 2007;52:823-9 [DOI] [PubMed] [Google Scholar]
- 22.Bower M, Jones W, Vessels B, et al. Nutritional support with endoluminal stenting during neoadjuvant therapy for esophageal malignancy. Ann Surg Oncol 2009;16:3161-8 [DOI] [PubMed] [Google Scholar]
- 23.Langer FB, Schoppmann SF, Prager G, et al. Temporary placement of self-expanding oesophageal stents as bridging for neo-adjuvant therapy. Ann Surg Oncol 2010;17:470-5 [DOI] [PubMed] [Google Scholar]
- 24.Pellen MG, Sabri S, Razack A, et al. Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer. Dis Esophagus 2012;25:48-53 [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44-51 [DOI] [PubMed] [Google Scholar]
- 26.Lopes TL, Eloubeidi MA. A pilot study of fully covered self-expandable metal stents prior to neoadjuvant therapy for locally advanced esophageal cancer. Dis Esophagus 2010;23:309-15 [DOI] [PubMed] [Google Scholar]
- 27.Martin R, Duvall R, Ellis S, et al. The use of self-expanding silicone stents in esophageal cancer care: optimal pre-, peri-, and postoperative care. Surgical endoscopy 2009;23:615-21 [DOI] [PubMed] [Google Scholar]
- 28.Siersema PD, van Hillegersberg R. Treatment of locally advanced esophageal cancer with surgery and chemoradiation. Current Opinion in Gastroenterology 2008;24:535-40 [DOI] [PubMed] [Google Scholar]
- 29.Koshy M, Esiashvilli N, Landry JC, et al. Multiple management modalities in esophageal cancer: combined modality management approaches. Oncologist 2004;9:147-59 [DOI] [PubMed] [Google Scholar]
- 30.Walsh TN, Grennell M, Mansoor S, et al. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival*. Diseases of the Esophagus 2002;15:121-4 [DOI] [PubMed] [Google Scholar]
- 31.Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 2007;102:2557-63 [DOI] [PubMed] [Google Scholar]
- 32.Braga M, Gianotti L, Gentilini O, et al. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Critical care medicine 2001;29:242-8 [DOI] [PubMed] [Google Scholar]
- 33.Heys SD, Walker LG, Smith I, et al. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg 1999;229:467-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudsk KA, Minard G, Croce MA, et al. A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg 1996;224:531-40; discussion 40-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 1992;216:172-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozzetti F, Braga M, Gianotti L, et al. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet 2001;358:1487-92 [DOI] [PubMed] [Google Scholar]
- 37.Jenkinson AD, Lim J, Agrawal N, et al. Laparoscopic feeding jejunostomy in esophagogastric cancer. Surg Endosc 2007;21:299-302 [DOI] [PubMed] [Google Scholar]
- 38.Margolis M, Alexander P, Trachiotis GD, et al. Percutaneous endoscopic gastrostomy before multimodality therapy in patients with esophageal cancer. Ann Thorac Surg 2003;76:1694-7; discussion 7-8. [DOI] [PubMed]
- 39.Ohnmacht GA, Allen MS, Cassivi SD, et al. Percutaneous endoscopic gastrostomy risks rendering the gastric conduit unusable for esophagectomy. Dis Esophagus 2006;19:311-2 [DOI] [PubMed] [Google Scholar]
- 40.Date RS, Clements WD, Gilliland R. Feeding jejunostomy: is there enough evidence to justify its routine use? Dig Surg 2004;21:142-5 [DOI] [PubMed] [Google Scholar]
- 41.Han-Geurts IJM, Hop WC, Verhoef C, et al. Randomized clinical trial comparing feeding jejunostomy with nasoduodenal tube placement in patients undergoing oesophagectomy. British Journal of Surgery 2007;94:31-5 [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell CA, Fullarton GM, Watt E, et al. Randomized clinical trial comparing self-expanding metallic stents with plastic endoprostheses in the palliation of oesophageal cancer. The British journal of surgery 2002;89:985-92 [DOI] [PubMed] [Google Scholar]
- 43.Shenfine J, McNamee P, Steen N, et al. A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer. Health Technol Assess 2005;9:iii, 1-121 [DOI] [PubMed] [Google Scholar]
- 44.Bergquist H, Wenger U, Johnsson E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis Esophagus 2005;18:131-9 [DOI] [PubMed] [Google Scholar]
- 45.Madhusudhan C, Saluja SS, Pal S, et al. Palliative stenting for relief of dysphagia in patients with inoperable esophageal cancer: impact on quality of life. Dis Esophagus 2009;22:331-6 [DOI] [PubMed] [Google Scholar]
- 46.Thompson AM, Rapson T, Gilbert FJ, et al. Endoscopic palliative treatment for esophageal and gastric cancer: techniques, complications, and survival in a population-based cohort of 948 patients. Surg Endosc 2004;18:1257-62 [DOI] [PubMed] [Google Scholar]
- 47.Karbowski M, Schembre D, Kozarek R, et al. Polyflex self-expanding, removable plastic stents: assessment of treatment efficacy and safety in a variety of benign and malignant conditions of the esophagus. Surgical endoscopy 2008;22:1326-33 [DOI] [PubMed] [Google Scholar]
- 48.Costamagna G, Shah SK, Tringali A, et al. Prospective evaluation of a new self-expanding plastic stent for inoperable esophageal strictures. Surgical endoscopy 2003;17:891-5 [DOI] [PubMed] [Google Scholar]
- 49.Holm AN, de la Mora Levy JG, Gostout CJ, et al. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointestinal endoscopy 2008;67:20-5 [DOI] [PubMed] [Google Scholar]
- 50.van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Alimentary pharmacology & therapeutics 2011;33:1292-301 [DOI] [PubMed] [Google Scholar]
- 51.Martin RC, Woodall C, Duvall R, et al. The use of self-expanding silicone stents in esophagectomy strictures: less cost and more efficiency. Ann Thorac Surg 2008;86:436-40 [DOI] [PubMed] [Google Scholar]


