Abstract
Objective
To investigate the relationship between plasma levels of small dense low-density lipoprotein cholesterol (sdLDL-C) and risk for incident coronary heart disease (CHD) in a prospective study among Atherosclerosis Risk in Communities (ARIC) study participants.
Approach and Results
Plasma sdLDL-C was measured in 11,419 men and women of the biracial ARIC study using a newly developed homogeneous assay. A proportional hazards model was used to examine the relationship between sdLDL-C, vascular risk factors, and risk for CHD events (n=1,158) over a period of ≈11 years. Plasma sdLDL-C levels were strongly correlated with an atherogenic lipid profile and were higher in diabetics than nondiabetics (49.6 vs. 42.3 mg/dL, p<0.0001). In a model that included established risk factors, sdLDL-C was associated with incident CHD with a hazard ratio (HR) of 1.51 (95%CI: 1.21–1.88) for the highest versus the lowest quartile, respectively. Even in individuals considered to be at low cardiovascular risk based on their LDL-C levels, sdLDL-C predicted risk for incident CHD (HR 1.61; 95% CI 1.04–2.49). Genome-wide association analyses identified genetic variants in 8 loci associated with sdLDL-C levels. These loci were in or close to genes previously associated with risk for CHD. We discovered 1 novel locus, PCSK7, for which genetic variation was significantly associated with sdLDL-C and other lipid factors.
Conclusions
sdLDL-C was associated with incident CHD in ARIC study participants. The novel association of genetic variants in PCSK7 with sdLDL-C and other lipid traits may provide new insights into the role of this gene in lipid metabolism.
Keywords: small dense LDL, incident CHD, GWAS, ARIC, triglyceride
Low-density lipoprotein cholesterol (LDL-C) is considered one of the most important risk factors for cardiovascular disease and remains the primary target for current cardiovascular risk reduction strategies.1,2 However many individuals with LDL-C within the normal range still develop cardiovascular disease.3,4 LDL particles are a heterogeneous population,5 and it has long been hypothesized that a subfraction of LDL, particularly small dense LDL (sdLDL), possesses increased atherogenic potential and thus contributes to this observation. A number of mechanisms have been proposed to explain the enhanced atherogenicity of sdLDL,6–10 including 1) a lower affinity for the LDL receptor, 2) facilitated entry into the arterial wall, 3) greater arterial retention due to increased binding to proteoglycans, and 4) greater susceptibility to oxidation. Since sdLDL particles are smaller and contain less cholesterol, increased levels of sdLDL also represent an increased number of atherogenic particles, which may not be reflected by the levels of LDL-C.
Two of the earliest and most widely used methods for LDL classification involved density and size determinations based upon ultracentrifugal and/or nondenaturing gradient density gel electrophoresis procedures. These resulted in the division of LDL particles somewhat arbitrarily into two categories for clinical assessment: sdLDL and large buoyant LDL (lbLDL). These also led to the development of a two phenotype classification system, with phenotype A (or pattern A) characterized as individuals with a predominance of lbLDL particles and phenotype B (or pattern B) as individuals with a predominance of sdLDL particles.11 This classification schema has been widely used, and pattern B has been recognized as a risk marker for cardiovascular disease. More recently, the efficacy of nuclear magnetic resonance methodology to determine both particle numbers and sizes of various lipoprotein fractions, including LDL, has been demonstrated.12
The distribution of LDL subfractions is determined by both genetic and environmental factors.13,14 Furthermore, the concentration of sdLDL is highly correlated with triglyceride level and is increased in individuals with diabetes or the metabolic syndrome.15 Therefore, it is plausible that genetic variants that affect circulating levels of sdLDL-C may also influence other lipid traits (e.g. triglycerides and HDL-C) and may aid in the identification of genes involved in the causal pathway linking atherogenic dyslipidemia characterized by sdLDL-C and CHD.
sdLDL has been found to be associated with increased risk for cardiovascular disease in cross-sectional studies16–18 as well as prospective observational studies.19–21 However, in most of these studies, sdLDL did not remain an independent risk predictor when adjusted for other lipid risk factor traits. Until recently, the methods available for the measurement of sdLDL were generally limited to nonquantitative, laborious, and/or highly complex techniques and therefore not readily adaptable to a large number of samples in a routine clinical laboratory environment. Recently, Ito and coworkers developed a simple homogeneous assay adaptable to autoanalyzers for the quantification of sdLDL-C.22 Thus far, few epidemiological studies have examined whether the cholesterol content of sdLDL can predict future cardiovascular events. In the present study, we measured sdLDL-C in 11,419 men and women of the Atherosclerosis Risk in Communities (ARIC) study. These participants were followed over a period of 11 years during which the incidence of coronary heart disease (CHD) was measured. The purpose of this study was to evaluate whether sdLDL-C is a better predictor of risk for CHD than LDL-C and other traditional or nontraditional cardiovascular risk factors. To better understand the genetic determinants of sdLDL-C and lbLDL-C, we investigated the association of both sdLDL-C and lbLDL-C levels with genetic markers spanning the genome.
Materials and methods
Materials and Methods are available in the online-only Data Supplement.
Results
Baseline Characteristics
Key baseline (visit 4) demographics of the 11,419 ARIC participants are described in Table 1. The mean age of the study cohort was 62.8 years; 78% of study participants were Caucasian, and 56% were female. Of the study participants, 58% had a history of smoking cigarettes; 16.9% had diabetes mellitus, and 44.6% were classified with metabolic syndrome according to Adult Treatment Panel III criteria.23 Prevalent CHD was present in 972 individuals at the baseline visit, and these individuals were excluded from analyses for incident events. In the remaining 10,225 individuals (excluding 222 who had missing data on incident CHD), incident CHD developed in 1158 participants over an average of 11 years of follow-up. The mean plasma sdLDL-C level was 43.5 mg/dL, and the mean sdLDL-C/LDL-C ratio was 0.35. Supplemental Table I (available online at http://atvb.ahajournals.org) shows the race- and gender-specific plasma lipid levels. Mean sdLDL-C levels were higher in Caucasians than African Americans and in men than women, whereas LDL-C was slightly higher in women than men but was not different between races. The proportion of LDL-C which was sdLDL-C was higher in Caucasians than African Americans and in men than women. Total cholesterol and high-density lipoprotein cholesterol (HDL-C) levels were higher in women than men, while triglyceride levels were higher in Caucasians than African Americans.
Table 1.
Description of 11,419 ARIC participants at baseline (ARIC visit 4)
| Characteristic | N=11,419 |
|---|---|
| Age, years | 62.83±5.67 |
| Race | |
| African American | 2,515 (22) |
| Caucasian | 8,904 (78) |
| Sex | |
| Female | 6,385 (56) |
| Male | 5,034 (44) |
| Ever smoked cigarettes | 6,635 (58) |
| Ever drank alcohol | 8,989 (79) |
| Statin use | 1,308 (11.5) |
| Prevalent CHD | 972 (8.7) |
| Diabetes mellitus | 1943 (16.9) |
| Metabolic syndrome | 5127 (44.6) |
| sdLDL-C, mg/dL | 43.48±20.76 |
| lbLDL-C, mg/dL | 79.22±28.52 |
| sdLDL-C/LDL-C | 0.35±0.1496 |
Data are presented as mean±SD or n (%). CHD: coronary heart disease; lbLDL-C: large buoyant low-density lipoprotein cholesterol; sdLDL-C: small dense low-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
Association of sdLDL-C with Cardiovascular Risk Factors
Table 2 shows the means or proportions of baseline traditional risk factors and other characteristics by sdLDL-C quartiles. Generally, individuals with sdLDL-C levels in the highest quartile had proatherogenic lipid profiles, were more likely to have diabetes, hypertension, and metabolic syndrome, and had higher body mass index (BMI) and plasma high-sensitivity C-reactive protein (hs-CRP) levels. Statin use was higher in those individuals with sdLDL-C levels in the 3rd and 4th quartile. sdLDL-C levels were not associated with smoking status.
Table 2.
Adjusted means±SE or proportions±SE of cardiovascular risk factors by quartiles of sdLDL-C and p-value for trend across quartiles, adjusted for age, race, and gender.*
| Characteristic | Q1 | Q2 | Q3 | Q4 | P-trend |
|---|---|---|---|---|---|
| Age, years* | 62.77±0.106 | 62.95±0.106 | 62.80±0.106 | 62.79±0.106 | 0.8500 |
| Male, %* | 0.40±0.038 | 0.42±0.038 | 0.49±0.038 | 0.45±0.038 | <.0001 |
| African American, %* | 0.30±0.041 | 0.23±0.044 | 0.19±0.048 | 0.14±0.055 | <.0001 |
| Total cholesterol, mg/dL | 172.08±0.557 | 194.61±0.556 | 207.09±0.558 | 229.94±0.559 | <.0001 |
| Triglycerides, mg/dL | 91.70±1.370 | 116.20±1.368 | 154.69±1.373 | 213.49±1.374 | <.0001 |
| LDL-C, mg/dL | 97.55±0.537 | 119.30±0.537 | 129.36±0.542 | 145.64±0.551 | <.0001 |
| HDL-C, mg/dL | 56.16±0.271 | 52.37±0.271 | 47.85±0.272 | 43.47±0.272 | <.0001 |
| BMI, kg/m2 | 27.80±0.103 | 28.46±0.103 | 29.20±0.103 | 29.79±0.103 | <.0001 |
| Smoking, % | 59±4.0 | 62±4.0 | 57±3.9 | 58±4.0 | 0.1125 |
| Diabetes, % | 11.0±5.8 | 13.0±5.6 | 17.0±5.1 | 23.0±4.6 | <.0001 |
| Hypertensive, % | 40.0±4.0 | 46.0±3.9 | 51.0±3.9 | 54.0±3.9 | <.0001 |
| Statin use, % | 9.0±6.7 | 11.0±6.0 | 12.0±5.8 | 12.0±5.7 | <.0001 |
| Metabolic syndrome, % | 23.0±4.5 | 33.0±4.0 | 50.0±3.8 | 73.0±4.3 | <.0001 |
| log hs-CRP | 0.75±0.020 | 0.84±0.020 | 0.94±0.020 | 1.05±0.021 | <.0001 |
BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; hs-CRP: high-sensitivity C-reactive protein; LDL-C: low-density lipoprotein cholesterol.
The correlation between sdLDL-C levels and various traditional and nontraditional cardiovascular risk factors are shown in Table 3. Strong positive correlations with sdLDL-C (|r|>0.50) were found for the lipid risk factors non-HDL-C, apolipoprotein (apo) B, LDL-C, total cholesterol, and log triglycerides. Circulating levels of lipoprotein-associated phospholipase A2 (Lp-PLA2) activity and lactate showed moderate positive correlations and HDL-C showed a moderate negative correlation with sdLDL-C (0.25<|r|<0.50). Weaker correlations with sdLDL-C were found for fasting plasma glucose, apo AI, lbLDL-C, and log hs-CRP. High-sensitivity cardiac troponin T was not significantly correlated with sdLDL-C.
Table 3.
Correlation of sdLDL-C with traditional and novel cardiovascular risk factors
| Risk Factor | n | Pearson R | P-value |
|---|---|---|---|
| Non-HDL-C | 11,419 | 0.721 | <.0001 |
| ApoB | 10,720 | 0.706 | <.0001 |
| Log triglycerides | 11,419 | 0.641 | <.0001 |
| Total cholesterol | 11,419 | 0.608 | <.0001 |
| LDL-C | 11,234 | 0.543 | <.0001 |
| Lp-PLA2 activity | 11,108 | 0.319 | <.0001 |
| HDL-C | 11,419 | −0.291 | <.0001 |
| Lactate | 11,417 | 0.253 | <.0001 |
| Fasting plasma glucose | 10,902 | 0.169 | <.0001 |
| ApoAI | 10,720 | −0.086 | <.0001 |
| lbLDL-C | 11,234 | −0.076 | <.0001 |
| Log hs-CRP | 11,202 | 0.072 | <.0001 |
| hs-cTroponin T | 11,130 | −0.013 | 0.1549 |
apo: apolipoprotein; HDL-C: high-density lipoprotein cholesterol; hs-CRP: high-sensitivity C-reactive protein; hs-cTroponin T: high-sensitivity cardiac troponin T; lbLDL-C: large buoyant low-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Lp-PLA2 lipoprotein-associated phospholipase A2.
sdLDL-C Levels and Incident CHD Events
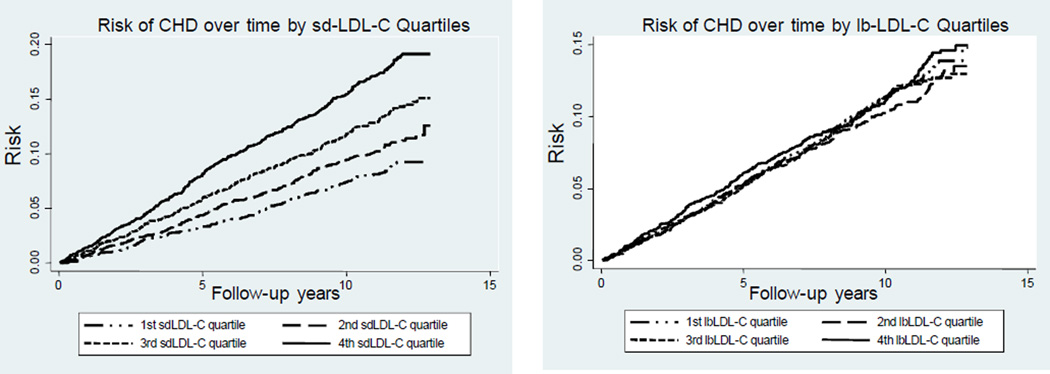
The cumulative incidence curves for CHD risk by sdLDL-C and lbLDL-C quartiles, adjusted by age, race, and gender, are shown in Figure 1. The incidence of CHD events increased proportionately over the follow-up years for participants in each consecutive quartile of sdLDL-C. In contrast, lbLDL-C did not exhibit a concentration-dependent relationship with future incident CHD events. sdLDL-C and LDL-C levels were moderately correlated (Supplemental Figure IA; r=0.54; available online at http://atvb.ahajournals.org) but often discordant. Supplemental Figure IB (available online at http://atvb.ahajournals.org) displays the prevalence and magnitude of this discordance. We examined concordant and discordant subgroups separately using a similar analysis approach as previously described by Otvos et al.24 We defined discordance as a difference of >24 percentile units (points outside the dashed lines in Supplemental Figure IB; available online at http://atvb.ahajournals.org). Supplemental Figure II (available online at http://atvb.ahajournals.org) shows the cumulative incidence curves for CHD risk for the subgroup with sdLDL-C>LDL-C discordance compared to the concordant and the discordant sdLDL-C<LDL-C subgroups. The sdLDL-C>LDL-C discordant subgroup showed the highest CHD risk compared to the concordant and discordant sdLDL-C<LDL-C subgroups.
Figure 1.
Cumulative incidence curves for risk of CHD by sdLDL-C and lbLDL-C quartiles, adjusted for age, race, and gender.
We used proportional hazards regression analyses to investigate the association of incident CHD with baseline levels of sdLDL-C and LDL-C modeled in quartiles, using quartile 1 as the referent group (Table 4). In the basic model adjusted for age, race, and gender (model 1), individuals with sdLDL-C levels in the highest quartile had a 2-fold higher risk for incident CHD compared to those in the lowest quartile (hazard ratio [HR] 2.00; 95% confidence interval [CI] 1.69–2.37). After additional adjustment for smoking, BMI, hypertension, HDL-C, log triglycerides, lipid-lowering medications, diabetes, diabetes medications, and log hs-CRP (model 2), risk for incident CHD was attenuated but remained significant (HR 1.51; 95% CI 1.21–1.88). sdLDL-C was not significantly associated with risk for incident CHD following further adjustment for other lipid risk factors, such as LDL-C, apo B, and total cholesterol. This may be due in part to over adjustment of the multivariable model and is not surprising given the strong correlations of sdLDL-C with these lipid risk factors. In comparison, individuals with LDL-C levels in the highest quartile had a 56% and 68% higher risk for incident CHD (HR 1.56; 95% CI 1.32–1.83 and HR 1.68; 95% CI 1.42–1.99) in the basic model (model 1) and more fully adjusted model (model 2), respectively.
Table 4.
Hazard ratio (95% confidence interval) for incident CHD by sdLDL-C and LDL-C quartiles
| Quartile of sdLDL-C* |
||||
|---|---|---|---|---|
| 2 | 3 | 4 | P† | |
| Model 1‡ | 1.19 (0.99–1.43) | 1.44 (1.21–1.72) | 2.00 (1.69–2.37) | <0.0001 |
| Model 2§ | 1.10 (0.90–1.33) | 1.21 (0.99–1.48) | 1.51 (1.21–1.88) | 0.0008 |
|
Quartile of LDL-C* |
||||
| 2 | 3 | 4 | P† | |
| Model 1‡ | 1.01 (0.85–1.21) | 1.15 (0.97–1.37) | 1.56 (1.32–1.83) | <0.0001 |
| Model 2§ | 1.08 (0.90–1.30) | 1.23 (1.03–1.47) | 1.68 (1.42–1.99) | <0.0001 |
sdLDL-C: small dense low-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
Lowest quartile (1) is reference.
P-values (Pr > chiSq) for linear hypothesis testing results of sdLDL-C quartiles.
Adjusted for age, sex, and race.
Adjusted for model 1 variables + smoking, body mass index, hypertension, high-density lipoprotein cholesterol, log(triglycerides), lipid-lowering medications, diabetes, diabetes medications, and log(hs-CRP).
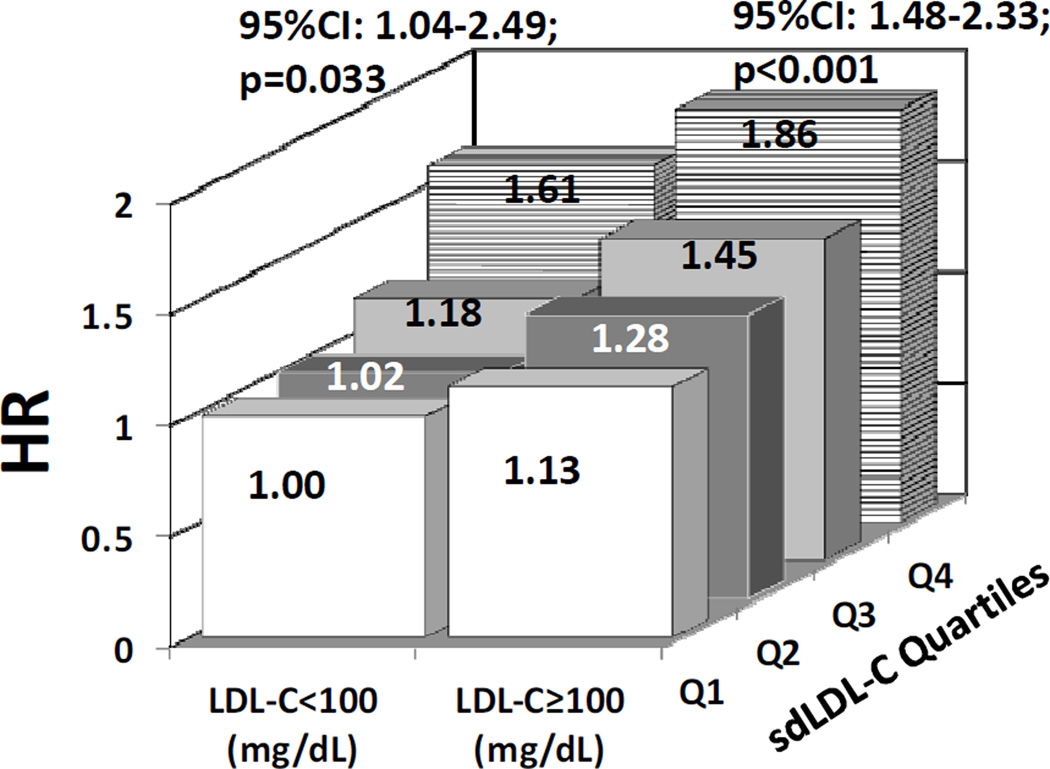
We further investigated the association of sdLDL-C with risk for incident CHD in participants stratified by LDL-C risk categories (i.e., LDL-C <100 mg/dL and LDL-C ≥100 mg/dL). In these analyses, we used a multivariable model (adjusting for age, gender, race, ever smoking, BMI, hypertension, diabetes, diabetes medication, and log hs-CRP), with quartile 1 for sdLDL-C in the LDL-C <100 mg/dL risk category as the referent group. Even in individuals with LDL-C levels <100 mg/dL, sdLDL-C was predictive of CHD risk across increasing sdLDL-C quartiles (Figure 2). Participants with LDL-C <100 mg/dL and sdLDL-C levels in the 4th quartile had a 61% increase in risk for incident CHD (HR 1.61; 95% CI 1.04–2.49) compared with individuals with sdLDL-C levels in the 1st quartile. In comparison, participants with LDL-C ≥100 mg/dL and sdLDL-C levels in the 4th quartile had an 86% increase in risk for incident CHD (HR 1.86; 95% CI 1.48–2.33) compared with the same referent group.
Figure 2.
Adjusted hazard ratios for incident CHD by sdLDL-C quartiles stratified by LDL-C risk categories, adjusted for age, sex, and race, smoking, BMI, hypertension, diabetes, diabetes medications, and log hs-CRP.
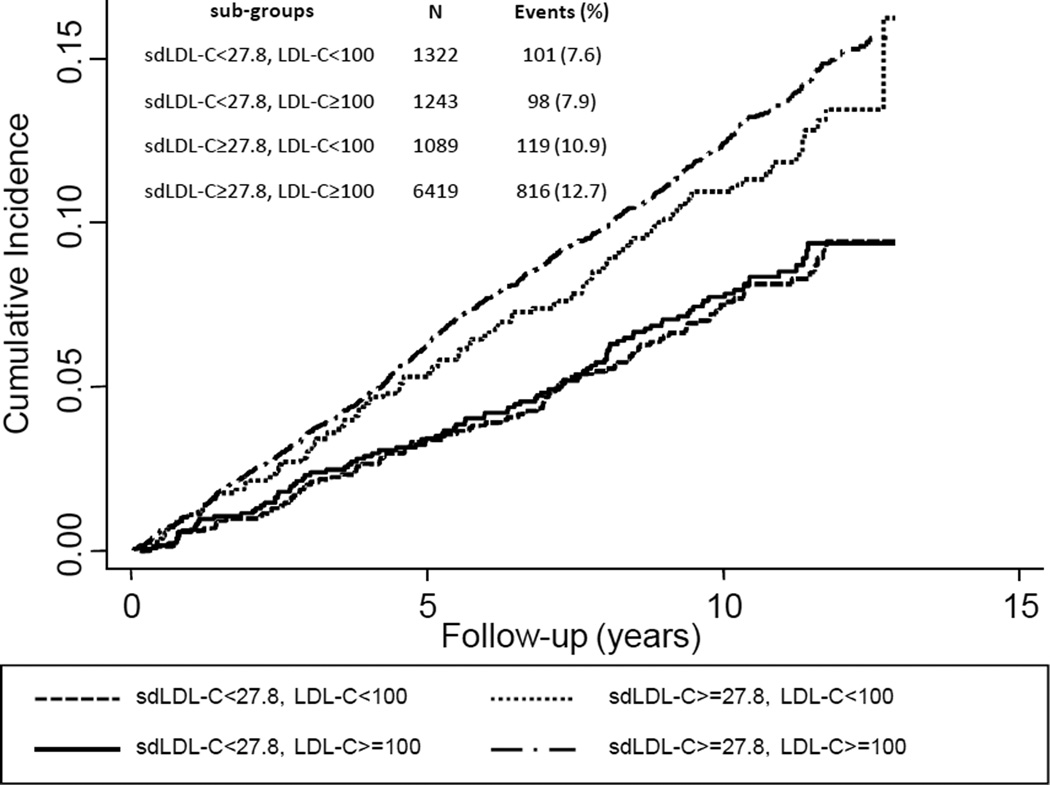
In additional analyses, we examined the effects on CHD risk of sdLDL-C discordance among ARIC participants with low LDL-C (<100 mg/dL; <25th percentile) or equivalently low sdLDL-C (<27.8 mg/dL; <25th percentile) (Figure 3). The cumulative incidence of CHD events was higher among individuals with low LDL-C but discordantly higher sdLDL-C (10.9%) compared to individuals with low sdLDL-C but discordantly higher LDL-C (7.9%). Not surprisingly, the cumulative incidence of CHD events was highest among individuals with concordantly higher levels of LDL-C and sdLDL-C (12.7%) and lowest among individuals with concordantly lower levels of LDL-C and sdLDL-C (7.6%).
Figure 3.
Cumulative incidence of cardiovascular events in sub-groups with LDL-C <100 mg/dL (<25th percentile) and/or sdLDL-C <27.8 mg/dL (<25th percentile), from proportional hazards models adjusted for age, gender, and race.
Genome-Wide Association Study of sdLDL-C
We performed genome-wide association study (GWAS) analyses of sdLDL-C and Table 5 summarizes our primary findings. In total, 127 single-nucleotide polymorphisms (SNPs) were significantly associated with sdLDL-C (p<5×10−8). These SNPs clustered at 8 different loci on chromosomes 1, 2, 7, 8, 11, and 19 and were located within 14 different genes (or gene clusters). With the exception of PCSK7, genetic variants within all of these genes have previously been found to be related to pathways involved in lipid metabolism and vascular inflammation (www.genome.gov).
Table 5.
Association of the top SNPs with sdLDL-C
| sdLDL-C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Location | Chromosome | # Sign SNPs |
Coded Allele |
Allele Frequency |
N | Beta | SEBeta | P | Gene |
| rs964184 | 11q23.3 | 11 | 12 | C | 0.8588 | 6979 | −5.70008 | 0.511384 | 7.46×10−29 | APOA5/A4/C3/A1 |
| rs4420638 | 19q13.32 | 19 | 6 | A | 0.8264 | 6979 | −5.45295 | 0.490271 | 9.77×10−29 | APOE/C1/C4/C2 |
| rs2075650 | 19q13.32 | 19 | 3 | A | 0.8611 | 6979 | −7.15912 | 0.748784 | 1.17×10−21 | TOMM40 |
| rs660240 | 1p13.3 | 1 | 10 | C | 0.7873 | 6979 | 3.45306 | 0.432418 | 1.40×10−15 | PSRC1/CELSR2/SORT1 |
| rs6589564 | 11q23.3 | 11 | 18 | C | 0.0723 | 6979 | 5.53037 | 0.700672 | 2.95×10−15 | BUD13 |
| rs2075290 | 11q23.3 | 11 | 5 | C | 0.0716 | 6979 | 5.48955 | 0.701319 | 4.98×10−15 | ZNF259 |
| rs2980853 | 8q24.13 | 8 | 38 | A | 0.5315 | 6979 | 2.63962 | 0.353669 | 8.42×10−14 | TRIB1 |
| rs1260326 | 2p23.3 | 2 | 3 | C | 0.5856 | 6979 | −2.60009 | 0.366214 | 1.25×10−12 | GCKR |
| rs7254892 | 19q13.32 | 19 | 1 | A | 0.0534 | 6979 | −10.9757 | 1.78507 | 7.82×10−10 | PVRL2 |
| rs562338 | 2p23–2p24 | 2 | 24 | A | 0.1811 | 6979 | −2.65678 | 0.464554 | 1.07×10−08 | APOB |
| rs508487 | 11q23-q24 | 11 | 1 | C | 0.9402 | 6979 | −4.95643 | 0.868883 | 1.17×10−08 | PCSK7 |
| rs4803760 | 19p13.2 | 19 | 1 | C | 0.7692 | 6979 | 2.73614 | 0.49648 | 3.57×10−08 | BCAM |
| rs1178977 | 7q11.23 | 7 | 3 | A | 0.8000 | 6979 | 2.55226 | 0.466442 | 4.46×10−08 | MLXIPL |
| rs6976930 | 7q11.23 | 7 | 2 | A | 0.2001 | 6979 | −2.54050 | 0.464764 | 4.60×10−08 | BAZ1B |
Association of PCSK7 SNP rs508487 Genotype with Circulating Lipids and CHD
A novel finding from the current GWAS analysis was the significant association of sdLDL-C with SNP rs508487 (at locus 11q23-q24) located in the PCSK7 gene. We investigated the effect of rs508487 genotype on circulating lipid levels (Table 6). Each copy of the minor allele at this SNP raised sdLDL-C by ~4 mg/dL and triglycerides by ~20 mg/dL. In contrast, each copy of the minor allele lowered lbLDL-C by ~3 mg/dL; rs508487 genotype had no significant effect on circulating LDL-C levels. Interestingly, 2 copies of the minor allele increased HDL-C by ~5 mg/dL and total cholesterol by ~14 mg/dL.
Table 6.
Association of PCSK7 SNP rs508487 genotype with circulating lipids
| Genotype |
||||
|---|---|---|---|---|
| Lipid, mg/dL (mean±SE) | CC | CT | TT | P |
| sdLDL-C | 44.3±20.6 | 48.5±21.4 | 51.5±23.4 | <0.0001 |
| lbLDL-C | 78.7±27.5 | 75.5±29.7 | 72.7±23.6 | 0.02 |
| sdLDL-C/LDL-C | 0.36±0.15 | 0.40±0.16 | 0.41±0.13 | <0.0001 |
| LDL-C | 123.0±32.8 | 124.0±32.6 | 124.2±33.8 | 0.75 |
| HDL-C | 50.2±16.4 | 48.6±16.1 | 54.9±13.4 | 0.03 |
| Total cholesterol | 201.5±35.6 | 205.0±35.0 | 215.3±40.4 | 0.02 |
| Triglycerides | 141.5±67.5 | 162.1±77.5 | 181.4±88.5 | <0.0001 |
HDL-C: high-density lipoprotein cholesterol; lbLDL-C: large buoyant low-density lipoprotein cholesterol; sdLDL-C: small dense low-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
Given that the PCSK7 variant (rs508487) is located in the chromosome 11 region harboring the APOA5-APOA4-APOC3-APOA1 gene cluster, we investigated whether the novel association result with PCSK7 is due to linkage disequilibrium with previously reported variants in this cluster, especially previously reported functional variants in APOA5. One variant, rs662799 (APOA5 T-1131C) was in significant linkage disequilibrium with rs508487 (R2=0.53). We repeated the association analysis between PCSK7 rs508487 and sdLDL-C in 6,069 individuals homozygous for the wild type allele at APOA5 rs662799, and the results were attenuated a bit but still nominally statistically significant (p=0.0027).
We investigated the relationship between PCSK7 SNP rs508487 genotype and CHD in the ARIC study and did not observe a significant association. However, the power for this particular analysis was limited due to the fact that the number of ARIC CHD cases with one or two minor alleles at this SNP was low. Therefore, we examined the association of rs508487 with 40,260 CHD cases from the CARDIoGRAM study and found that rs508487 was significantly associated with CHD (Odds Ratio=1.13; 95% confidence interval, 1.06–1.21, P=0.00017).
We next analyzed rare variants on the Illumina exome chip designated as nonsynonymous, splicing, or stop gain in the PCSK7 gene for association with sdLDL-C. We found a total of 7 nonsynonymous PCSK7 variants among Caucasian ARIC participants, resulting in amino acid substitutions in the wild type PCSK7 protein. Since these variants had minor allele frequencies below 1%, we analyzed them collectively for their effect on sdLDL-C. Individuals who were carriers of any of the rare PCSK7 variants had a significant increase in circulating levels of sdLDL-C (~ 7.5 mg/dL; P=0.012) and triglycerides (ln(TG) ~0.145; P=0.043) compared to non-carriers (Table 7).
Table 7.
Change in circulating lipids among Caucasian carriers of rare nonsynonymous PCSK7 variants
| Lipid | Change (β) | SE | P |
|---|---|---|---|
| sdLDL-C (mg/dL) | 7.53 | 3.00 | 0.012 |
| Ln (triglycerides) | 0.144 | 0.071 | 0.043 |
| HDL-C (mg/dL) | −3.58 | 2.12 | 0.091 |
sdLDL-C: small dense low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol.
Discussion
In the current study, we investigated the relationship between plasma levels of sdLDL-C and risk of incident CHD in the predominantly biracial ARIC study cohort using a newly developed automated homogeneous sdLDL-C assay. Elevated plasma sdLDL-C levels were associated with increased risk of incident CHD in a multivariable model (hazard ratio (HR) 1.51; 95%CI: 1.21–1.88) and even in individuals considered to be at low cardiovascular risk based on their LDL-C levels, sd-LDL-C predicted risk for incident CHD (HR 1.61; 95% CI 1.04–2.49). Using genome-wide association (GWA) analyses, we discovered one novel locus, PCSK7, for which genetic variation was significantly associated with sdLDL-C levels and other lipid traits. Subsequent examination in the CARDIoGRAM Study showed a significant association of the PCSK7 SNP rs508487 with CHD.
sdLDL-C and Risk for Incident CHD
Among ARIC participants, the mean baseline plasma sdLDL-C level was 43.5 mg/dL, which represented on average 35% of the total LDL-C concentration. sdLDL-C and sdLDL-C/LDL-C ratio were higher in Caucasians than African -Americans and higher in men than women. Our findings related to circulating sdLDL-C levels seem in general agreement with a report from the Framingham Offspring Study, which showed that men had higher sdLDL-C levels and a higher percentage of LDL-C as sdLDL-C compared to women.25 Although we found higher mean sdLDL-C and sdLDL-C/LDL-C ratio overall than those reported in the Framingham Offspring Study, these differences may be in part due to differences in study populations (e.g., the ARIC cohort has a higher prevalence of obesity and metabolic syndrome than the Framingham Offspring Study) and sdLDL-C assay methodologies.
Plasma levels of sdLDL-C were adversely associated with cardiovascular lipid risk factors, a finding consistent with previous reports showing a correlation of sdLDL with an atherogenic lipid profile. We also found significant correlations between sdLDL-C and nonlipid risk factors, such as fasting glucose and lactate levels. Even though sdLDL-C was associated with diabetes and metabolic syndrome in previous studies, we report a remarkable increase in prevalence of metabolic syndrome among individuals with sdLDL-C levels in the highest quartile (73%) compared with those in the lowest quartile (23%). sdLDL-C was also correlated with inflammatory markers such as Lp-PLA2 activity and hs-CRP.
Over the 11-year follow-up period of this study, 1158 (11.3%) participants developed CHD. The cumulative incidence curves clearly illustrate the direct relation between sdLDL-C levels and CHD risk, whereas we did not find a similar relation between lbLDL-C and CHD. These results suggest that the sdLDL subfraction is a major contributor to the risk for incident CHD that is associated with LDL-C. Circulating levels of sdLDL-C were significantly associated with increased risk for CHD in a model adjusted for age, sex, and race and in a more fully adjusted model that also included smoking, BMI, hypertension, HDL-C, triglycerides, lipid-lowering medications, diabetes, diabetes medications, and hs-CRP. However, sdLDL-C was not an independent predictor of incident CHD when we further adjusted for other lipid risk factors, such as LDL-C, apo B, and total cholesterol, which is not surprising given the strong correlations of sdLDL-C with these other lipid risk factors, and our results are in agreement with previous studies reporting that sdLDL was not an independent predictor of cardiovascular disease.19–21 Interestingly, sdLDL-C showed predictive power for CHD risk even in individuals with optimal LDL-C levels as defined in the current guidelines (<100 mg/dL).23 Several investigators have emphasized that the number of particles as measured by nuclear magnetic resonance is more important for assessment of cardiovascular risk than LDL subclass, LDL particle size, or LDL-C concentration.26,27 Since sdLDL particles contain less cholesterol than lbLDL particles, there are more sdLDL particles than lbLDL particles at a given LDL-C concentration. Whether the total number of particles or the cholesterol payload per particle is more important to cardiovascular risk remains a topic of discussion. However, it is important to note that the particle number theory does not take into account the accumulating evidence pointing to different atherogenic properties of LDL subclasses. A limitation of this study is the fact that we did not have particle number information available and thus, we were not able to address this question specifically.
Genetics of sdLDL-C
GWAS analysis identified a large number of SNPs, clustered at 8 different loci on chromosomes 1, 2, 7, 8, 11 and 19 that were significantly associated with sdLDL-C. With the exception of PCSK7, genetic variants located in all the genes associated with sdLDL-C levels have been reported previously in GWAS studies of blood lipid levels.28 Our GWAS findings are in general agreement with an earlier report by Chasman and coworkers who employed a comprehensive GWAS analysis to identify largely similar loci that affect the nuclear magnetic resonance–based measures of concentration and size of LDL, HDL and VLDL in women.29 Although the study by Chasman et al did not find a significant association with LDL particle size or concentration at the PCSK7 locus, this apparent discrepancy may be due to notable differences between the two studies, such as differences in methodologies to measure lipoprotein fractions, genotyping methods and study populations. Indeed, a recent report from the Multi-Ethnic Study of Atherosclerosis (MESA) compared the identical automated assay of sdLDL-C as was used in our study to nuclear magnetic resonance-derived small LDL concentrations with regards to risk prediction for incident CHD.30 The authors showed that the new automated assay of sdLDL-C identified risk of CHD whereas the nuclear magnetic resonance-derived small LDL concentrations did not convey a significant risk of CHD in the MESA cohort. Therefore, if these two different methodologies show different associations with cardiovascular risk it is plausible that they may also lead to different GWAS findings.
Genetic variants within a number of the genes associated with sdLDL-C in our study have previously been found to be associated with increased risk for cardiovascular disease in meta-analyses of GWA studies.31,32 SNP rs4420638, which is located in the APOE-APOC1-APOC4-APOC2 gene cluster, was also associated with Lp-PLA2 activity and CHD in a meta-analysis of GWA studies from 5 community-based studies.33 In addition, we have previously shown an association of the SNP rs780094 in GCKR with metabolic syndrome prevalence and incident diabetes in the ARIC study.34 rs780094 was also significantly associated with sdLDL-C (p=4.08×10−12) in our current study.
Our findings have important implications in light of recent observations from mendelian randomization studies investigating genetic determinants of HDL-C levels and risk for incident CHD. Unlike data from human mendelian diseases, which support a causal role for LDL-C in risk for CHD,35,36, evidence for a causal role of HDL-C from mendelian randomization studies is inconsistent and complicated by the fact that most SNPs associated with HDL-C levels affect multiple lipid traits. Voight and coworkers recently showed that a genetic risk score consisting of 14 SNPs exclusively associated with HDL-C was not associated with risk for myocardial infarction, in contrast to a genetic risk score for LDL-C.37 These investigators had previously shown that a number of SNPs associated with HDL-C were also associated with other lipid traits such as triglycerides and LDL-C.38 The SNPs associated with HDL-C that were most strongly associated with increased risk for myocardial infarction and that influenced other lipid traits were located in or near the APOA5-APOA4-APOC3-APOA1, TRIB1, and LPL genes or gene clusters. In the current study, we showed that these genes are also associated with sdLDL-C or sdLDL-C/LDL-C ratio (see Supplemental Table II [available online at http://atvb.ahajournals.org]).
Association of PCSK7 SNP-rs508487 Genotype with Circulating Lipids
A novel finding from the current GWAS analysis is the significant association of sdLDL-C with SNP rs508487 (at locus 11q23-q24), located in the PCSK7 gene. Investigation of the effect of rs508487 genotype on circulating lipid levels showed that each copy of the minor allele at this SNP raised sdLDL-C by ~4 mg/dL. It is important to note that genetic variation at the PCSK7 locus was not associated with LDL-C levels in our study. Since LDL-C is a more commonly used lipid measurement, it is plausible that genetic variations in the PCSK7 gene have not been associated with circulating lipids in previous GWAS studies.
PCSK7 encodes subtilisin-like/kexin proprotein convertase type 7 (PCSK7), a calcium-dependent serine endoprotease. PCSK7 has previously been implicated as a mediator of adipogenesis39 and in the processing of VEGF-D, a critical step for binding of the angiogenic receptor VEGFR-2.40 Furthermore, recent data show that internalization of PCSK7 from the plasma membrane is mediated by clathrin-coated vesicles,41 which are also implicated in the internalization of other cellular receptors such as the LDL receptor and various scavenger receptors. Although the physiologic role of PCSK7 is not clearly understood, it is plausible that PCSK7 could be involved in the processing of LDL and/or scavenger receptors, thereby modulating circulating lipid levels. Alternatively, recent studies suggest a potential role of protein convertases, including PCSK7, in lipid metabolism through proteolytic activation of angiopoietins and proteolytic inactivation of lipases.42,43 In contrast to PCSK9, another member of the proprotein convertase family, to our knowledge PCSK7 has not been previously associated with cardiovascular lipid risk factors in other GWA studies. However, we should be cautious in the interpretation of our findings regarding the association of PCSK7 genotype with circulating lipids. A limitation of our study is that we did not measure protein or mRNA levels of PCSK7. Furthermore, the PCSK7 SNP rs508487 is in close proximity to the APOA5-APOA4-APOC3-APOA1 gene cluster, which has also been shown to affect circulating triglyceride levels. However, our exome chip data show that rare variants in the PCSK7 gene, which lead to amino acid substitutions in the PCSK7 protein, are associated with sdLDL-C and other lipid traits. Additional in vitro or animal studies using transgenic or PCSK7-knockout mouse models are needed to investigate the potential role of PCSK7 in lipid metabolism. Our findings also highlight the important issue of pleiotropy as PCSK7 was associated with circulating levels of sdLDL-C, triglycerides and HDL-C.
Conclusions
In summary, our results showed that sdLDL-C was highly correlated with an atherogenic lipid profile and, in contrast to lbLDL-C, predicted future CHD events in ARIC participants. Furthermore, sdLDL-C predicted risk for incident CHD even in individuals who would be considered at low cardiovascular risk based on their LDL-C level. This new homogenous sdLDL-C assay could be readily implemented in most routine clinical chemistry laboratories, provided that its clinical value can be confirmed in future studies. GWAS analysis identified significant associations of sdLDL-C with genetic variants in 14 different genes, all but 1 of which have been previously linked to cardiovascular disease risk. Our GWAS findings, together with findings from previous studies showing genetic variants in the same genes associated with other lipid traits, highlight the importance of pleiotropy in the development of cardiovascular disease. The novel finding of a significant association of sdLDL-C with genetic variants in PCSK7, a member of the subtilisin-like/kexin proprotein convertase family, provide new insights into the role of this gene in lipid metabolism.
Supplementary Material
Significance.
Low-density lipoprotein cholesterol (LDL-C) is considered one of the most important risk factors for cardiovascular disease and remains the primary target for current cardiovascular risk reduction strategies. LDL particles are a heterogeneous population, and it has long been hypothesized that a subfraction of LDL, small dense LDL (sdLDL), possesses atherogenic potential. In the current study, we investigated the relationship between plasma levels of sdLDL-C and risk of incident CHD in the biracial ARIC study cohort. Elevated plasma sdLDL-C levels were associated with increased risk of incident CHD, even in individuals considered to be at low cardiovascular risk based on their LDL-C levels. Using genome-wide association (GWA) analyses, we discovered one novel locus, PCSK7, for which genetic variation was significantly associated with sdLDL-C levels and other lipid traits. Together these findings provide new insights into the role of the PCSK7 gene in lipid metabolism and risk of cardiovascular disease.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Support for the exome chip genotyping and centralized calling was provided by Building on GWAS for NHLBI-diseases: the U.S. CHARGE consortium through the National Institutes of Health (NIH) American Recovery and Reinvestment Act of 2009 (ARRA) (5RC2HL102419) (PI: E. Boerwinkle). Genome-wide association support was funded by R01HL087641, R01HL59367, R01HL086694, National Human Genome Research Institute contract U01HG004402, and National Institutes of Health contract HHSN268200625226C. The infrastructure was partly supported by grant UL1RR025005, a component of the NIH and NIH Roadmap for Medical Research.
Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development Career Development Grant.
Abbreviations
- apo
apolipoprotein
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- GWAS
genome-wide association study
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- hs-CRP
high-sensitivity C-reactive protein
- lbLDL
large buoyant low-density lipoprotein
- lbLDL-C
large buoyant low-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- Lp-PLA2
lipoprotein-associated phospholipase A2
- sdLDL
small dense low-density lipoprotein
- sdLDL-C
small dense low-density lipoprotein cholesterol
- SNP
single-nucleotide polymorphism
Footnotes
Disclosures
Dr. Hoogeveen received a research grant from Denka Seiken, Ltd. and all reagents for the sdLDL-C measurements were provided by Denka Seiken, Ltd.
Contributor Information
Ron C. Hoogeveen, Baylor College of Medicine and Methodist DeBakey Heart and Vascular Center, Houston, TX.
John W. Gaubatz, Baylor College of Medicine and Methodist DeBakey Heart and Vascular Center, Houston, TX.
Wensheng Sun, Baylor College of Medicine and Methodist DeBakey Heart and Vascular Center, Houston, TX.
Rhiannon C. Dodge, University of Texas Health Science Center at Houston, Houston, TX.
Jacy R. Crosby, University of Texas Health Science Center at Houston, Houston, TX.
Jennifer Jiang, Baylor College of Medicine and Methodist DeBakey Heart and Vascular Center, Houston, TX.
David Couper, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Salim S. Virani, Michael E. DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, and Methodist DeBakey Heart and Vascular Center, Houston, TX.
Sekar Kathiresan, Center for Human Genetic Research & Cardiovascular Research Center, Massachusetts General Hospital and Department of Medicine, Harvard Medical School, Boston, MA.
Eric Boerwinkle, University of Texas Health Science Center at Houston, Houston, TX.
Christie M. Ballantyne, Baylor College of Medicine and Methodist DeBakey Heart and Vascular Center, Houston, TX.
References
- 1.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 2.Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebocontrolled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 4.Cholesterol Treatment Trialists C. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. The Lancet. 376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]
- 6.Anber V, Griffin BA, McConnell M, Packard CJ, Shepherd J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis. 1996;124:261–271. doi: 10.1016/0021-9150(96)05842-x. [DOI] [PubMed] [Google Scholar]
- 7.Dejager S, Bruckert E, Chapman MJ. Dense low density lipoprotein subspecies with diminished oxidative resistance predominate in combined hyperlipidemia. J Lipid Res. 1993;34:295–308. [PubMed] [Google Scholar]
- 8.Hurt-Camejo E, Camejo G, Rosengren B, Lopez F, Wiklund O, Bondjers G. Differential uptake of proteoglycan-selected subfractions of low density lipoprotein by human macrophages. J Lipid Res. 1990;31:1387–1398. [PubMed] [Google Scholar]
- 9.Nordestgaard BG, Zilversmit DB. Comparison of arterial intimal clearances of LDL from diabetic and nondiabetic cholesterol-fed rabbits. Differences in intimal clearance explained by size differences. Arteriosclerosis. 1989;9:176–183. doi: 10.1161/01.atv.9.2.176. [DOI] [PubMed] [Google Scholar]
- 10.Sattar N, Petrie JR, Jaap AJ. The atherogenic lipoprotein phenotype and vascular endothelial dysfunction. Atherosclerosis. 1998;138:229–235. doi: 10.1016/s0021-9150(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 11.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 12.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Austin MA. Genetic and environmental influences on LDL subclass phenotypes. Clin Genet. 1994;46:64–70. doi: 10.1111/j.1399-0004.1994.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 14.Austin MA, Talmud PJ, Farin FM, Nickerson DA, Edwards KL, Leonetti D, McNeely MJ, Viernes HM, Humphries SE, Fujimoto WY. Association of apolipoprotein A5 variants with LDL particle size and triglyceride in Japanese Americans. Biochim Biophys Acta. 2004;1688:1–9. doi: 10.1016/j.bbadis.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Gazi I, Tsimihodimos V, Filippatos T, Bairaktari E, Tselepis AD, Elisaf M. Concentration and relative distribution of low-density lipoprotein subfractions in patients with metabolic syndrome defined according to the National Cholesterol Education Program criteria. Metabolism. 2006;55:885–891. doi: 10.1016/j.metabol.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Lowdensity lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–1921. [PubMed] [Google Scholar]
- 17.Campos H, Genest JJ, Jr, Blijlevens E, McNamara JR, Jenner JL, Ordovas JM, Wilson PW, Schaefer EJ. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb. 1992;12:187–195. doi: 10.1161/01.atv.12.2.187. [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Kwiterovich PO, Jr, Smith HH, Bachorik PS. Association of plasma triglyceride concentration and LDL particle diameter, density, and chemical composition with premature coronary artery disease in men and women. J Lipid Res. 1993;34:1687–1697. [PubMed] [Google Scholar]
- 19.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 20.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 21.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 22.Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem. 2011;57:57–65. doi: 10.1373/clinchem.2010.149559. [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105–113. doi: 10.1016/j.jacl.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, Cupples LA, Wilson PW, Schaefer EJ. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56:967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-Density Lipoprotein Particle Concentration and Size as Determined by Nuclear Magnetic Resonance Spectroscopy as Predictors of Cardiovascular Disease in Women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 27.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, O'Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chasman DI, Pare G, Mora S, Hopewell JC, Peloso G, Clarke R, Cupples LA, Hamsten A, Kathiresan S, Malarstig A, Ordovas JM, Ripatti S, Parker AN, Miletich JP, Ridker PM. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009;5:e1000730. doi: 10.1371/journal.pgen.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:196–201. doi: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myocardial Infarction Genetics Consortium. Kathiresan S, Voight BF, Purcell S, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grallert H, Dupuis J, Bis JC, et al. Eight genetic loci associated with variation in lipoprotein-associated phospholipase A2 mass and activity and coronary heart disease: meta-analysis of genome-wide association studies from five community-based studies. Eur Heart J. 2012;33:238–251. doi: 10.1093/eurheartj/ehr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bi M, Kao WH, Boerwinkle E, Hoogeveen RC, Rasmussen-Torvik LJ, Astor BC, North KE, Coresh J, Kottgen A. Association of rs780094 in GCKR with metabolic traits and incident diabetes and cardiovascular disease: the ARIC Study. PLoS One. 2010;5:e11690. doi: 10.1371/journal.pone.0011690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest. 2003;111:1795–1803. doi: 10.1172/JCI18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 37.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croissandeau G, Basak A, Seidah NG, Chretien M, Mbikay M. Proprotein convertases are important mediators of the adipocyte differentiation of mouse 3T3-L1 cells. J Cell Sci. 2002;115:1203–1211. doi: 10.1242/jcs.115.6.1203. [DOI] [PubMed] [Google Scholar]
- 40.McColl BK, Paavonen K, Karnezis T, Harris NC, Davydova N, Rothacker J, Nice EC, Harder KW, Roufail S, Hibbs ML, Rogers PA, Alitalo K, Stacker SA, Achen MG. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007;21:1088–1098. doi: 10.1096/fj.06-7060com. [DOI] [PubMed] [Google Scholar]
- 41.Declercq J, Meulemans S, Plets E, Creemers JW. Internalization of proprotein convertase PC7 from plasma membrane is mediated by a novel motif. J Biol Chem. 2012;287:9052–9060. doi: 10.1074/jbc.M111.306407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei X, Shi F, Basu D, Huq A, Routhier S, Day R, Jin W. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem. 2011;286:15747–15756. doi: 10.1074/jbc.M110.217638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Afroza H, Rader DJ, Jin W. Angiopoietin-like protein 3 inhibits lipoprotein lipase activity through enhancing its cleavage by proprotein convertases. J Biol Chem. 2010;285:27561–27570. doi: 10.1074/jbc.M110.144279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.