Abstract
Background:
Alopecia areata (AA) is a common hair disorder of unknown etiology and prognosis with no definitive cure.
Aims and Objective:
(i) To study the efficacy and immunomodulatory action of 88% phenol on hair growth on test area in AA. (ii) To study various epidemiological factors in relation to AA.
Materials and Methods:
A total of 50 subjects presenting with nonscarring patchy hair loss on scalp were enrolled in this prospective open label study to receive 88% phenol at 3 weekly intervals in a tertiary care hospital. Efficacy was assessed using scoring system for density, pigmentation, and texture of growing hair.
Results:
Texture and pigmentation of hair growth was significantly improved at 9 week, while density of hair growth showed steady improvement, which was significant from 9 week onwards. About 78% of patients showed good to excellent response while none of them showed poor response.
Conclusion:
88% phenol was found to be efficacious with all patients showing hair regrowth. 88% phenol can be considered as a treatment of choice for stable AA due to its ease of application, easy availability, and low cost.
Keywords: Alopecia areata, contact sensitization, phenol
INTRODUCTION
Alopecia areata (AA) is a chronic inflammatory disease, which affects the hair follicles and sometimes the nails. [1] The onset may be at any age and there is no known race or sex preponderance. It is characterized by circumscribed nonscarring areas of baldness on the scalp, eyebrows, eyelashes, beard, moustache area, or hairy skin anywhere on the body affecting both sexes and almost all age groups. [2]
Exact etiology of AA is not known; but factors responsible are autoimmunity, genetic constitution, the atopic state, nonspecific immune reactions, and possibly emotional stress. [3]
Various modalities of treatment are available in the form of local stimulators with caustics, intralesional and topical corticosteroid therapies, topical contact sensitizers like 1-chloro-2, 4-dinitrobenzene (DNCB), diphencyprone, psoralens followed by ultraviolet A exposure, systemic corticosteroids, topical minoxidil and immunostimulation have all been used with variable success. [4,5,6,7] Topical and intra lesional steroid can cause atrophy of the skin, telangiectasia, and in addition to these oral steroids have systemic side-effects viz. hypothalamic-pituitary-adrenal axis suppression. [8,9] Topical sensitizers cause whole body sensitization, which again has risk of anaphylaxis. While 88% phenol is cheap, easy to apply, less painful procedure, requiring 0.5 ml application only once in 3 week with minimal side-effects. The purpose of this study is to determine the efficacy of topical 88% phenol and to evaluate acceptability and safety in AA in our patients.
MATERIALS AND METHODS
Study design
The study was performed in accordance with Good Clinical Practices and was approved by Institutional Review Board. This open labeled prospective study was carried out in the outpatient clinic of a tertiary care hospital in Mumbai. All patients gave a written, informed consent for induction in the trial, while parents gave consent in case of minor.
Patient selection
A total of 50 patients with clinical diagnosis of AA were included in the study. Patients were in the age group of 7-45 years having <5 stable patches of AA on the scalp. Patients were excluded from the study if they had: (i) AA on areas other than scalp, (ii) diffuse AA, and (iii) known cardiac, renal, hepatic problems. Pregnant and lactating females and those with unrealistic expectation were also the criteria for exclusion.
Initial assessment
A detailed clinical history and physical examination was performed by a dermatologist. At the baseline, relevant attention was paid to history pertaining to AA, i.e. age of onset, duration, personal or family history of associated endocrine or autoimmune condition. Number, size, site, and extent of patches were recorded. Clinical close up photographs of the scalp were taken at baseline visit.
Prognosis of each patient was assessed according to score given below.
Routine investigations such as blood cell counts, erythrocyte sedimentation rate, blood glucose levels, and chest X-ray were done. Liver and renal function tests were done along with serum lipid profile.
Treatment regime
Full strength liquefied phenol (carbolic acid) was procured from hospital pharmacy and was prepared by keeping bottle containing phenol crystals in hot water until dissolution of crystals. This was further diluted with water to make 88% phenol solution (88 ml liquefied pure phenol in 100 ml water).
The area to be treated was degreased by scrubbing with savlon, followed by spirit and acetone. In a small glass container, 0.5-1 ml 88% phenol was taken which, was then applied gently with thin cotton tipped applicator by giving uniform smooth strokes until an ivory white uniform frosting was seen. Feathering of the borders was done by painting of the phenol from the periphery of the lesion into the surrounding normal skin. No neutralization of phenol was done in any of the patients, as it is not required in phenol peels. All patients were monitored for half an hour for pulse rate and heart rate after the procedure. Three applications of 88% phenol were done at 3 weekly intervals for each patient.
Clinical assessment of efficacy
The patients were advised to follow-up every 3 week interval, on each follow-up density, pigmentation, texture of hair was assessed as follows: [Tables 1–3].
Table 1.
Scoring of texture of regrown hair

Table 3.
Scoring for density of hair regrowth
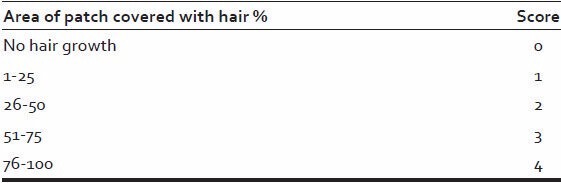
Table 2.
Scoring of pigmentation of regrown hairs

One of the patch was left untreated (except in case of patients with a single patch of alopecia) to serve as a control which was assessed as per scoring system.
A composite score was calculated based on the scores obtained for pigmentation, density, and texture each [Table 4].
Table 4.
Scoring of overall response (1+2+3) at the end of the study

Color photographs of the patients were taken showing four views of the patients' scalp hair: (i) Frontal, (ii) temporal, (iii) mid-pattern, and (iv) vertex.
Statistical analyses
The analysis of texture, pigmentation, and density was done by using two-tailed t-test. P < 0.05 was considered to be statistically significant.
Side effects
At each visit, assessment was done for side-effects of topical phenol application, i.e., pigmentation, redness, infection, scarring, or any systemic complaints.
RESULTS
A total of 50 patients were included in the study, which was carried in the outpatient Department of Dermatology of tertiary care teaching institute.
Patient profile and demographic parameters are given in Tables 5–7.
Table 5.
Baseline profile of patients with relation to age and sex
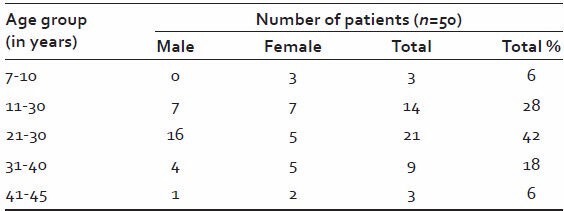
Table 7.
Conditions associated with alopecia areata
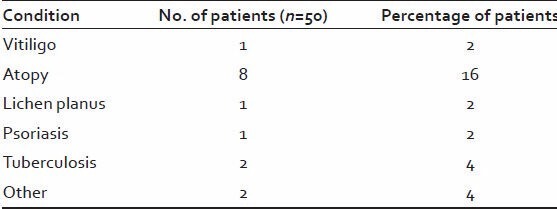
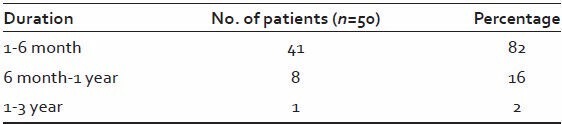
Table 6.
Duration of the disease
Majority of patients (42%) were in age group 21-30 years. The male: female was 1.27:1.
41 patients (82%) reported to have the alopecia of <6 months duration.
History of atopic diathesis was noted in 8 out of 50 patients.
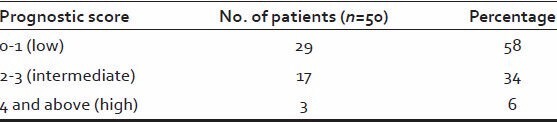
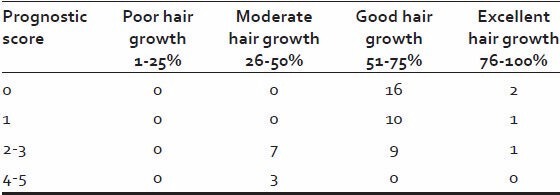
Majority of patient (58%) had a low prognostic score while high prognostic score seen only in 3 (6%) patients [Table 8].
Table 8.
Prognostic score
Evaluation of clinical response is based on the texture, pigmentation, and density of regrown hairs.
All patients after application of 88% phenol on test area developed immediate frosting for 10-15 min and mild to moderate burning sensation only for 15-30 s. The next day skin became dark-brown to black in color and started peeling at places along with crusting. Most of the crusts fell off within 5-10 days when re-epithelization occurred revealing a shiny, erythematous skin. Clinical response was seen in the form of fine partially pigmented vellus hairs, which gradually started growing in size and becoming thicker in diameter and darker in color [Tables 9–11].
Table 9.
Texture of regrown hair
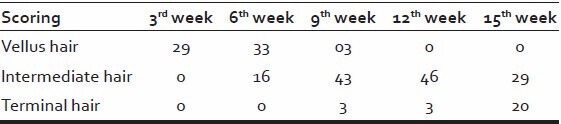
Table 11.
Density of regrown hair
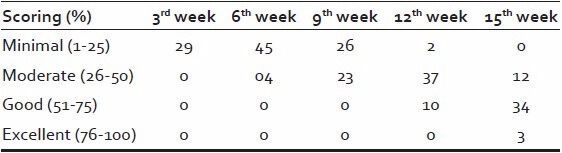
Table 10.
Pigmentation of regrown hair
Patient showed initial clinical response with the growth of vellus hair in 29 patients (58%) by the end of 3rd week [Figure 1]. This vellus hair gradually transformed into terminal hairs such that at the end of 12th week, [Figure 2] 46 (92%) had intermediate hair and 6% had terminal hair and at the end of 15th week 29 patients (58%) showed intermediate hair and 20 patients (40%) showed terminal hair growth.
Figure 1.
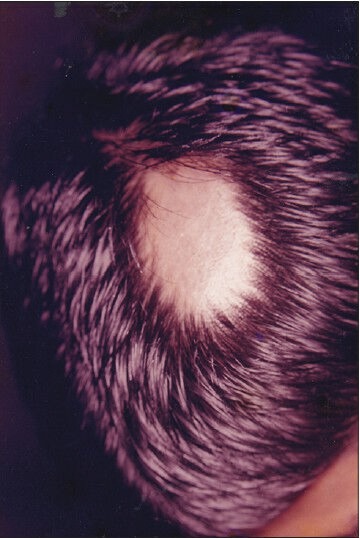
Patient 1 - Pretreatment photograph showing a single patch of alopecia areata
Figure 2.
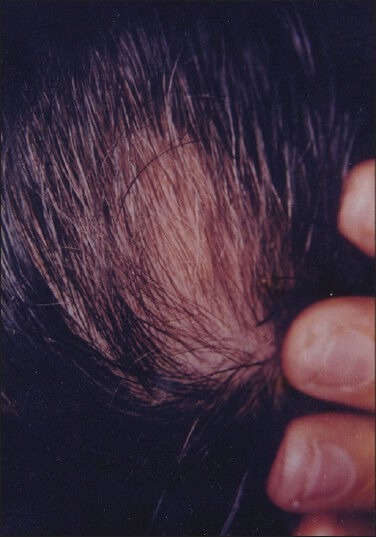
Patient 1 - Posttreatment photograph after 9 weeks of phenol treatment showing significant hair re-growth
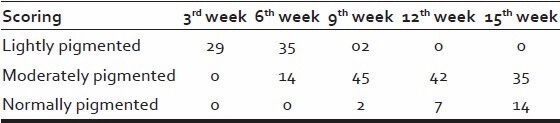
It was observed that pigmentation of hair began by the 3rd week in 58% patients and gradually increased over the weeks and hence that by the end of 15th week, 35 patients (70%) had moderately pigmented and 14 patients (28%) had normally pigmented hair.
Out of 50, 29 patients (58%) showed initiation of clinical response in the form of hair growth by the end of 3rd week. Majority of the patients 37 (74%) showed moderate (26-50%) hair growth by 12th week. Good to excellent density of hair growth was evident in 37 (74%) patients by the end of 15th week.
Out of 29 (58%) patients with a low prognostic score, all showed good hair growth. While all the three patients with a high prognostic score [4,5] showed only moderate hair growth. None of the patients with a higher prognostic score showed good or excellent hair growth [Table 12].
Table 12.
Relation between prognostic score and response to treatment
Texture and pigmentation of hair growth was significantly improved up to 9th weeks [Figures 3–5], but showed no further improvement after 12th week, it has been stabilized. Density of hair growth showed steady improvement, which was significant from 9th week onwards [Table 13].
Figure 3.
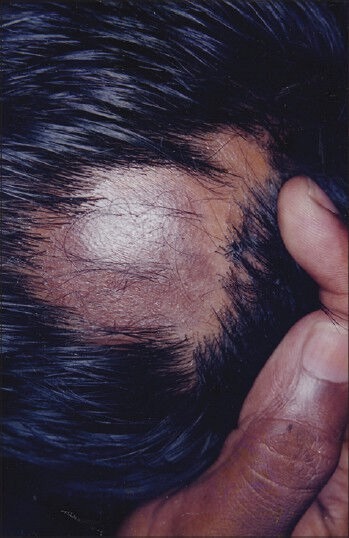
Patient 2 - Baseline clinical photograph showing a single smooth patch of alopecia
Figure 5.
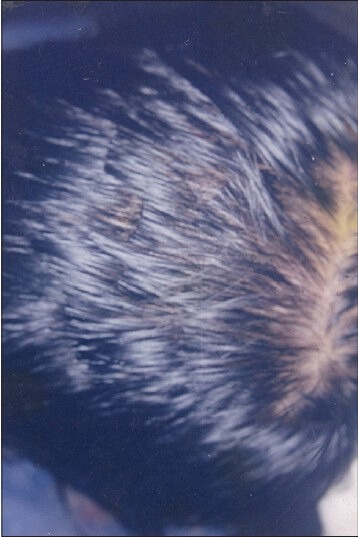
Patient 2 - At week 15 showing complete hair growth
Table 13.
Statistical analysis of texture, pigmentation, density of regrown hair*
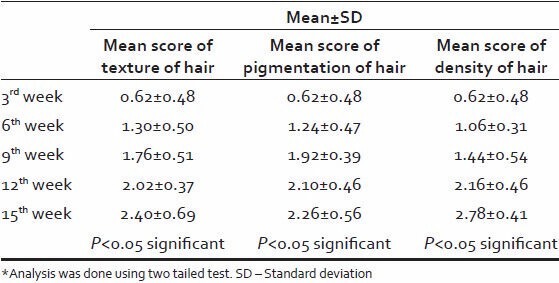
Figure 4.
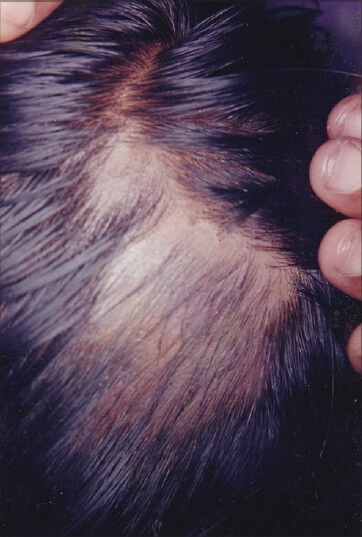
Patient 2 - Postphenolization photograph at week 9 demonstrating growth of terminal hairs
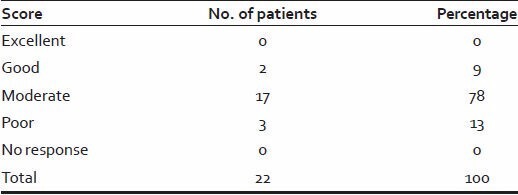
Only two nontested areas showed good response, 17 nontested areas showed moderate response, while none of them showed excellent response [Table 14].
Table 14.
Overall assessment of nontest area (at the end of the study)
Five patients developed hypopigmentation, which lasted only for 3-4 weeks while three patients showed hyper pigmentation, which lasted for 6-8 weeks, persistent erythema, remained for 4 weeks. Secondary infections due to improper wound care were seen in only one patient.
DISCUSSION
A total of 50 informed patients of AA completed the study in the outpatient Department of Dermatology of a teaching hospital.
The patients (28 males and 22 females) were in the age group ranging from 7 to 45 years (mean age 25.4 years). The duration of the disease was <6 months in the majority of cases (82%).
The statistical analysis of increase in texture, pigmentation, density was found to be significant at every 3 weekly interval, establishing efficacy of phenol. Texture and pigmentation of hair growth was found to be significantly improved in 3rd -9th weeks but at the 12th week it was stabilized and again after 12th week it has shown significant improvement. Density of hair growth from 9th week onwards has been showed significant improvement.
On assessing the overall response (texture + pigmentation + density) at the end of the study, all patients showed response to 88% phenol. Majority of the patients (78%) showed good to excellent response while none of them showed poor response.
Patients were evaluated using a prognostic score. It was found that out of the 29 (58%) patients with low prognostic score all showed good hair growth and none showed poor hair growth, while all the three patients with high prognostic score showed only moderate hair growth. None of these three showed good or excellent hair growth. This correlates well with the observation of Bruillard and Shapiro who suggested that low prognostic score was suggestive of good prognosis.
Most common side-effect observed was hypopigmentation, which was noted in 10% of patients, which remained for 3-4 weeks. It is a well-known fact that after facial phenol peels melanin synthesis is impaired temporarily which can result in hypopigmentation.
Three patients developed hyperpigmentation, which lasted for 6-8 weeks. Persistent erythema was seen in only one patient, which remained for 4 weeks. Secondary bacterial infection occurred as a complication only in one patient due to improper wound care on part of the patient who later dropped out from the study. This is contrary to the findings of Savant and Shenoy who found secondary bacterial infection in 8% of patients. No systemic side-effects were noted.
Rosenberg and Drake introduced contact immunotherapy in 1976. [10] The contact allergens that have been used in the treatment of AA include DNCB, squaric acid dibutylester and 2, 3 diphenylcyclopropenone. [11,12] Phenol (carbolic acid) is a contact irritant, which acts by way of “antigenic competition” and immunomodulation thereby decreasing the immune attack on pigmented anagen hairs. [13] Our search of literature revealed only two reports of use of phenol in AA. In 1937, Paul studied application of phenol on one patch of AA refractory to UVA therapy and documented excellent response. [14] Another study was by Savant and Shenoy who documented the response to 88% phenol in 69 patches of AA, but did not explain the details regarding the density, pigmentation, and texture of hair regrowth. [13] Hence, we could not find any comparative data for our observations.
Our study has established the efficacy and safety of 88% phenol in the therapy of AA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Muller SA, Winkelmann RK. Alopecia areata. An evaluation of 736 patients. Arch Dermatol. 1963;88:290–7. doi: 10.1001/archderm.1963.01590210048007. [DOI] [PubMed] [Google Scholar]
- 2.Anderson I. Alopecia areata: A clinical study. Br Med J. 1950;2:1250–2. doi: 10.1136/bmj.2.4691.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonagh AJ, Messenger AG. The pathogenesis of alopecia areata. Dermatol Clin. 1996;14:661–70. doi: 10.1016/s0733-8635(05)70392-2. [DOI] [PubMed] [Google Scholar]
- 4.Hull SM, Pepall L, Cunliffe WJ. Alopecia areata in children: Response to treatment with diphencyprone. Br J Dermatol. 1991;125:164–8. doi: 10.1111/j.1365-2133.1991.tb06064.x. [DOI] [PubMed] [Google Scholar]
- 5.Rokhsar CK, Shupack JL, Vafai JJ, Washenik K. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751–61. doi: 10.1016/s0190-9622(98)70048-9. [DOI] [PubMed] [Google Scholar]
- 6.Claudy AL, Gagnaire D. PUVA treatment of alopecia areata. Arch Dermatol. 1983;119:975–8. [PubMed] [Google Scholar]
- 7.Ranchoff RE, Bergfeld WF, Steck WD, Subichin SJ. Extensive alopecia areata. Results of treatment with 3% topical minoxidil. Cleve Clin J Med. 1989;56:149–54. doi: 10.3949/ccjm.56.2.149. [DOI] [PubMed] [Google Scholar]
- 8.Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674–5. [PubMed] [Google Scholar]
- 9.Sharma VK. Pulsed administration of corticosteroids in the treatment of alopecia areata. Int J Dermatol. 1996;35:133–6. doi: 10.1111/j.1365-4362.1996.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg EW, Drake L. In discussion of Dunaway DA. Alopecia areata. Arch Dermatol. 1976;112:256. [Google Scholar]
- 11.Happle R, Hausen BM, Wiesner-Menzel L. Diphencyprone in the treatment of alopecia areata. Acta Derm Venereol. 1983;63:49–52. [PubMed] [Google Scholar]
- 12.Singh G, Lavanya M. Topical immunotherapy in alopecia areata. Int J Trichology. 2010;2:36–9. doi: 10.4103/0974-7753.66911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savant SS, Shenoy S. Chemical peeling with phenol: For the treatment of stable vitiligo and alopecia areata. Indian J Dermatol Venereol Leprol. 1999;65:93–8. [PubMed] [Google Scholar]
- 14.Bechet PE. Extensive alopecia areata and result of treatment. Dermatologica. 1937;37:1073–4. [Google Scholar]


