Abstract
Background
Planar cell polarity (PCP) signaling regulates the coordinated polarization of cells and is required for the normal development and function of many tissues. Previous studies have identified conserved PCP genes, such as Van gogh-like 2 (Vangl2) and Prickle (Pk), in the regulation of coordinated orientation of inner ear hair cells and female reproductive tract development. Testin shares a PET-LIM homology with Pk. It is not clear whether Testin acts in PCP processes in mammals.
Results
We identified Testin as a Vangl2-interacting protein through a 2-hybrid screen with a cochlea cDNA library. Testin is enriched to cell-cell boundaries in the presence of Vangl2 in cultured cells. Genetic inactivation of Testin leads to abnormal hair cell orientation in the vestibule and cellular patterning defects in the cochlea. In addition, Testin genetically interacts with Vangl2 to regulate hair cell orientation in the cochlea and the opening of the vaginal tract.
Conclusions
Our findings suggested Testin as a gene involved in coordinated hair cell orientation in the inner ear and in female reproductive tract development. Furthermore, its genetic interaction with Vangl2 implicated it as a potential molecular link, responsible for mediating the role of Vangl2-containing membranous PCP complexes in directing morphologic polarization.
Keywords: Testin, Planar cell polarity, Vangl2, Inner ear, Female reproductive tract, Hair cells
Introduction
Planar cell polarity (PCP) refers to coordinated polarization of neighboring cells within the plane of a cell sheet. In vertebrates, PCP is regulated by a set of conserved genes known as core PCP genes, including Celsr, Van gogh-like (Vangl), Frizzled (Fz) receptor, and Prickle (Pk) genes (Wallingford, 2012). In vertebrates, PCP signaling is required for diverse cellular processes. Defects in PCP signaling cause abnormalities in many organs and tissues, such as the loss of coordinated orientation of inner ear sensory hair cells and a defective convergent extension (CE) of the cochlear duct (Curtin et al., 2003; Montcouquiol et al., 2003; Lu et al., 2004; Wang et al., 2005; Jones et al., 2008), lung branching (Ansley et al., 2003; Paudyal et al., 2010; Yates and Dean, 2011) and dermis (Devenport and Fuchs, 2008) abnormalities, kidney diseases (Kramer-Zucker et al., 2005), axonal defects (Tissir and Goffinet, 2010; Armstrong et al., 2011; Banerjee et al., 2011; Berger-Muller and Suzuki, 2011; Shimizu et al., 2011; Shafer et al., 2012), and defective closures of the palate (Topczewski et al., 2011), ventricular septum (Sheldahl et al., 2003; Phillips et al., 2005; Etheridge et al., 2008; Paudyal et al., 2010; Henderson and Chaudhry, 2011) and the neural tube (Kibar et al., 2001; Murdoch et al., 2001a; Murdoch et al., 2001b; Hamblet et al., 2002; Curtin et al., 2003; Lu et al., 2004; Ciruna et al., 2006; Paudyal et al., 2010). Individual PCP genes have been associated with additional processes, such as loss-of-function mutations in Vangl2 with cellular polarization during postnatal female reproductive tract development in mice (Vandenberg and Sassoon, 2009).
The inner ear sensory organs, i.e., the one in the cochlea and the five in the vestibule, show distinctive forms of PCP in vertebrates (Kelly and Chen, 2007). At the apical surface of each sensory hair cell, microvilli-derived stereocilia are arranged in rows of increasing length, with the tallest sterocilia positioned toward the periphery. In addition, there is a single primary cilium, known as the kinocilium, eccentrically placed near the tallest stereocilia at one edge of the cellular apex. All of the hair cells within each of the six inner ear sensory organs are coordinately polarized (Kelly and Chen, 2007). Hair cells in the cochlea and in the three vestibular cristae are uniformly oriented, while hair cells in the vestibular saccule and utricle have opposite orientations along a line of polarity reversal (Kelly and Chen, 2007). Among the conserved core PCP genes, mutations in Vangl2 (Montcouquiol et al., 2003), Celsr1 (Curtin et al., 2003), Fz (Wang et al., 2006), or Dvl genes (Wang et al., 2005) cause the disruption of coordinated hair cell orientation of in the cochlea. A vestibular PCP defect was also observed in Vangl2 and Fz mutants (Wang et al., 2006). It is thought that the membrane proteins Vangl2 and Fz form membrane-associated protein complexes with cytoplasmic PCP proteins to mediate intercellular interactions and to coordinate the polarity of neighboring cells (Wang et al., 2005; Montcouquiol et al., 2006; Wang et al., 2006; Wu and Mlodzik, 2008). Indeed, asymmetric localization of Vangl2, Fz, and Dvl proteins along the PCP axis is observed in the inner ear sensory organs (Wang et al., 2005; Wang et al., 2006), and the polarized localization of membrane PCP proteins is lost when PCP signaling is disrupted (Wang et al., 2005; Montcouquiol et al., 2006; Wang et al., 2006; Deans et al., 2007). On the other hand, the membrane-associated PCP protein complexes are normally polarized in several PCP mutants that are defective in directing the intrinsic polarization of cells (Jones et al., 2008; Grimsley-Myers et al., 2009; Sipe and Lu, 2011), which suggests that membrane PCP proteins are not sufficient to direct morphological polarization but likely recruit and interact with other cellular factors to direct morphological polarizations. However, very little is known about such cellular factors in vertebrates.
To further explore vertebrate PCP regulation, we performed a two-hybrid screen to identify proteins which interact with transmembrane PCP protein Vangl2. We used the C-terminal cytoplasmic domain of Vangl2 and a mouse embryonic cochlear cDNA library. The screen identified several proteins (Li et al., 2012) including Testin. Testin shares a PET and LIM domain homology with a known PCP protein, Pk. In Drosophila, Pk functions in an intercellular feedback loop crucial to the establishment of PCP in the eye and epidermis by interacting with Vang and modulating Frizzled-Dishevelled signaling (Tree et al., 2002; Das et al., 2004; Jenny et al., 2005). While interference with Pk1 causes CE defects in zebrafish and ascidians (Carreira-Barbosa et al., 2003; Veeman et al., 2003; Jiang et al., 2005), both over-expression and depletion of Testin also inhibit activin-induced CE of animal caps and cause gastrulation CE defects in Xenopus (Dingwell and Smith, 2006). The impact of Testin modulation on these CE processes implicates a potential role for Testin in vertebrate PCP signaling. Here, we present data suggesting that Testin is a mammalian gene involved in coordinated orientation of hair cells in the inner ear and in the development of the female reproductive tract, and demonstrate a role for Testin in PCP regulated processes in mice.
Results
Testin interacts with Vangl2 in the yeast two-hybrid assay and is recruited by Vangl2 and Dvl1 to their subcellular locations
To further explore the vertebrate PCP pathway, we performed a yeast two-hybrid screen (Li et al., 2012). Vangl2 is predicted to consist of 4 transmembrane domains with a short N-terminal cytoplasmic domain and a long C-terminal cytoplasmic domain. Several mutations at the C-terminal cytoplasmic domain cause the loss-of-function for Vangl2 in PCP signaling (Torban et al., 2004a; Torban et al., 2004b), which implicates the functional importance of Vangl2 C-terminal region. The yeast two-hybrid screen was carried out using the C-terminal domain of Vangl2, Vangl2C, as the bait against a mouse cDNA library made from embryonic day 15 (E15) cochlear tissues (Li et al., 2012). In a minimum culture medium that lacks histidine and adenine, the presence of Vangl2C or Testin alone did not activate the expression of reporter genes which allow for cell growth independent of histidine or adenine. As a consequence, there was no cell growth on the minimum plates (Fig. 1A, B). When both Vangl2C and Testin were present, the cells grew in the minimal medium and turned blue in the presence of galactosidase substrates (Fig. 1B). This result suggested that reporter genes are expressed in the presence of both Vangl2C and Testin, or Vangl2C and Testin brought the Gal4 DNA binding domain (Gal4-BD) to the Gal4 DNA activation domain (Gal4-AD) to activate reporter gene expression.
Fig.1.
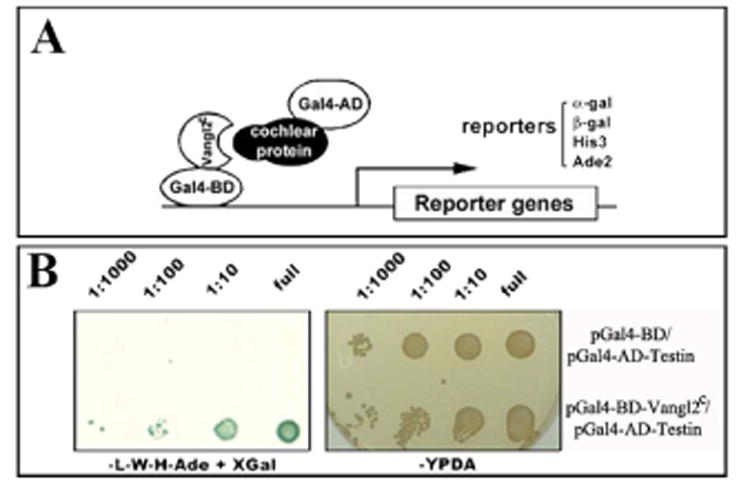
Testin interacts with Vangl2 in the yeast two-hybrid assay.
(A) Schematic diagram of the two hybrid screen in which the C-terminal cytoplasmic tail of Vangl2, Vangl2C, was fused in-frame with Gal4 DNA binding domain (gal4-BD) and a cochlear cDNA was fused to Gal4 DNA activation domain (Gal4-AD). (B) Yeast cells were serially diluted and replicated on rich media YPDA (yeast media containing yeast extract, peptone, dextrose plus adenine) plates and minimal media plates lacking Leucine (L), Tryptophan (W), Histidine (H), Adenosine (Ade). The survival of cells on the minimum media depends on the presence of the two plasmids (Gal4-BD and Gal4-AD) and the expression of reporter genes. pGBTK7 plasmid contains Gal4-DB only while pVangl2-C contains Vangl2C fused to Gal4-BD.
The mouse Testin protein consists of a PET domain, a ∼110 amino acid motif found in the N-terminal regions of LIM domain proteins Prickle (Pk), Espinas, and Testin (PET), and three LIM domains (Bekman and Henrique, 2002; Katoh and Katoh, 2003). In mice, Testin shares homology with Pk1 and Pk2 (Katoh and Katoh, 2003). Pk1 and Pk2 are mammalian homologs of the Drosophila PCP protein Pk, which is known to interact with Drosophila core PCP proteins Van gogh and Dishevelled (Jenny et al., 2003; Jenny et al., 2005). Genetic analysis of Pk1 and Pk2, however, has not concluded essential roles of Pk in mammalian PCP processes, likely due to the overlapping functions of Pk1, Pk2, and other mammalian functional homologs. Testin shares the common PET and LIM domains with Pk1 and Pk2 (Katoh and Katoh, 2003), and has been shown to regulate gastrulation in Xenopus (Dingwell and Smith, 2006). In particular, the knocking down of Testin causes characteristic convergent extension defects in Xenopus (Dingwell and Smith, 2006). It is thought that Testin may act similarly or redundantly with Pk1 and Pk2 in vertebrate PCP signaling (Dingwell and Smith, 2006). To test whether Testin may be a mammalian protein involved in PCP processes and to confirm its interaction with Vangl2 as indicated by the yeast two-hybrid assay, we performed coimmunoprecipitation using extracts from cultured cells co-transfected with GFP-tagged Testin (GFP-Testin) and Ha-tagged Vangl2 (HA-Vangl2), but failed to mutually pull down either of the proteins.
It is possible that the co-immunoprecipitation conditions used do not accommodate the interaction between the two proteins in vitro. To further examine the potential interaction or association of Testin and Vangl2 as implicated by the 2-hybrid assay, we transfected IMCD3 cells with Testin-GFP and HA-Vangl2 and examined their localization in the cells (Fig. 2). When transfected alone, Testin showed a general distribution in the cells, with some membrane localization (Fig. 2A), while Vangl2 accumulated at the plasma membrane (Fig. 2B). In cells co-transfected with Vangl2 and Testin (Fig. 2D-F), Testin was enriched at the cellular contact where Vangl2 was also enriched. The localization of Testin at the cellular contacts was observed in 62% of the pairs of cells co-transfected with Vangl2 (N=37 pairs), compared with 18.9% of the pairs of cells transfected with Testin alone (N=37 pairs) (Fig. 2A, D-F). Furthermore, the signal for Testin-GFP at the cellular contacts of co-transfected cell pairs was drastically enhanced (100%) in comparison to that of the localization of Testin-GFP at the contacts of cells transfected with Testin-GFP alone (Fig. 2A, D). This result suggests that the cellular distribution of Testin can be affected by Vangl2 and is recruited by Vangl2 to cell-cell contacts.
Fig. 2.
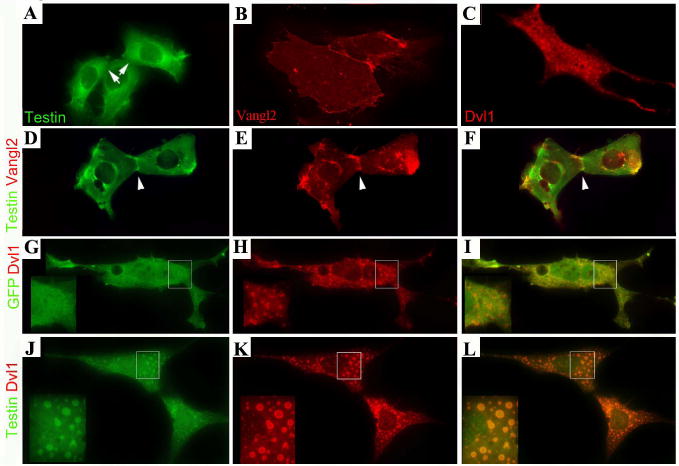
Testin is localized to the same subcellular compartments as Vangl2 and Dvl1.
(A-C) IMCD3 cells transfected with Testin-GFP (A), HA-Vangl2 (B), or HA-Dvl1 (C). The arrows (A) indicate the cellular boundaries of Testin-transfected cells. (D-F) IMCD3 cells transfected with Testin-GFP (green) and HA-Vangl2 (red). The arrowhead marks the cellular boundary with significantly enriched Testin signals in the presence of Vangl2. (G-I) IMCD3 cells transfected with GFP (green) and HA-Dvl1 (red). The inserts are the larger images of the boxed areas in each panel to better visualize the localization of Dvl1 to the vesicular structures and the uniform distribution of GFP in the cells. (J-L) IMCD3 cells transfected with Testin-GFP (green) and HA-Dvl1 (red). The inserts are the larger images of the boxed areas in each panel to visualize the enrichment of Testin-GFP to Dvl1-positive vesicles.
In addition to interacting with Vang proteins, Pk family members are also known to interact with Dishevelled proteins (Jenny et al., 2003; Jenny et al., 2005). We therefore also examined the localization of Testin-GFP and HA-tagged Dvl1 (HA-Dvl1) in IMCD3 cells (Fig. 2). As reported previously (Ahmad-Annuar et al., 2006; Terabayashi et al., 2009; Shafer et al., 2012), Dvl1 displayed a distinctive vesicular localization pattern in transfected cells (Fig. 2C). In cells co-transfected with GFP and HA-Dvl1 (Fig. 2G-I), GFP was uniformly distributed in the cells and was not enriched in the Dvl1-containing vesicles. In all of the cells (N>100 cells, 100%) co-transfected with Testin-GFP and Dvl1-HA, Testin-GFP was specifically re-localized to the vesicular structures consisting of Dvl1 (Fig. 2J-L), in contrast to cells transfected with only Testin-GFP (Fig. 2A) or cells co-transfected with GFP and Dvl1 (Fig. 2G-I). These data together indicate that Testin is specifically recruited by Dvl1.
Together, the data from the 2-hybrid assay and the localization experiments in cultured cells suggested that Testin could interact with Vangl2, and could be recruited to subcellular compartments by Vangl2 or Dvl1.
Testin is required for cellular patterning and interacts with Vangl2 genetically in regulating precisely coordinated orientation of hair cells in the cochlea
The findings of the recruitment of Testin to Dvl1 cellular structures and its enrichment to the same cellular contact by Vangl2 support the possibility that Testin may be involved in the same cellular processes as Dvl1 and Vangl2. To explore its potential role in PCP processes, we examined the expression of Testin in the cochlea during the establishment of PCP (Fig. 3), and accessed the loss-of-function of Testin in the inner ear and its genetic interaction with Vangl2 (Fig. 4).
Fig. 3.
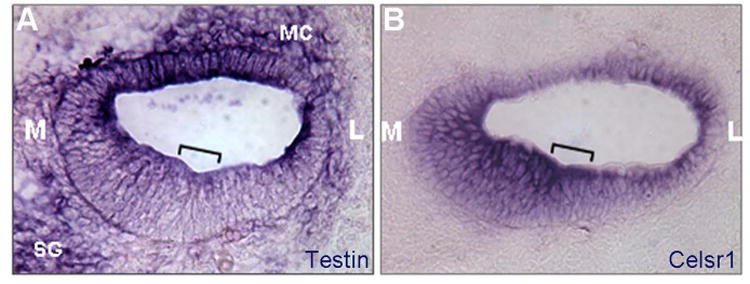
Testin is expressed in the inner ear during development.
(A-B) E14.5 cochlear sections probed for Testin (A) or Celsr1 (B). The bracket in each panel marks the developing organ of Corti. M: medial or center of the cochlear spiral; L: Lateral or periphery of the cochlear spiral; MC: mesenchyme cells; SG: spiral ganglion neurons.
Fig. 4.
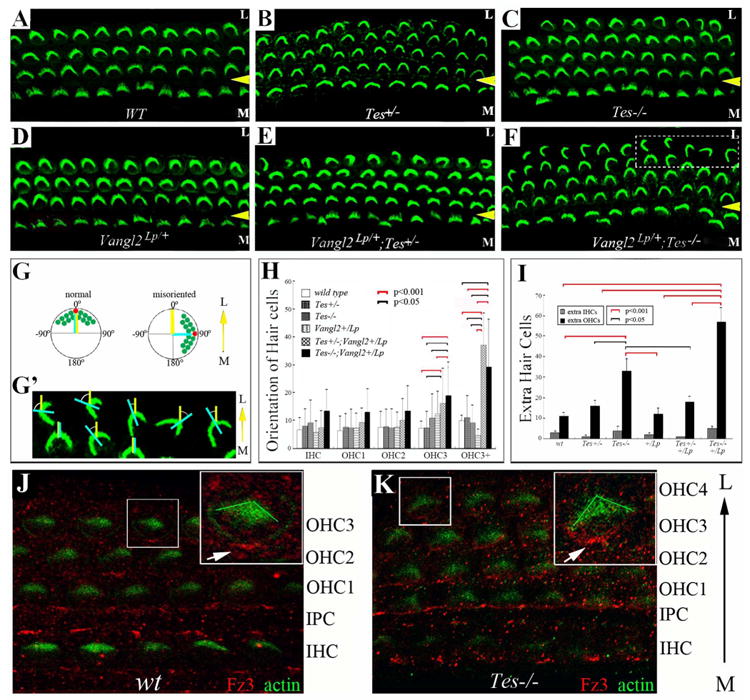
Testin is involved in cellular patterning and hair cell orientation in the cochlea.
(A-F) Cochlear whole mounts were prepared from wild type (WT) (A), Testin+/- (Tes+/-) (B), Testin-/- (Tes-/-) (C), Vangl2Lp/+ (D), Vangl2Lp/+;Tes+/- (E), and Vangl2Lp/+;Tes-/- (F) animals at P6. The samples were stained for phalloidin to visualize actin-rich stereocilia (green) of hair cells. The yellow arrowheads mark the outer pillar cell region that separates the inner (IHCs) from outer hair cells (OHCs). M: medial region of the cochlea duct; L: lateral region of the cochlear duct. The control organ of Corti consists of one row of inner and three rows of outer hair cells (A). Extra rows of outer hair cells were seen in the heterozygous and homozygous Testin mutants (B, C), as well as in Testin and Vangl2 compound mutants (E, F). (G-G′) Schematic diagrams illustrating the measurement of hair cell orientation. The yellow line in (G,G′) represents the medial-to-lateral radius of the cochlear spiral, and the light blue line in (G,G′) is the bisecting line of the V-shaped stereocilia bundle of hair cells. The angle formed between the yellow and light blue lines is the angle measured to represent the orientation of each hair cell. The measurement of the hair bundle orientation is illustrated in (G′) using hair cell images from the boxed area in (F). (H) The orientation of each row of hair cells from comparable mid-apical region of the cochlea was measured, quantified, and plotted. (I) Extra hair cells were counted and plotted for the genotypes included in (A-F). Pairs of groups with p value <0.005 for their difference were indicated by brackets in (H, I). The red and black brackets indicate the p values are less than 0.001 or 0.005, respectively (H,I). (J-K) Fz3 localization in wild type (J) and Testin-/- (Tes-/-) (K) cochleae at P0 was visualized using an antibody against Fz3.
We could not validate the specificity of the antibodies against Testin, and used in-situ hybridization (ISH) to assess the expression of Testin transcript in the developing cochlea (Fig. 3). The developing organ of Corti at E14-15, PCP gene Celsr1 was expressed in the entire cochlear epithelium, with higher expression levels observed in the region medial to the developing organ of Corti (Fig. 3B). Testin transcripts were detected in the cochlear epithelium, with higher levels seen in the region medial to the developing organ of Corti and in the roof of the cochlear duct (Fig. 3A). Testin transcripts were also observed in the mesenchyme cells surrounding the cochlear epithelium and in the spiral ganglion cells, in contrast to Celsr1 transcripts that were only seen in the cochlear epithelium (Fig. 3).
We further examined the cochleae isolated from postnatal day 6 (P6) control, Testin mutant (Drusco et al., 2005), and Testin and Vangl2 compound mice (Fig. 4). The looptail (Lp) mutation in Vangl2 gene causes the loss of function of Vangl2 in PCP signaling (Torban et al., 2004a; Torban et al., 2004b). The homozygous looptail mutant mice show characteristic PCP defects, including mis-orientation of hair cells and a shortened and widened sensory region with cellular patterning defects in the cochlea resulting from a CE defect (Montcouquiol et al., 2003). In the wild type control littermates at P6, hair cells are patterned into 4 rows along the entire length of the cochlear duct and oriented along the same direction, with the vertex of each hair bundle pointing from the medial (M) to the lateral (L) side of the cochlear spiral (Fig. 4A). In Testin+/- and Testin-/- animals, the orientation of the hair bundles does not show a statistically significant difference from that of the wild-type controls (Fig. 4B, C, and H). Cellular patterning in Testin+/- and Testin-/- animals from the mid-apical to apical region, however, appears abnormal in comparison to that of the wild-type controls (Fig. 4A-C). In several PCP mutants, defective CE causes shortening of the cochlear duct and consequently the widening of the tissue, with the appearance of extra rows of hair cells concentrated toward the apical region of the cochlear duct (Montcouquiol et al., 2003; Wang et al., 2005). The patterning defect of hair cells in Testin-/- mutants is statistically significant but only mildly so (Fig. 4I). The associated length reduction in core PCP mutants was not detected in Testin-/- mutants, likely due to the milder patterning defect and the fact that the sensitivity of the length measurement for a spiral structure could be influenced by variations in fixation and mounting of the tissue.
The additional loss of function of one copy of Vangl2 has a statistically significant effect on hair cell orientation in the Testin mutant background. Within the sensory region, the third row of outer hair cells in Testin-/-;Vangl2Lp/+ animals showed deviations in orientation in comparison to wild-type, Testin+/-, Testin-/-, and Vangl2Lp/+ littermates (Fig. 4A-H). Furthermore, a defect in hair cell orientation was observed in the extra row of hair cells in both the Testin+/-;Vangl2Lp/+ and Testin-/-;Vangl2Lp/+ animals (Fig. 4H).
PCP signaling in vertebrates involves a characteristically polarized enrichment of membrane associated protein complexes consisting of the core PCP proteins Fz3/6 and/or Vangl2, which presumably propagate the directional information across the tissue and coordinate polarity among neighboring cells. The membrane enrichment of the complexes is lost in core PCP mutants (Wang et al., 2005; Montcouquiol et al., 2006; Wang et al., 2006) but is maintained in mutants of other PCP genes, including Fuzzy and Inturned genes, which act downstream of the core PCP genes (Park et al., 2006; Heydeck et al., 2009; Dai et al., 2011), and cilia genes that act parallel to the core PCP genes (Jones et al., 2008). No quantitatively significant hair cell orientation defect in Testin-/- mutants was detected (Fig. 4C, H), which implies that core PCP proteins are likely localized normally in Testin-/- mutants. Indeed, the examination of core PCP protein Fz3 in the cochlea using immunostaining showed a characteristically asymmetric localization of the Fz3 protein in both the wild type control and in Testin-/- cochleae at E18.5 (Fig. 4J, K).
Together, the analysis revealed that Testin is required for cellular patterning and that there exists a genetic interaction between Testin and Vangl2 in the regulation of hair cell orientation. Furthermore, the examination of Fz3 localization in Testin-/- cochlea implicated that Testin may act downstream of core PCP genes in these processes.
Deletion of Testin leads to characteristic PCP defects in the vestibular sensory organs
In addition to the cochlea, the vestibular sensory organs in the inner ear also show coordinated hair cell orientations within each sensory organ. In the utricle and saccule, hair cells are oriented towards or opposing to each other across a line of polarity reversal (Deans et al., 2007). In the three cristae ampullaris, all of the hair cells are oriented in the same direction (Kelly and Chen, 2007). In several known PCP mutants, hair cell orientation defects in the vestibular organs are reported (Wang et al., 2006). In particular, the Vangl2Lp/+ animals show head bobbing, which suggests potential vestibular abnormalities. Posterior cristae isolated from Vangl2Lp/+ animals were smaller and the hair cells were misoriented (Wang et al., 2006).
We examined the vestibular organs isolated from Testin mutant mice (Figs. 5, 6). To visualize the orientation of hair cells in the vestibular organs, we used an antibody against α-Spectrin, which is enriched in the cuticular plate of hair cells excluding the region underneath the kinocilium, known as the fonticulus (Figs. 5, 6). Characteristic PCP defects were observed in the posterior crista and saccule in Testin-/- mice (Figs. 5, 6). In the posterior cristae from the wild type animals, all of the hair cells were oriented in the same direction along the PCP axis perpendicular to the long axis of the crista (Fig. 5A-C, G, H). In Testin-/- animals, hair cells were generally orientated in opposite directions on each of the two sides of the posterior crista, and hair cells within the same side of the posterior crista were not all oriented along the same direction (Fig. 5D-F, I).
Fig. 5.
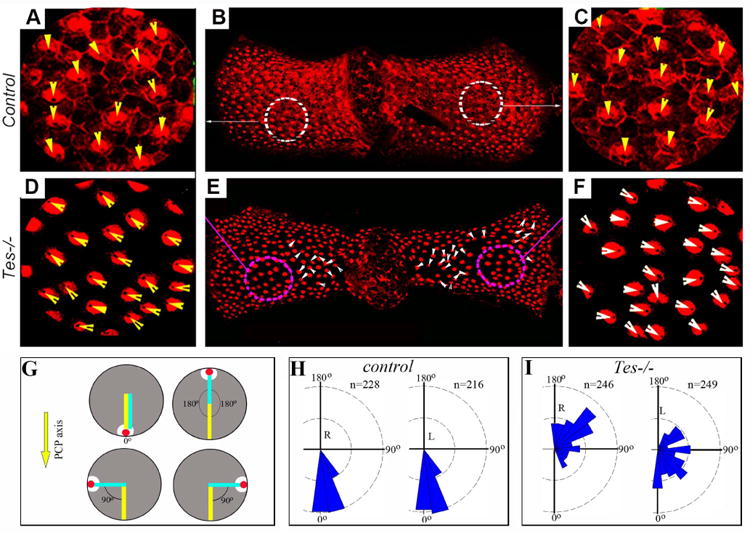
Testin is required for hair cell orientation in the posterior crista.
(A-F) Posterior cristae were prepared from control (A-C) and Testin-/- (Tes-/-) (D-F) animals and stained for an antibody against α-Spectrin. At the apical surface of the vestibular hair cells, the basal body region beneath the kinocilium is devoid of α-Spectrin staining. The arrowheads mark the orientation of hair cells. (G-I) A diagram for hair cell orientation measurement in the posterior crista (G) and plots of hair cell orientation in wild type control (H) and Testin-/- (I) posterior cristae. The red dot and the white area represent the position of the kinocilium and the fonticulus devoid of α-Spectrin staining in a hair cell, respectively (G). The orientation of hair cells from each side of the crista was plotted separately (H, I). 3 cristae for each genotype were used for quantification. R: right side; L: left side.
Fig. 6.
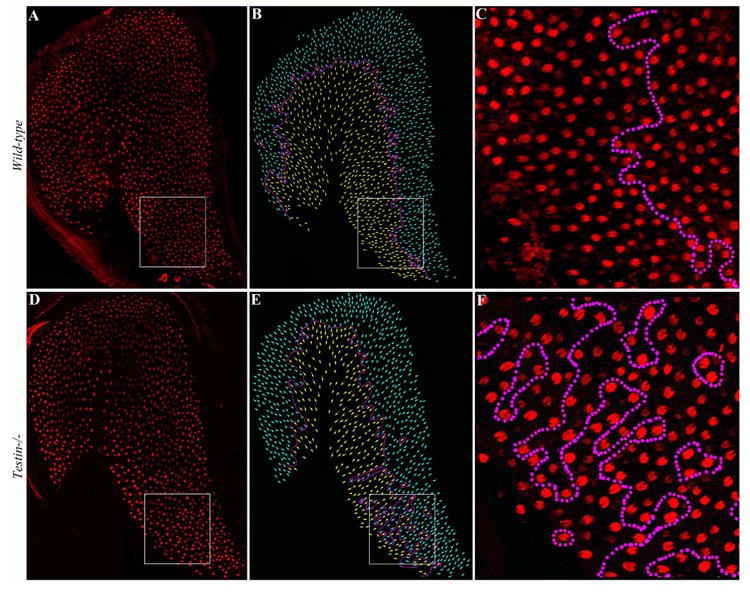
Testin is required for precisely coordinated orientation of hair cells in the saccule.
(A-F) Saccule whole mounts prepared from wild type control (A-C) and Testin-/- (Tes-/-) E18.5 mice (D-F) stained for α-Spectrin to visualize the orientation of hair cells. In the wild type saccule, the orientations of hair cells on either side of the line of polarity reversal are coordinated (A-C, the dotted magenta line). In the Testin-/- saccule (D-F), the orientation of hair cells is mostly coordinated along a distinct line of polarity reversal excluding one area (D, E, boxed region) where the line of polarity reversal is lost (F).
In the wild type saccule (Fig. 6), hair cells were oriented in opposite directions across the line of polarity reversal (Fig. 6A-C). In Testin-/- mutants (Fig. 6D-F), this line of polarity reversal was observed in most regions of the saccule but became disrupted at one region (Fig. 6E, F, n=3).
The observed hair cell mis-orientation in the vestibular organs from Testin-/- animals suggests that Testin is required for the regulation of the precisely coordinated hair cell orientation in the posterior crista and the saccule of the vestibule.
Testin genetically interacts with Vangl2 to regulate female reproductive tract (FRT) development
During the process of generating Testin and Vangl2 compound mutants to test the genetic interaction between Testin and Vangl2 in the cochlea, it was noted immediately that many of the females failed to breed. Upon further examination, we found that those animals did not have externally visible female reproductive tracts (FRT). The FRT in mice consists of bilateral ovaries, two oviducts (OT), two uteruses, a cervix, and a vagina that opens externally. Genotyping assays for the male-specific gene Sry were carried out and confirmed that those animals were genotypically females. We quantified this phenotype in Vangl2Lp/+, Testin-/-, Testin+/-; Vangl2Lp/+, and Vangl2Lp/+;Testin-/- female animals (Fig. 7A). The wild type (N=100) and Testin-/- (N=30) females do not have closed FRT (Fig. 7A, and data not shown). 7% of the Vangl2Lp/+ females (N=100) had a closed reproductive tract while 33% of the Testin+/-; Vangl2Lp/+ (N=30) or Testin-/-; Vangl2Lp/+ (N=30) animals had the same phenotype, indicating a strong genetic interaction of the two genes in the development of FRT.
Fig. 7.
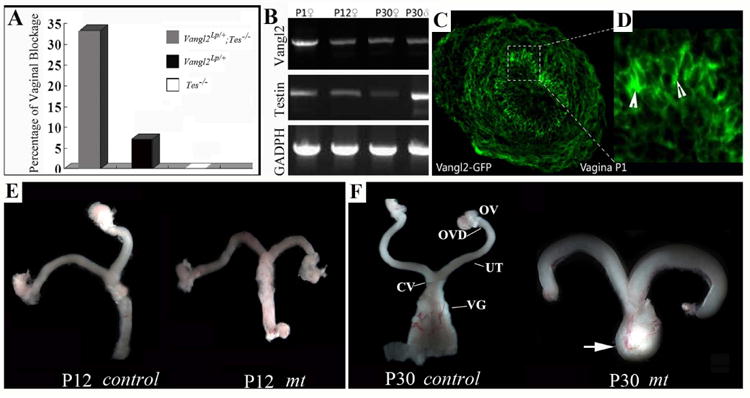
Testin genetically interacts with Vangl2 to regulate the normal development of the female reproductive tract in mice.
(A) Female mice without an externally visible female reproductive tract (FRT) were quantified and plotted. (B) The analysis of Testin and Vangl2 transcripts in FRTs from P1 to P30 and in Testis at P30. RT-PCR reactions were carried out to detect the transcripts. GADPH was used as a house-keeping gene control. (C,D) A cross section of the distal FRT from a postnatal day 1 (P1) animal shows the cellular localization of Vangl2-GFP. Vangl2 (green) was observed on the lateral side of the FRT epithelial cells (C,D). (E,F) Dissected female reproductive tracts from control and Vangl2Lp/+;Testin-/- mutant animals at P12 (E) and P30 (F). OV: ovary; OVD: Oviduct; UT: uterus; CV: cervix; VG: vagina.
We further examined the expression of Vangl2 and Testin transcripts in the FRTs of wild type controls, Vangl2 mutants, and Vangl2 and Testin compound mutants (Fig. 7B-F). Both Vangl2 and Testin RNA transcripts were detected in FRT from postnatal day 1 (P1) to P30 and the expression of both genes is higher at birth (Fig. 7B). Furthermore, Vangl2 protein is located to the lateral side of the FRT epithelial cells (Fig. 7C, D). The dissected FRTs from the control and mutant female animals appeared to be similar at postnatal day 12 (Fig. 7E). However, in contrast to the control females (N=100) in which FRT opens by P30, 1/3 of the Testin and Vangl2 compound mutant females (N=30 each for Testin+/-;Vangl2Lp/+ and Testin-/-;Vangl2Lp/+) and 7% of the Vangl2Lp/+ females (N=100) had a closed FRT at P30 (Fig. 7F). The FRTs in these females were greatly enlarged and filled with fluid (Fig. 7F).
Together, the data suggested that Testin and Vangl2 interact genetically to regulate the maturation or opening of the FRT in mice.
Conclusions and Discussion
Studies in Drosophila have identified a conserved genetic pathway that is required for various forms of PCP. In vertebrates, a similar set of genes, including Fz, Dvl, Vangl2, and Celsr1, regulate many PCP processes (Barrow, 2006). It appears that the vertebrate PCP pathway uses a similar mechanism of polarized sorting of membrane-associated complexes to propagate the polarity signal and to recruit intracellular effectors for morphological polarization (Jones and Chen, 2008). In particular, Vangl2 has emerged as a central player essential for all of the known PCP processes. However, the molecular mechanisms underlying the roles of Vangl2 and other conserved PCP genes in PCP signaling remain elusive. In this study, we identified Testin as an interacting protein with Vangl2 by a two-hybrid screen, and demonstrated that Testin is required in several processes involving known PCP genes.
The requirement for Testin in inner ear development
Testin is a structural homolog of Drosophila PCP protein Pk, consisting of a characteristic PET domain and three LIM domains (Bekman and Henrique, 2002; Katoh and Katoh, 2003). Moreover, a functional study in Xenopus implicated that Testin plays a role in gastrulation involving several PCP processes (Dingwell and Smith, 2006). We sought to determine whether Testin functions in mammalian PCP regulation after we identified Testin by its interaction with Vangl2 in the two-hybrid screen we carried out with Vangl2 C-terminal domain (Fig. 1).
The sensory organs in the inner ear display distinct forms of epithelial PCP. In addition, CE occurs during terminal differentiation of the cochlear epithelium (Wang et al., 2005). As a consequence, defective PCP signaling also causes hair cell patterning defects in a shortened cochlear duct (Montcouquiol et al., 2003; Wang et al., 2005). We examined the inner ears of Testin mutant mice to test whether Testin is essential for any PCP processes in the inner ear (Figs. 4-6), and to determine the genetic interaction between Vangl2 and Testin during inner ear PCP processes (Fig. 4).
Deletion of the third exon of the Testin gene leads to the elimination of the protein in homozygous knockout animals (Drusco et al., 2005). Depletion of Testin in the mouse inner ear causes two inner ear phenotypes: a patterning defect of cochlear hair cells and the mis-orientation of vestibular hair cells (Figs. 4-6). In Vangl2, Fz, or Dvl core PCP mutants, defective CE causes the shortening of the cochlear duct and widening of the sensory organ, or the appearance of extra rows of hair cells in a shortened cochlear duct (Montcouquiol et al., 2003; Wang et al., 2005). In cochleae from Testin-/- animals, the precise pattern of 4 rows of sensory hair cells was disrupted and extra hair cells were present (Fig. 4). This phenotype, albeit statistically significant in comparison to control animals, is still mild. The associated shortening of the cochlear duct in PCP mutants was not detected in Testin-/- animals. It is possible that the patterning defect is due to mild deregulation of CE in Testin-/- animals, and the quantification of extra hair cells is more sensitive than the measurement of minor changes in cochlear length, which could be affected by several factors in sample preparation. Alternatively, other potential roles of Testin may account for the patterning abnormalities observed in Testin-/- animals. It is noted that the patterning defect is limited to the mid-apical to apical region of the cochlear duct; similar to what has been observed in core PCP mutants (Montcouquiol et al., 2003; Wang et al., 2005). This may be the consequence of the differentiation gradient of the organ of Corti (Chen and Segil, 1999) and the extension of the cochlear duct from the base to the apex (Wang et al., 2005).
The mis-orientation of hair cells in the vestibular organs was distinct and conclusive of the role of Testin in regulating precisely coordinated hair cell orientation (Figs. 5, 6). In the posterior crista from Testin-/- animals, the uniform orientation of hair cells was disrupted (Fig. 5), while the organ was normal in appearance. In the saccules isolated from Testin-/- animals, the line of polarity reversal was consistently disrupted in one area (Fig. 6). These data together indicated that Testin plays a role in regulating hair cell orientation in the vestibule and implicated Testin's potential role in cochlear CE.
Notably, despite the patterning defect in the cochleae from Testin mutants, hair cell orientation appears to be normal in Testin mutants (Fig. 4B, C, and H). The lack of apparent cochlear hair cell orientation defects may be due to the overlapping roles of Pk homologs, and the different sensitivities of individual PCP processes to the loss of Testin in the cochlea. We crossed Testin mutant mice with Vangl2 looptail mutants to test whether the inactivation of another gene in the same pathway would reveal a masked requirement for Testin in regulating cochlear hair cell orientation (Fig. 4E, F). Indeed, cochlear hair cells showed orientation defects in Testin+/-;Vangl2Lp/+ and Testin-/-;Vangl2Lp/+ animals (Fig. 4).
Together, the data suggested that Testin is a mammalian gene involved in PCP processes in the inner ear, including coordinated hair cell orientation and CE.
Testin in female reproductive tract development
An important feature of mammalian development is the generation of sexually dimorphic reproductive tracts from the Müllerian and Wolffian ducts. In females, Müllerian ducts develop into the oviduct, uterus, cervix and vagina, whereas Wolffian ducts regress. A number of genes have been identified to be essential for FRT development, such as Lim1, Pax2, Emx2, Wnt4, Alk3, Hoxa10/11/13, P63, Wnt7a, Wnt5a (Mericskay et al., 2004), and Vangl2 (Vandenberg and Sassoon, 2009).
Among the genes known for FRT development, Vangl2 appears to be required for the polarization of epithelial cells in the FRT (Vandenberg and Sassoon, 2009), and Wnt5a (Mericskay et al., 2004) is considered to be a Wnt molecule for PCP signaling. The requirement of Vangl2 and Wnt5a in FRT development implicates the involvement of PCP signaling or a signaling cascade consisting of PCP genes. In this study, we found a genetic interaction between Vangl2 and Testin which enhances the vaginal blockage phenotype originally observed in VanglLp/+ females.
The potential role of Testin in vertebrate PCP signaling
The genetic interactions of Testin with Vangl2 and its role in regulating hair cell orientation and FRT development suggested Testin could be a mammalian gene involved in PCP processes in multiple tissues, including regulating the establishment of polarity features at the apical surface of the epithelial cells and cellular morphogenesis in epithelial tissues to create an opening in the tubular tissues.
Notably, Testin has been well known to be associated with actin cytoskeleton structures (Coutts et al., 2003; Garvalov et al., 2003; Griffith et al., 2005; Rotter et al., 2005; Boeda et al., 2007). Its biochemical properties and cellular functions suggest that Testin may be directly involved in cellular polarization during PCP signaling. Testin appears to have different conformations corresponding to its various localizations and associations (Zhong et al., 2009). It can bind to a variety of cytoskeleton proteins, including paxillin (Garvalov et al., 2003), talin, zyxin, VASP, Mena (Coutts et al., 2003; Griffith et al., 2005), EVH, and alpha-II–Spectrin (Rotter et al., 2005; Boeda et al., 2007), and also regulates a plethora of cell behaviors. In rat and chicken fibroblasts, Testin is associated with actin stress fibers and promotes cell spreading on fibronectin (Coutts et al., 2003). In Hela cells, Testin is localized to focal adhesions and regulates the actin cytoskeleton (Garvalov et al., 2003; Griffith et al., 2005). Our cellular localization data (Fig. 2) showed that Testin has a general cytoplasmic localization, and its membrane enrichment is greatly enhanced when Vangl2 is over-expressed and can be recruited to Dvl1 domains (Fig. 2). It is possible that Testin shares an overlapping role with Pk in establishing and propagating the polarity signal across a tissue. Given the extensive evidence of Testin association with actin structures, Testin may concurrently act as a link between membranous PCP complexes and the actin cytoskeleton, allowing it to regulate cellular morphogenesis. Conversely, Pk genes may also play a role in directly linking membranous PCP complexes with the actin cytoskeleton. Growing evidence has indicated a tumor suppressor role for Testin for loss of cell polarity when Testin levels are abolished or reduced (Drusco et al., 2005; Zhu et al., 2012), consistent with its role in directly regulating cytoskeleton polarization.
In summary, our current study showed that Testin is involved in the coordinated orientation of hair cells in the inner ear and regulates FRT development. The interaction of Testin with known PCP protein Vangl2 along with its well-established association with actin cytoskeleton structures suggested a role for Testin in linking the membranous PCP complexes with actin cytoskeletons in regulating cellular morphological polarization.
Experimental Procedures
Mouse strains and animal care
Animal care and use was in accordance with US National Institutes of Health (NIH) guidelines and was approved by the Animal Care and Use Committee of Emory University. LPT/Le mice or Vangl2 looptail mutant mice were obtained from the Jackson Laboratories (Torban et al., 2004a; Torban et al., 2004b). Vangl2–green fluorescent protein (GFP) transgenic mice were generated as reported (Qian et al., 2007). Testin mutant mice were gifts from Drusco and colleagues (Drusco et al., 2005). Vangl2 looptail mutant and Testin mutant strains were maintained on B6/129 background.
Yeast two-hybrid analysis
We utilized the Matchmaker Gal4 yeast two-hybrid system (Clontech, USA) and a cDNA library from embryonic day 15 mouse cochlear epithelia constructed in Gal4-AD (Li et al., 2011). A 283-aa C-terminal cytoplasmic tail of Vangl2 served as the bait. The plasmid pGal4-BD/Vangl2C alone with the empty Gal4-AD vector does not activate reporter expression. Yeast two-hybrid assays were performed as described in the Clontech protocol. We co-transfected pGal4-BD/Vangl2C and the cDNA library DNA into yeast reporter cells and screened for colonies that grew on histidine- and adenine-deficient plates and turned blue under the conditions for α-galactosidase (α-gal) and β-gal assays.
Plasmid construction, cell culture and plasmid transfection
HA-tagged Vangl2 (HA-Vangl2) and GFP-tagged Testin (Testin-GFP) were used for cell cultures. Dvl1-HA was a gift from Dr. Gerd Walz (Germany). IMCD3 cells were used for transfection and co-localization experiments.
Inner ear dissection, immunostaining, and imaging
Inner ear dissection, staining and imaging were performed as described previously (Jones et al., 2008). Briefly, the inner ears were isolated from mice at various developmental stages, fixed in 4% paraformaldehyde overnight at 4°C. The sensory organs were further dissected and subjected to staining. Rhodamine or Alexa-Fluor-488 conjugated phalloidin dyes (Invitrogen, 1:1000) were used to stain actin-rich structures such as stereocilia bundles and the cuticular plate of hair cells. An anti-α-Spectrin antibody (1:200, Chemicon, MAB1622) was used to depict the polarity of vestibular hair cells by revealing the eccentric position of the kinocilium. An antibody against Fz3 (a gift from J. Nathans, Johns Hopkins University, Maryland, USA) was used to visualize the subcellular localization of membrane associated PCP complexes. The following microscopes were used for image acquisition: Olympus SZX12 upright microscope and Zeiss LSM510 confocal microscope.
Quantification of hair cell orientation and statistical analysis
The number of inner or outer hair cells in each cochlea was counted by scoring phalloidin+ hair bundles. To determine stereocilia bundle orientation in the cochlea, we drew a line from the vertex of the “V”-shaped stereocilia bundle through the middle of the “V”-shaped stereocilia (bisecting line). The angle formed between this line and the line parallel to the diagonal or the mediolateral axis of the cochlea spiral was measured and used for quantifications. In wild type animals, this angle is close to 0°. For histogram plots of distribution of the angles, the angles form at the right side or the left side of the line representing mediolateral axis are designated to be positive (+) or negative (−), respectively. For quantification of the average deviation from the normal orientation (angle=0°), the angles were quantified without positive (+) or negative (−) designations to avoid cancellation of the angle deviations. At least 50 hair cells in each row at the medial-apical regions were quantified for each sample. For average deviations, the absolute rotation away from the 0° orientation was determined regardless of direction. The measurement of hair cell angels in the crista was performed as diagramed in figure 5.
Data collected from each experimental group with at least 3 samples per genotype are expressed as mean ± S.E.M. SPSS (13.0) was used for statistic analyses.
Histology of female reproductive tracts
Female reproductive tracts (FRT) were isolated from postnatal day 1 (P1), P12, and P30 animals, fixed in 4% paraformaldehyde in PBS at 4°C overnight for imaging.
Salient features of the manuscript.
Identified Testin as a Vangl2-interacting protein in a 2-hybrid screen;
Enrichment of Testin in PCP protein-containing compartments in culture cells;
Genetic inactivation of Testin in mice leads to misorientation of hair cells in the vestibule;
Testin interacting with Vangl2 genetically to coordinate hair cell orientation in the cochlea;
Testin is required for precise patterning of the organ of Corti;
Testin interacting with Vangl2 genetically during female reproductive tract development in mice
Acknowledgments
We would like to thank Lanbo Deng (Shanghai, China) for quantification of hair cell orientation, Dr. Nathans (Johns Hopkins University) for Fz3 antibody, and Anne Lin (Johns Hopkins University) for editing the manuscript. This study is supported by NIH research grants RO1 DC007423 and DC005213 to P. C., NSFC grant 81028003/H1305 to P. C. and F. C., NSFC grant 81000413/H1305 to D.R., International Cooperation projects- of Shanghai Science and Technology Committee (10410700800) to F.C and P.C., Shanghai Rising-Star Program (A type) 11QA1401100 to D.R., Key Basic Research Project of the Science and Technology Commission of Shanghai Municipality (10JC1402500) to F.C. This research was supported in part by the Neuronal Imaging Core of the Emory Neuroscience NINDS Core Facilities grant P30NS055077.
Grant support information:
NIH/NIDCD RO1 DC007423
NIH/NIDCD RO1 DC005213
HFSP RGP0012-2012
NIH/NINDS Core Facilities grant P30NS055077
References
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Armstrong A, Ryu YK, Chieco D, Kuruvilla R. Frizzled3 is required for neurogenesis and target innervation during sympathetic nervous system development. J Neurosci. 2011;31:2371–2381. doi: 10.1523/JNEUROSCI.4243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Gordon L, Donn TM, Berti C, Moens CB, Burden SJ, Granato M. A novel role for MuSK and non-canonical Wnt signaling during segmental neural crest cell migration. Development. 2011;138:3287–3296. doi: 10.1242/dev.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow JR. Wnt/PCP signaling: A veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol. 2006;17:185–193. doi: 10.1016/j.semcdb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Bekman E, Henrique D. Embryonic expression of three mouse genes with homology to the Drosophila melanogaster prickle gene. Mech Dev. 2002;119(Suppl 1):S77–81. doi: 10.1016/s0925-4773(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Berger-Muller S, Suzuki T. Seven-pass transmembrane cadherins: roles and emerging mechanisms in axonal and dendritic patterning. Mol Neurobiol. 2011;44:313–320. doi: 10.1007/s12035-011-8201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeda B, Briggs DC, Higgins T, Garvalov BK, Fadden AJ, McDonald NQ, Way M. Tes, a specific Mena interacting partner, breaks the rules for EVH1 binding. Mol Cell. 2007;28:1071–1082. doi: 10.1016/j.molcel.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AS, MacKenzie E, Griffith E, Black DM. TES is a novel focal adhesion protein with a role in cell spreading. J Cell Sci. 2003;116:897–906. doi: 10.1242/jcs.00278. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dai D, Zhu H, Wlodarczyk B, Zhang L, Li L, Li AG, Finnell RH, Roop DR, Chen J. Fuz controls the morphogenesis and differentiation of hair follicles through the formation of primary cilia. J Invest Dermatol. 2011;131:302–310. doi: 10.1038/jid.2010.306. [DOI] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- Deans MR, Antic D, Suyama K, Scott MP, Axelrod JD, Goodrich LV. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J Neurosci. 2007;27:3139–3147. doi: 10.1523/JNEUROSCI.5151-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell KS, Smith JC. Tes regulates neural crest migration and axial elongation in Xenopus. Dev Biol. 2006;293:252–267. doi: 10.1016/j.ydbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Drusco A, Zanesi N, Roldo C, Trapasso F, Farber JL, Fong LY, Croce CM. Knockout mice reveal a tumor suppressor function for Testin. Proc Natl Acad Sci U S A. 2005;102:10947–10951. doi: 10.1073/pnas.0504934102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvalov BK, Higgins TE, Sutherland JD, Zettl M, Scaplehorn N, Kocher T, Piddini E, Griffiths G, Way M. The conformational state of Tes regulates its zyxin-dependent recruitment to focal adhesions. J Cell Biol. 2003;161:33–39. doi: 10.1083/jcb.200211015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith E, Coutts AS, Black DM. RNAi knockdown of the focal adhesion protein TES reveals its role in actin stress fibre organisation. Cell Motil Cytoskeleton. 2005;60:140–152. doi: 10.1002/cm.20052. [DOI] [PubMed] [Google Scholar]
- Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci. 2009;29:15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Chaudhry B. Getting to the heart of planar cell polarity signaling. Birth Defects Res A Clin Mol Teratol. 2011;91:460–467. doi: 10.1002/bdra.20792. [DOI] [PubMed] [Google Scholar]
- Heydeck W, Zeng H, Liu A. Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev Dyn. 2009;238:3035–3042. doi: 10.1002/dvdy.22130. [DOI] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. Embo J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Jones C, Chen P. Chapter eight primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol. 2008;85:197–224. doi: 10.1016/S0070-2153(08)00808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Identification and characterization of human PRICKLE1 and PRICKLE2 genes as well as mouse Prickle1 and Prickle2 genes homologous to Drosophila tissue polarity gene prickle. Int J Mol Med. 2003;11:249–256. [PubMed] [Google Scholar]
- Kelly M, Chen P. Shaping the mammalian auditory sensory organ by the planar cell polarity pathway. Int J Dev Biol. 2007;51:535–547. doi: 10.1387/ijdb.072344mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Li S, Esterberg R, Lachance V, Ren D, Radde-Gallwitz K, Chi F, Parent JL, Fritz A, Chen P. Rack1 is required for Vangl2 membrane localization and planar cell polarity signaling while attenuating canonical Wnt activity. Proc Natl Acad Sci U S A. 2012;108:2264–2269. doi: 10.1073/pnas.1013170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001a;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Rachel RA, Shah S, Beermann F, Stanier P, Mason CA, Copp AJ. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics. 2001b;78:55–63. doi: 10.1006/geno.2001.6638. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Paudyal A, Damrau C, Patterson VL, Ermakov A, Formstone C, Lalanne Z, Wells S, Lu X, Norris DP, Dean CH, Henderson DJ, Murdoch JN. The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC Dev Biol. 2010;10:87. doi: 10.1186/1471-213X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter B, Bournier O, Nicolas G, Dhermy D, Lecomte MC. AlphaII-spectrin interacts with Tes and EVL, two actin-binding proteins located at cell contacts. Biochem J. 2005;388:631–638. doi: 10.1042/BJ20041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell. 2012;20:177–191. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Sato M, Tabata T. The Wnt5/planar cell polarity pathway regulates axonal development of the Drosophila mushroom body neuron. J Neurosci. 2011;31:4944–4954. doi: 10.1523/JNEUROSCI.0154-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe CW, Lu X. Kif3a regulates planar polarization of auditory hair cells through both ciliary and non-ciliary mechanisms. Development. 2011;138:3441–3449. doi: 10.1242/dev.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabayashi T, Funato Y, Fukuda M, Miki H. A coated vesicle-associated kinase of 104 kDa (CVAK104) induces lysosomal degradation of frizzled 5 (Fzd5) J Biol Chem. 2009;284:26716–26724. doi: 10.1074/jbc.M109.039313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Planar cell polarity signaling in neural development. Curr Opin Neurobiol. 2010;20:572–577. doi: 10.1016/j.conb.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Topczewski J, Dale RM, Sisson BE. Planar cell polarity signaling in craniofacial development. Organogenesis. 2011;7:255–259. doi: 10.4161/org.7.4.18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torban E, Kor C, Gros P. Van Gogh-like2 (Strabismus) and its role in planar cell polarity and convergent extension in vertebrates. Trends Genet. 2004a;20:570–577. doi: 10.1016/j.tig.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004b;279:52703–52713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Vandenberg AL, Sassoon DA. Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via van gogh-like 2. Development. 2009;136:1559–1570. doi: 10.1242/dev.034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LL, Dean CH. Planar polarity: A new player in both lung development and disease. Organogenesis. 2011;7:209–216. doi: 10.4161/org.7.3.18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhu J, Wang Y, Zhou J, Ren K, Ding X, Zhang J. LIM domain protein TES changes its conformational states in different cellular compartments. Mol Cell Biochem. 2009;320:85–92. doi: 10.1007/s11010-008-9901-7. [DOI] [PubMed] [Google Scholar]
- Zhu J, Li X, Kong X, Moran MS, Su P, Haffty BG, Yang Q. Testin is a tumor suppressor and prognostic marker in breast cancer. Cancer Sci. 2012;103:2092–2101. doi: 10.1111/cas.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]


