Abstract
Background:
Sarcoidosis is a systemic granulomatous disease of unknown etiology. Lungs and lymphatics are the principal sites affected by this disease. The disorder is often not suspected by physicians.
Materials and Methods:
This was a retrospective study done on 140 transbronchial lung biopsies received for histopathological examination in the Department of Pathology for 1 year in a multispeciality tertiary care hospital, in Delhi.
Results:
Out of 140 transbronchial lung biopsies studied, 13 cases of sarcoidosis were diagnosed histopathologically. In these patients a clinical, pathological, and radiological corelation was done. And a final diagnosis of sarcoidosis was given after excluding other granulomatous lesions.
Conclusion:
Transbronchial lung biopsies have become an important tool in the diagnosis of sarcoidosis in present time. Hence sarcoidosis should be considered as a differential diagnosis when dealing with granulomatous lesions in lung biopsies.
KEY WORDS: Granulomatous lesion, sarcoidosis, transbronchial lung biopsies
INTRODUCTION
Sarcoidosis is a multisystem disorder of unknown etiology. Although prevalent at all ages, in India it predominantly occurs during fourth to fifth decade of life.[1] The most common organs affected are lungs, eyes, and skin.[2] Pulmonary involvement is the commonest.[3] Patients with pulmonary disease, complain of nonproductive cough, dyspnea, chest pain, and wheezing.[2] Diagnosis of pulmonary sarcoidosis relies on the presence of noncaseating granulomas on histopathological specimens and clinicoradiological corelation. But other entities like mycobacterial and fungal infections, occupational diseases like chronic beryllium disease and pneumoconiosis, hypersensitivity alveolitis, and Wegener's granulomatosis have to be excluded. The disease is often self-limiting but sometimes it presents with multiorgan involvement and can even be life threatening. This disease has been under reported in India. In this study, we have emphasized that sarcoidosis is an important entity in differential histopathological diagnosis in transbronchial lung biopsy specimens.
MATERIALS AND METHODS
This was a retrospective study done on 140 transbronchial lung biopsies received in the Department of Pathology for 1 year in a multispeciality tertiary care Hospital, in Delhi. Histopathological examination of the biopsies was done. The biopsies showing non caseating granuloma were studied. Besides H and E staining, special stains for fungi like Grocotts and PAS and ZN staining for tuberculosis was done. Clinical history, radiological findings and other data were analyzed. A clinicopathological and radiological corelation was carried out. A strict histopathological criteria for diagnosis of sarcoidosis was followed. The important diseases that can produce similar histopathological appearances were excluded.
RESULTS
Out of 140 transbronchial lung biopsies studied, 37 cases had malignancies, 10 had tuberculosis, and 18 had other benign lung lesions like pneumonia, bronchitis, and pnemonitis. A total of 62 biopsies were inconclusive due to small size of biopsies and in 13 cases noncaseating epitheloid granulomas were seen with no peripheral rimming of lymphocytes [Figure 1]. The granulomas were composed of tightly clustered epithelioid cells, often with multinucleate giant cells [Figure 2]. These granulomas were seen either as single or discrete lesion or as confluent masses [Figure 3]. They were seen in the bronchial mucosa. Some of the granulomas showed hyalinization. ZN staining for tuberculosis and PAS and Grocotts stain for fungal profile were negative.
Figure 1.
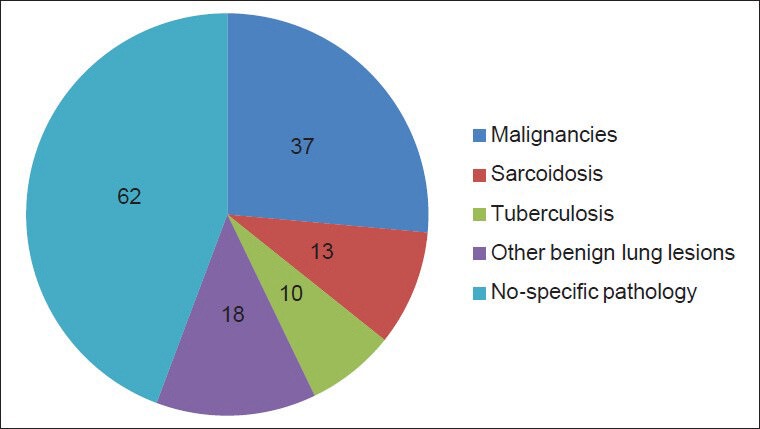
Pie chart showing distribution of different lung lesions in 140 transbronchial lung biopsies specimen included in this study
Figure 2.
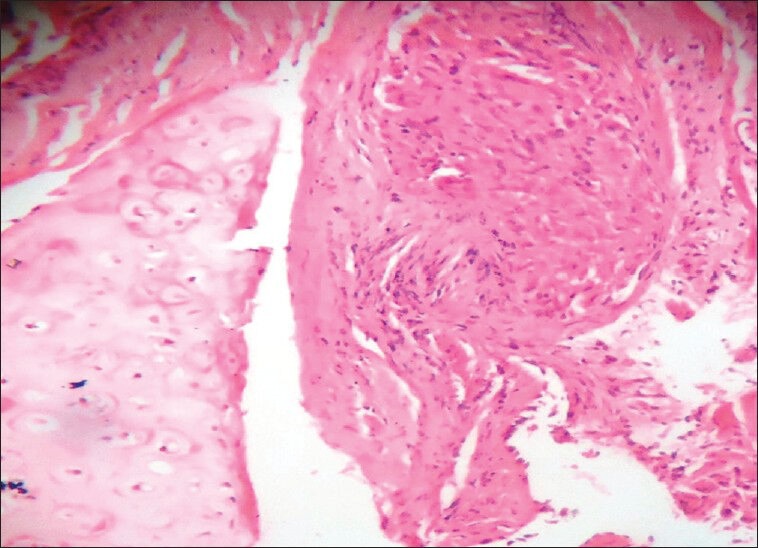
Histopathology of pulmonary sarcoid granulomas composed of noncaseating granulomas without peripheral rimming of lymphocytes [at higher magnification]
Figure 3.
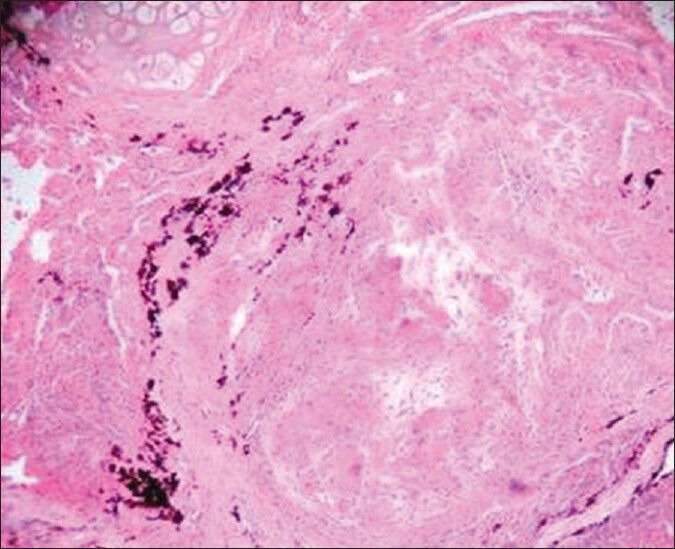
Histopathology of Pulmonary sarcoidosis – Numerous large epitheloid granulomas [at lower magnification]
In these patients a clinical, pathological, and radiological corelation was done and a final diagnosis of sarcoidosis was given. Out of 13 patients, 8 patients belonged to 30-50 years age group, 4 patients were in 51-60 years age group, and 1 patient was <30 years of age. There was a female preponderance, 9 patients out of 13 patients were females [Table 1].
Table 1.
Age and sex distribution of patients with sarcoidosis

DISCUSSION
Sarcoidosis, also called sarcoid, Besnier-Boeck disease, or Besnier-Boeck-Schaumann disease, is characterized by granulomas that can form nodules in multiple organs. Although the etiology of sarcoidoisis remains unknown, several lines of evidence suggest that it is a disorder of immune regulation in genetically predisposed individuals exposed to certain environmental agent's.[4] It is more common in males in India, but there is a slight female predominance in rest of the world.[1] Sarcoidosis was found to be more common in females in our study. Similar observation was made by Singh et al.[5] Majority of the Indian patients present it in fourth to fifth decade of life. In India, sarcoidosis is an under-diagnosed disease. Among the few earlier studies reported, sarcoidosis constituted 10-12/1000 new registration annually at Respiratory Unit at Kolkata and 61.2/100,000 new cases at VPCI.[6] In our study, we have tried to find out prevalence of sarcoidosis cases from all transbronchial lung biopsies over a year and the prevalence of sarcoidosis was found to be 13/140 (9.1%) biopsies.
The characteristic radiological findings in patients with pulmonary sarcoidosis are bilateral hilar lymphadenopathy.[7] Other findings include reticulonodular infiltrates, pulmonary infiltrates, and extensive fibrocystic changes and scarring causing honey combing. In our study, the characterized X-ray finding was of bilateral hilar adenopathy, pulmonary infiltratres, and presence of nodular opacities.
The salient histopathological findings of sarcoidosis were presence of noncaseating granulomas. These granulomas tend to be most prevalent around bronchovascular and the fibrous septae containing pulmonary veins. The lymphangitic distribution is very characteristic of sarcoidosis.[7] Granulomas may be seen as single discrete lesions or as confluent masses. As our study was on small transbronchial lung biopsies specimen, the granulomas were seen mainly near the bronchial mucosa and interstitium and the characteristic lymphovascular pattern could not be so well appreciated. These granulomas were tight, well-formed granulomas, nonnecrotizing and naked granulomas.
Inclusions frequently seen in giant cells are asteroid bodies, schaumann/conchoids bodies and calcium oxalate crystals, these findings are nonspecific and not diagnostic of sarcoidosis.[8] In our study, due to small size of biopsies only conchoidal bodies were seen in some of biopsies.
The common disease, which had to be excluded, was tuberculosis. Pulmonary tuberculosis usually shows areas of caseation necrosis, in which ZN staining for AFB is positive, in our cases both the findings were negative. Other entities to be excluded were fungal infections, Wegeners granulomatosis, puemocoinosis and entities like hypersensitivity alveolitis. PAS and Grocott stain for fungal elements were negative in our biopsies.
Wegeners granulomatosis of lungs is characterized with liquefactive and/or coagulative necrosis, large number of eosinophils, scanty lymphocytes, and plasma cells. Well-formed granulomas are rare here.[9] Leucocytoclastic angitis is another important feature. In our cases there were granulomas but no necrosis, or plasma cells or eosinophils. Thus this entity was excluded.
Occupational diseases like pnuemoconiosis and hypersensitivity alveolitis were excluded with a relevant history of occupational exposure, environmental exposure, and medical history.
Thus the diagnosis of sarcoidosis is based on a compatible clinical, radiological findings and histopathological evidence of noncaseating granuloma in tissue biopsy specimens, and exclusion of all other diseases that produce a similar picture. Other diagnostic modalities are estimation of serum ACE activity,[1] serum calcium, and gallium scanning, but these investigations are not commonly done.
For asymptomatic patients, no treatment is required. In symptomatic patient's corticosteroids have been the mainstay of treatment.[10] In patients with severe disease methotrexate, azathothioprine and TNF- α are effective.
CONCLUSION
The prevalence of pulmonary sarcoidosis in India is much more than that has been reported. Due to lack of awareness and nonavailability of investigations for diagnosis, the disease was under reported in the past. Transbronchial lung biopsies have become an important tool in the diagnosis of sarcoidosis in present time and it has brought out those cases, which were not diagnosed previously. Hence this entity should be considered when reporting transbronchial lung biopsies and differentiated from all granulomatous lesions. Exclusion of tuberculosis to diagnose sarcoidosis is important in particular because corticosteroids form the mainstay of treatment for sarcoidosis. Early diagnosis of sarcoidosis definitely has a good prognosis. Thus biopsy is a necessary diagnostic modality for diagnosis of pulmonary sarcoidosis before treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sharma SK, Mohan A. Sarcoidosis in India: Not So Rare! Journal Indian Academy of Clinical Medicine] 2004;5:12–21. [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 3.Wu JJ, Schiff KR. Sarcoidosis. Am Fam Physician. 2004;70:312–22. [PubMed] [Google Scholar]
- 4.Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361:1111–8. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 5.Singh RB, Babu KS. Pulmonary sarcoidosis in a south Indian hospital: Clinical and lung function profile. Indian J Chest Dis Allied Sci. 1999;41:145–51. [PubMed] [Google Scholar]
- 6.Gupta SK. Proceedings of the symposium Delhi: VP Chest Institute; 2002. Epidemiology of Sarcoidosis; pp. 27–31. [Google Scholar]
- 7.Criado E, Sánchez M, Ramírez J, Arguis P, de Caralt TM, Perea RJ, et al. Pulmonary sarcoidosis: Typical and Atypical manifestations at High-Resolution CT with Pathologic Correlation. Radiographics. 2010;30:1567–86. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 8.Rosen Y. Pathology of Sarcoidosis. Semin Respir Crit Care Med. 2007;28:36–52. doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 9.Heffner DK. Wegener's granulomatosis is not a granulomatous disease. Ann Diag Pathol. 2002;6:329–3. doi: 10.1053/adpa.2002.35752. [DOI] [PubMed] [Google Scholar]
- 10.Baughmann RP. Therapautic options for sarcoidosis: New and old. Curr Opin Pulm Med. 2002;8:464–9. doi: 10.1097/00063198-200209000-00021. [DOI] [PubMed] [Google Scholar]


