Abstract
Stereotactic body radiation therapy (SBRT) is a novel form of external beam radiation therapy. It is used to treat early and locally recurrent nonsmall cell lung cancer (NSLC) in medically inoperable patients. It uses high dose, hypofractionated radiotherapy, with targeting of the tumor by precise spatial localization, thus minimizing injury to surrounding tissues. It can be safely used to ablate NSLC in both central and peripheral locations. We present two cases of delayed esophageal perforation after SBRT for locally recurrent central NSLC. The perforations occurred several months after the therapy. They were treated with covered esophageal stents, with mortality, due to the perforation in one of the patients. SBRT should be judiciously used to ablate centrally located NSLC and patients who develop episodes of esophagitis during or after SBRT, need to be closely followed with endoscopy to look for esophageal ulcerations. These ulcers should be closely followed for healing as these may degenerate into full thickness perforations several months after SBRT.
KEY WORDS: Covered esophageal stents, esophageal perforation, lung cancer, stereotactic body radiation therapy
INTRODUCTION
Stereotactic body radiation therapy (SBRT) is a novel form of external beam radiation therapy and is used to treat early and locally recurrent Non-small cell lung cancer (NSLC), in otherwise medically inoperable patients.[1,2,3] It uses high dose, hypo-fractionated radiotherapy with targeting of the tumor by precise spatial localization, thus minimizing injury to surrounding tissues. It has been safely used to ablate NSLC in both central (defined as a lesion within 2 cm of the main stem bronchus) and peripheral locations.[1,3] Esophageal toxicity is particularly concerning complication when SBRT is used to ablate lesions in central locations. Esophageal lesions commonly occur in the form of mild to severe esophagitis and rarely as esophageal ulcerations or trachea-esophageal fistulas.[4,5] We present two cases of delayed esophageal perforation after SBRT for locally recurrent central NSLC.
CASE REPORT
An 85-year old male with medical history of hypertension, type-2 diabetes, and coronary artery disease underwent a right lower lobectomy for a T2bN0M0 Stage 1b squamous cell carcinoma of the lung. After 1 year, the patient developed a local recurrence adjacent to the inferomedial aspect of the right upper lobe. A PET scan performed showed no evidence of disease elsewhere. The patient was considered unfit for a completion pneumonectomy. The recurrence was treated by SBRT using a Cyber knife Stereotactic Radiosurgery System (Accuracy, Sunnyvale, CA) with a prescription dose of 50 Gy in five fractions given every other day. Five months after the SBRT, the patient presented acutely with an empyema of the right chest. Flexible bronchoscopy showed a well-healed lower lobe bronchus. A barium swallow showed an extravasation of the oral contrast into the right pleural space [Figure 1]. An upper gastrointestinal endoscopy revealed a single 2.5 cm perforation in the distal thoracic esophagus and no tumor ingrowth. The patient was treated with a Wall flex fully covered esophageal stent (Boston Scientific, Natick, MA), sealing the esophageal perforation [Figure 1]. The patient also needed an open drainage of his right chest. However, the patient did not do well and succumbed to the esophageal perforation.
Figure 1.
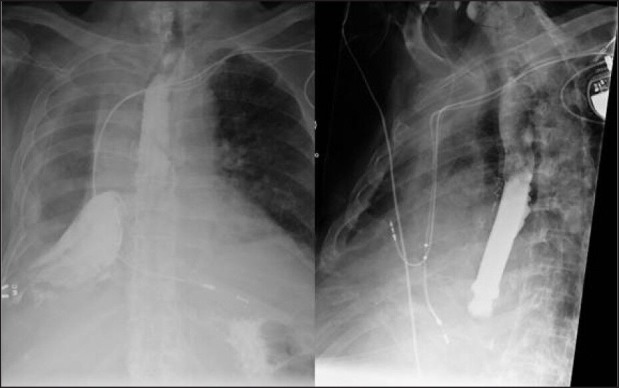
Barium swallow showing extravasation of oral contrast into the right pleural cavity due to an esophageal perforation and treatment of the perforation with a covered esophageal stent
A 73-year old female with medical history of hypertension underwent a right lower lobectomy for a T1N0M0 Stage 1A adenocarcinoma of the lung. After 4 years, the patient developed a new primary in the right upper lobe. A completion pneumonectomy and four cycles of adjuvant chemotherapy with cisplatin and paclitaxel were given for this T2N2M0 stage 3 adenocarcinoma of the right upper lobe. The patient did well for 2 years, after which she developed a regional recurrence near the right main stem bronchus stump. The patient was given SBRT to the recurrence site with a Cyber knife Stereotactic Radiosurgery System (Accuracy, Sunnyvale, CA), with a prescription dose of 50 Gy in five fractions given every other day. Seven months after SBRT, the patient presented with a hydropneumothorax in the postpneumonectomy space on routine surveillance chest x-ray. Bronchoscopy showed a well-healed bronchial stump. The CT scan of the chest showed oral contrast extravasation into the right pneumonectomy space [Figure 2]. An upper esophagogastroscopy confirmed two esophageal perforations at 22 and 37 centimeters from the incisors. Both were treated with a Wall flex fully covered esophageal stents (Boston Scientific, Natick, MA), and the right chest was drained with a tube thoracotomy. The patient did well, with retrieval of the stent at 5 weeks post placement.
Figure 2.
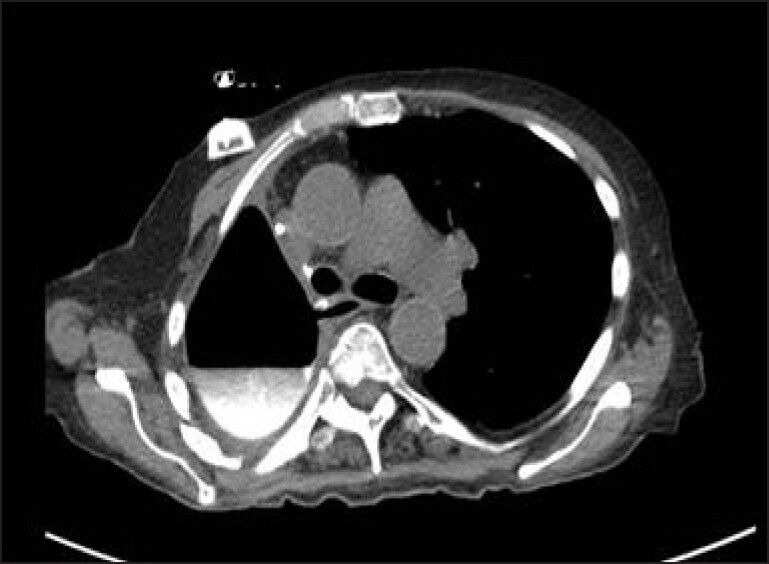
CT scan of the chest showing extravasation of oral contrast into the right pleural space, due to an esophageal perforation
DISCUSSION
Surgery is the preferred modality for local tumor control in early NSCL or locally recurrent NSLC, with traditional external beam radiotherapy used as a salvage modality in medically inoperable patients, with a poor survival outcome.[6] However, high-dose radiation doses appear to enhance local tumor control, leading to a better cancer-specific survival than those treated with lower doses typically used in traditional external beam radiation therapy.[7] It works through a mechanism called radioablation and is comparable to ablative effects seen after surgical resection. However, dose escalation is limited because increased doses of radiation result in increased toxicity and damage to surrounding pulmonary parenchyma. Stereotactic body radiation therapy (SBRT) uses high doses of radiation, which is oligo-fractionated and precisely delivered to the tumor by precise spatial localization, thus minimizing injury to surrounding tissues. It has been effectively used to treat tumors in others parts of the body such as intracranial tumors. Its application was limited in intrathoracic tumors due to respiratory motion preventing precise targeting of the tumor. With the advent of respiratory gating, it has been safely used in intrathoracic neoplasms such as NSLC.[1] While it was commonly used to ablate peripheral lung lesions, there was initial concerns with safety of SBRT in central location of NSLC (defined as <2 cm from the main bronchus), due to possible toxicity to hilar structures such as the main stem bronchus, esophagus and the heart.[2] With a combination of dosage reduction of radiation per fraction and precise respiratory gating, SBRT has been used to treat central NSLC with no significant increase in toxicity.[1,3] SBRT has also been effectively used to treat local recurrence of NSLC.[3]
The centrally located right lung mass received a prescription dose of 50 Gy in five fractions given every other day. The dose prescription, margin expansion, and dose-volume limits were derived from RTOG (radiation therapy oncology group) 0813, which is a Phase I/II trial for early stage, centrally located (i.e. within 2 cm of the bronchial tree), NSCLC in medically inoperable patients.[8] The main thrust of this trial is to address SBRT for centrally located NSCLC. Timmerman, et al. have described a higher rate of toxicity when delivering 60–66 Gy in three weekly fractions for these central tumors (mainly respiratory, hemoptysis, and pericardial effusion),[3] which is the rationale for their exclusion in RTOG protocols 0236 and 0618. The design of RTOG 0813 is intended to dose escalate to determine the maximum tolerable dose (MTD) for SBRT to these centrally located NSCLC tumors. The starting RT dose for the study was 10 Gy × 5 fractions every 2 days, which was our choice of prescription dose for the patient. When addressing critical organ dose-volume limits, the trial suggests a total maximum dose of 27.5 Gy to less than 5 cc of radiated esophagus and a maximum point dose of no more than 105% of prescription dose. However, the trial does recognize that treatment targets adjacent to the esophagus, trachea, bronchi, and heart will necessarily result in higher doses received by portions of those critical structures and to respect the 105% point dose maximum. In our patients, the volume of the esophagus receiving less than 27.5 Gy was 10.5 cc but the maximum point dose within the esophagus was 104.6% (52.3 Gy). In time, with additional data, more refined dose-volume constraints will be necessary to help prevent late sequel in these organs.
A wide spectrum of adverse effects is seen in SBRT ranging from generalized weakness, chest wall pain, rib fractures, brachial plexopathy, pneumonia and bronchitis, life threatening hemoptysis, bronchial artery pseudo aneurysm, pericarditis, and pericardial effusions. Esophageal toxicity is particularly concerning, especially when SBRT is used for centrally located lesions. Unlike the pulmonary parenchyma, which is a parallel functioning tissue, where every unit can function independent of the other, the esophagus is a serially functioning tissue, and damage to even a small volume of the esophagus from the radiation has serious consequences due to lack of redundancy. Most esophageal toxicities range from esophagitis to severe esophageal ulcerations, leading to a tracheoesophageal fistula.[4,5] Our cases presented as free thoracic esophageal perforation in a delayed fashion. Retrospective questing of both the patients did reveal history compatible with esophagitis during and after SBRT in both the patients, but none requiring inpatient care. These perforations raise the question of safety of SBRT when used to ablate centrally located NSLC (<2 cm from the main bronchus). Alternative explanations such as local tumor growth causing an esophageal perforation are unlikely, as there was no tumor in-growth seen on endoscopy. We theorize that SBRT, when used in central locations, may lead to esophagitis with ulcerations, which may lead to esophageal perforation several months after SBRT. Patients, who develop a new hydro-pneumothorax in the setting of a pneumonectomy or lobectomy and with concomitant use of SBRT to treat local recurrences, should be investigated for an esophageal pleural fistula in addition to the traditional cause for such a presentation, namely the bronchopleural fistula.
CONCLUSION
Stereotactic body radiation therapy should be judiciously used to ablate centrally located NSLC and patients who develop episodes of esophagitis during or after SBRT, need to be closely followed with endoscopy to look for esophageal ulcerations. These ulcers should be closely followed for healing as these may degenerate into full thickness perforations several months after SBRT.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nuyttens JJ, van der Voort van Zyp NC, Praag J, Aluwini S, van Klaveren RJ, Verhoef C, et al. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiother Oncol. 2012;102:383–7. doi: 10.1016/j.radonc.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–71. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56:126–35. doi: 10.1016/s0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–55. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 6.Qiao X, Tullgren O, Lax I, Sirzén F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. 2003;41:1–11. doi: 10.1016/s0169-5002(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 7.Jeremic B, Classen J, Bamberg M. Radiotherapy alone in technically operable, medically inoperable, early-stage (I/II) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:119–30. doi: 10.1016/s0360-3016(02)02917-6. [DOI] [PubMed] [Google Scholar]
- 8.Bethesda (MD): National Library of Medicine (US). 2008 Sep 9; [updated 2013 Sep 9; cited 2013 Dec 22]. National Cancer Institute; Radiation therapy Oncology Group. Seamless Phase I/II Study of Stereotactic Lung Radiotherapy (SBRT) for Early Stage, Centrally Located, Non-Small Cell Lung Cancer (NSCLC) in Medically Inoperable Patients. In: ClinicalTrials.gov [Internet] Available from: http://clinicaltrials.gov/show/NCT00750269 NLM Identifier: NCT00750269 . [Google Scholar]


