Abstract
Importance
Recognition of sagging eye syndrome (SES) as the cause of chronic or acute acquired diplopia may avert neurologic evaluation and imaging in most cases.
Objectives
To determine whether SES results from inferior shift of lateral rectus (LR) extraocular muscle (EOM) pulleys and to investigate anatomic correlates of strabismus in SES.
Design and Setting
We used magnetic resonance imaging to evaluate rectus EOMs, pulleys, and the LR– superior rectus (SR) band ligament at an eye institute.
Participants
Patients with acquired diplopia suspected of having SES. We studied 56 orbits of 11 men and 17 women (mean [SD] age of 69.4 [11.9] years) clinically diagnosed with SES. Data were obtained from 25 orbits of 14 control participants age-matched to SES and from 52 orbits of 28 younger controls (23[4.6] years).
Main Outcome Measures
Rectus pulley locations compared with age-matched norms and lengths of the LR-SR band ligament and rectus EOMs. Data were correlated with facial features, binocular alignment, and fundus torsion.
Results
Patients with SES commonly exhibited blepharoptosis and superior sulcus defect. Significant infero-lateral LR pulley displacement was confirmed in SES, but the spectrum of abnormalities was extended to peripheral displacement of all other rectus pulleys and lateral displacement of the inferior rectus pulley, with elongation of rectus EOMs (P < .001). Symmetrical LR sag was associated with divergence paralysis esotropia and asymmetrical LR sag greater than 1mm with cyclovertical strabismus. The LR-SR band was ruptured in 91% of patients with SES.
Conclusions and Relevance
Widespread rectus pulley displacement and EOM elongation, associated with LR-SR band rupture, causes acquired vertical and horizontal strabismus. Small-angle esotropia or hypertropia may result from common involutional changes in EOMs and orbital connective tissues that may be suspected from features evident on external examination.
Orbital connective tissues degenerate with aging, 1,2 creating clinical consequences, including aponeurotic blepharoptosis as well as limited supraduction due to inferior displacement of the horizontal rectus pulleys.3–5 One of the ligaments interconnecting the extraocular muscle (EOM) pulleys is the lateral rectus (LR)– superior rectus (SR) band, originating on the lateral border of the SR pulley and terminating on the superior border of the LR pulley. Degeneration of the LR-SR band has been postulated to permit inferior sag of the LR pulley, causing esotropia, cyclovertical strabismus, or both.2,6,7 It has been hypothesized that bilateral inferior shift of the LR may result mechanically in divergence paralysis esotropia (DPE), also termed divergence insufficiency, divergence insufficiency esotropia, and divergence paresis esotropia, that is characterized by esotropia at distance fixation, fusion at near fixation, and normal saccadic velocities in adduction and abduction.6,7 Asymmetrical inferior shift of the LR pulley has been postulated to produce cyclovertical strabismus (CVS). If these suppositions of the sagging eye syndrome (SES) are correct, clinical strabismus should correlate closely with anatomical changes. Characterization of SES as a cause of acquired diplopia would be important because such a presentation is typically considered indicative of an acute neurologic event, necessitating extensive and often costly investigations.8,9 Conversely, a mechanical etiology presents a more favorable prognosis for surgical treatment.6,7
We used magnetic resonance imaging (MRI) to evaluate rectus EOMs, pulleys, and the LR-SR band ligament to test the foregoing hypothesis in patients with acquired diplopia suspected of having SES. We predicted that the LR pulley would be lower in the hypotropic than in the hypertropic eye in CVS, whereas both LR pulleys would be displaced inferiorly by a similar amount in DPE. We further predicted that the LR-SR band ligaments would be bilaterally symmetrically elongated in DPE but asymmetrically more elongated in the hypotropic than hypertropic eye in CVS, and we predicted that the hypotropic eye in CVS would be excyclotropic.
METHODS
This study examined by orbital MRI 28 patients (11 men and 17 women) of mean (SD) age 69.4(11.9) years who had clinical symptoms of acquired diplopia. Patients who consented to research approved by the institutional review board and complying with the Declaration of Helsinki underwent complete clinical examination, including best corrected visual acuity (logMAR), refractive error, stereopsis (Titmus Fly Stereotest), motility examination, Hess screen testing, slitlamp and fundoscopic evaluation, photography in diagnostic gaze positions, and saccade examination. Divergence paralysis esotropia, defined as orthophoria or asymptomatic esophoria of 10Δ or less at 33 cm, with symptomatic distance esotropia measuring at least twice the near esophoria, was present in 11 patients.7 Cyclovertical strabismus, defined as vertical deviation greater than 2Δ with or without cyclotropia, was present in 17 patients. Exclusion criteria included superior oblique (SO) palsy, thyroid eye disease, trauma, history of strabismus surgery, and significant myopic degeneration, which may be suggestive of the “heavy eye” syndrome.4
Heterotropia was measured at distance and near by prism and cover testing. Fundus torsion was objectively determined by slitlamp measurement of the fovea to optic disc angle.10,11 External ocular adnexa were examined to evaluate baggy eye-lids, deep superior sulcus deformity, aponeurotic ptosis, and blepharoplasty scars.
The mean (SD) age of patients with DPE (3 men and 8 women) was 71.8(11.1) years, whereas that of patients with CVS (7 men and 10 women) was 67.8(2.4) years (P=.20). In patients with DPE, mean (SD) esotropia was 11.5(10.6) Δ for distance and 1.3(3.1) Δ for near viewing. The esotropia was vertically comitant, so that there were no significant “V” or “A” patterns. Ten patients had pure CVS, and 7 more had CVS with associated esotropia. The mean (SD) hypertropia of these patients was 9.9(9.4) Δ.
High-resolution fast spin-echo T1- or T2-weighted sequence as demonstrated by MRI was performed in central gaze using surface coils in a 1.5-T scanner (Signa; General Electric) as published.5,12 Each scanned eye fixated on a central target. Using sets of 2-mm-thick quasi-coronal image planes perpendicular to the long axis of the orbit, cross sections of the rectus and SO EOMs were outlined manually and analyzed in a normalized, oculocentric coordinate system to identify pulley locations, quantified by their horizontal and vertical coordinates relative to globe center. Rectus pulley positions were compared with published norms obtained using the identical technique.5,12 Maximum quasi-coronal SO cross sections were determined to detect, and consequently exclude if present, any cases of neurogenic SO atrophy due to SO palsy.
Quasi-coronal MRI of 52 orbits of 28 young controls (mean [SD]age, 23.0[4.6] years)and of 25 orbits of 14 controls (64.7[4.6] years) with no ocular disease who were age-matched to the patients with SES was performed to characterize the LR-SR band.
Horizontal rectus EOM lengths were determined from axial MRI, and vertical rectus EOM lengths were determined from quasi-sagittal MRI parallel to the long axis of the orbit. Lengths were obtained from manually drawn curved paths bi secting EOM width from orbital apex to scleral insertion. Horizontal rectus lengths were determined in 24 orbits of 12 patients with SES (12 orbits with DPE and 12 orbits with SES), with normative comparison in 28 orbits of 17 younger controls (mean [SD] age, 32.3[15.5] years) and 7 orbits of 5 older controls (57.6[24.7] years).
Quasi-sagittal MRI was performed to evaluate SR and IR lengths in 18 orbits of 9 patients with SES (mean [SD] age, 64.3[4.5] years), with normative comparison to 64 orbits of 34 young controls (23.6[3.8] years) and 15 orbits of 9 older controls (62.0[5.4] years). The group with SES comprised 7 patients with CVS and 2 with DPE.
RESULTS
ADNEXA
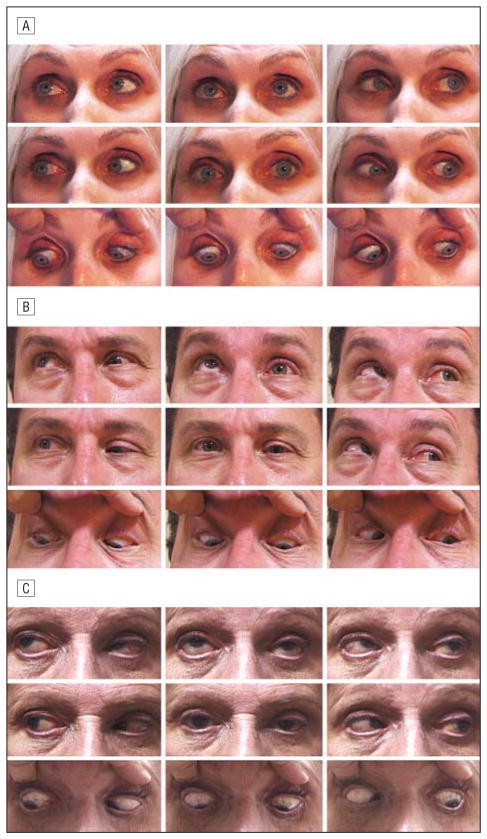
Superior sulcus deformity was evident in 18 of 28 patients (64%) with SES, whereas 8 (29%) exhibited high upper eyelid crease and aponeurotic blepharoptosis (Figure 1A-C). Eight patients (29%) had previously undergone blepharoplasty, brow-lift, or face-lift.
Figure 1.
Sagging eye syndrome. A, Deep superior sulcus and left hypotropia distance esotropia. There is marked bilateral limitation of supraduction. B, Left ptosis, left hypotropia, and marked limitation of supraduction in the right eye. C, Left aponeurotic ptosis and deep superior sulcus are more prominent in the left eye, and there is left hypotropia.
VISUAL FINDINGS
The mean (SD) visual acuity of patients with DPE was 0.08(0.11) logMAR vs 0.04(0.16) logMAR in patients with CVS (P=.15). The mean (SD) spherical equivalent refractive error was −0.60(2.53) diopter (D) in CVS vs −1.78(2.72) D in DPE (P=.05). The mean (SD) stereo threshold was 371(780) arcsec in CVS and 216(260) arcsec in DPE (P=.27). Horizontal saccades were clinically normal in all participants.
MRI OF RECTUS PULLEY POSITIONS
Patients with strabismus exhibited significant (P <.005) displacement of both the medial rectus (MR) and LR pulleys away from the orbital center compared with younger and older controls (Table 1). In DPE, the LR pulley was 4.0 mm more lateral and the MR pulley was approximately 2.4 mm more medial than in control orbits while also being significantly infero placed by a mean of 5.9 and 3.4 mm, respectively, from positions of younger controls. The LR pulley in DPE was also approximately 4.0 mm inferior to that of older controls (P <.005) (Table 1 and Figure 2). In DPE, the IR pulley was displaced approximately 5.0mm temporally and approximately 3.0mm inferiorly compared with both younger and older controls.
Table 1.
Rectus Pulley Positions Relative to Globe Center
| Rectus, Mean (SD), mm
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Medial
|
Superior
|
Lateral
|
Inferior
|
|||||
| Group | Lateral | Superior | Lateral | Superior | Lateral | Superior | Lateral | Superior |
| DPE | −12.15 (0.69)a | −3.51 (2.0)a | −2.31 (3.52) | 10.18 (0.69) | 14.13 (1.56)a | −6.2 (1.6)a | 1.36 (2.27)a | −15.17 (1.17)a |
| CVS | ||||||||
| Hypertropic | −12.8 (1.39) | −2.2 (2.03) | −2.8 (2.6) | 10.9 (1.2) | 14.4 (1.49)b | −6.1 (3.7)b | 0.2 (1.68)b | −15.6 (2.27)b |
| Hypotropic | −12.8 (1.51)c | −2.7 (1.6)c | −2.8 (3.23) | 10.5 (1.23)c | 14 (1.54)c | −8.8 (3.5)c | 0.8 (1.88)c | −16.6 (1.91)c |
| Controls | ||||||||
| Older | −14.5 (0.9)a | −1.05 (1.11) | −2.06 (1.85) | 11.5 (1.11) | 10.13 (0.8)a,b,c | −2.03 (1.7)a,c | −5.14 (0.9)a,b,c | −12.6 (0.83)a,b,c |
| Younger | −14.4 (1.01)a,c | −0.1 (1.24)a,c | −2.3 (0.9) | 11.8 (0.84)c | 10.1 (0.6)a,b,c | −0.3 (1.03)a,b,c | −5.4 (0.9)a,b,c | −12.2 (1.01)a,b,c |
Abbreviations: CVS, cyclovertical strabismus; DPE, divergence paralysis esotropia.
Significant differences between DPE and control groups (P < .005).
Significant differences for the hypertropic eye of CVS vs controls.
Significant differences for the hypotropic eye of CVS vs controls.
Figure 2.
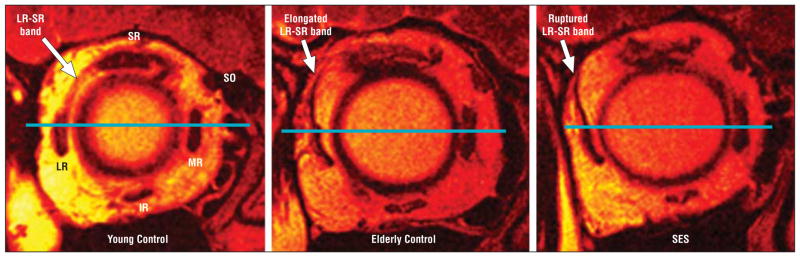
Fast spin-echo T2-weighted sequence quasi-coronal plane magnetic resonance imaging. Left, Younger control participant showing lateral rectus (LR)–superior rectus (SR) band. Note the normal morphology of LR muscle with respect to a horizontal reference line drawn through the globe center. Middle, Elderly control participant demonstrated marked elongation of LR-SR band associated with LR muscle sag. Right, Rupture of LR-SR band in sagging eye syndrome (SES) with resultant LR sag. IR indicates inferior rectus; MR, medial rectus; and SO, superior oblique.
In CVS, the MR pulley of the hypotropic eye was located 2.6 mm inferior and 1.6 mm temporal to that of younger controls (P < .005) but similar to that of older controls (Table 1). The SR pulley in CVS was approximately 1.0 mm inferior to that of younger controls (P < .005) but not significantly different from that of older controls. The MR and SR pulleys in the hypertropic eyes of patients with CVS were normally located.5 The LR pulley in the hypotropic eye in CVS was displaced approximately 4.0 mm temporally and 6.0 to 8.0 mm inferiorly compared with both younger and older controls (P < .005 for both) (Table 1), whereas the LR pulley in the hypertropic eye was displaced approximately 4.0 mm inferiorly, which was significantly different only from younger but not older controls. However, the LR pulley in the hypertropic eye in CVS was significantly displaced temporally by 4.0 mm compared with both younger and older controls (P < .005) (Table 1). The IR pulley was displaced temporally by 4.0 to 5.0 mm in both the hypotropic and the hypertropic eyes in CVS, which was significantly different from both younger and older controls (P < .005) (Table 1).
The difference in LR pulley infraplacement in the hypotropic eye vs hypertropic eye in CVS was significant (P = .02), with the eye with greater infraplacement (sag) always being hypotropic. Except for this difference in LR sag, pulley locations in the hypertropic and the hypotropic eyes were statistically similar (P > .05). The hypotropic eye with greater LR sag had a mean (SD) excycloposition of 11.7° (6.1°), while the eye with less LR sag had only 6.7° (5.0°) (P = .01.)
In patients with DPE, LR sag was bilaterally symmetrical, differing a mean (SD) of only 0.3 (0.1)mm(P = .80). By contrast, in CVS, the LR sag asymmetry was a mean(SD) of 2.6 (1.8)mm(P = .04).The difference in asymmetry between DPE and CVS was significant (P <.001).For patients with DPE, the interocular difference in LR sag was always less than 0.5 mm, whereas in CVS, the difference always exceeded 1mm and was greater in the hypotropic than in the hypertropic eye. The LR sag in the hypertropic eye in CVS was similar to that in DPE, whereas the hypotropic eye in CVS had significantly greater sag. There was greater sag (P = .02)and more nasal position of the MR pulley (P = .02) in DPE than in CVS (Table 1). Infraplacement of the IR pulley was greater in CVS than in DPE (P = .03).
RECTUS EOM LENGTHS
Axial MRI demonstrated that the LR was markedly elongated in SES (Figure 3). In younger controls, LR length was a mean (SD) of 32.6 (5.6) mm, not significantly different from the length of 30.9 (14.2) mm in older controls (Table 2). However, the LR was approximately 50% longer, at 45 to 47 mm, in both DPE and CVS (P < .005) (Table 2). The MR was elongated to a lesser degree than the LR in SES. In younger controls, MR length was a mean (SD) of 31.0 (5.5) mm, not significantly different from the length of 29.2 (7.2)m min older controls (Table 2). However, the MR was approximately 25% longer, at 38 to 39 mm, in both DPE and CVS (P < .005) (Table 2). The LR was significantly more elongated than the MR in SES (P < .001) (Figure 2). The optic nerve was tortuous in 9 of 12 patients, a finding observed only in SES (Figure 3).
Figure 3.
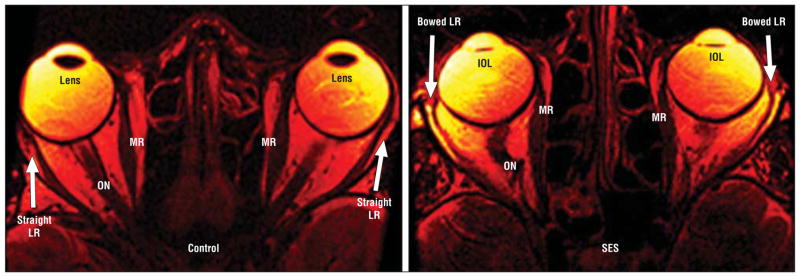
Fast spin-echo T2-weighted sequence axial magnetic resonance imaging. Left, Control participant illustrates medial rectus (MR) and lateral rectus (LR) configuration. Right, Marked elongation and thinning of LR more than in sagging eye syndrome (SES). IOL indicates intraocular lens; and ON, optic nerve.
Table 2.
Rectus Muscles and LR-SR Band
| Rectus Length, Mean (SD), mm
|
LR-SR Band
|
||||||
|---|---|---|---|---|---|---|---|
| Group | Medial | Superior | Lateral | Inferior | Length, Mean (SD), mma | Angle, Mean (SD), dega | Rupture, % |
| DPE | 37.8 (16.4) | 40.7 (1.2) | 45.3 (16.0) | 37.0 (4.7) | 12.7 (5.6) | 22.4 (5.6) | 64 |
| CVS | 39.4 (6.2) | 43.4 (3.9) | 46.8 (5.8) | 42.1 (5.7) | 14.1 (5.6) | 23.6 (13.1) | 91 |
| Older controls | 29.2 (7.2) | 37 (4.0) | 30.9 (14.2) | 39.3 (4.3) | 12.4 (2.9) | 17.6 (7.2) | 0 |
| Younger controls | 31.0 (5.5) | 36.1 (2.9) | 32.6 (5.6) | 39.4 (2.8) | 8.5 (1.5) | 5.7 (8.9) | 0 |
Abbreviations: CVS, cyclovertical strabismus; DPE, divergence paralysis esotropia; LR-SR, lateral rectus–superior rectus.
Values reported only for cases without LR-SR band rupture.
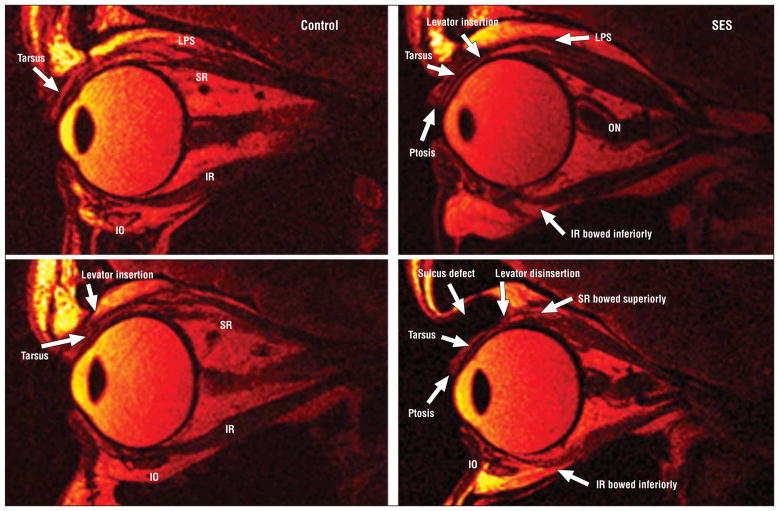
Quasi-sagittal MRI (Figure 4) demonstrated that the SR in both subgroups of SES was 3.0 to 6.0 mm longer than in both groups of control participants, in whom mean age did not significantly influence SR length (Table 2). Length of the IR was similar in controls and in SES.
Figure 4.
Fast spin-echo T2-weighted sequence quasi-sagittal magnetic resonance imaging. Left column, Morphology and length of the superior rectus (SR) and inferior rectus (IR) muscles in a young control participant. The insertion of the levator palpebrae superioris (LPS) to the tarsus and normal eyelid anatomy is visible. Top right, Aponeurotic ptosis and bowing of the IR muscle in sagging eye syndrome (SES). The LPS is minimally attached to the tarsus. The optic nerve (ON) is convoluted. Bottom right, Another case of SES showing levator disinsertion, ptosis, and marked superior sulcus defect. The SR and IR are markedly elongated and bowed. IO indicates inferior oblique.
MRI OF LR-SR BAND
Length of the LR-SR band was measured by digital tracing (Figure 2). The ligament was approximately 12 mm longer in older than in younger controls (Table 1) (P < .005). However, no control participant had LR-SR band rupture or dehiscence. In younger controls, the LR-SR band could be visualized as far posteriorly as 2.9 (1.5) mm posterior to the globe–optic nerve junction compared with 3.8 (2.0)mm in older controls (P = .01). The mean (SD) angulation of the LR with respect to the vertical was 5.7° (8.9°) in younger controls but significantly greater at 17.6° (7.2°) in older controls (P <.001).
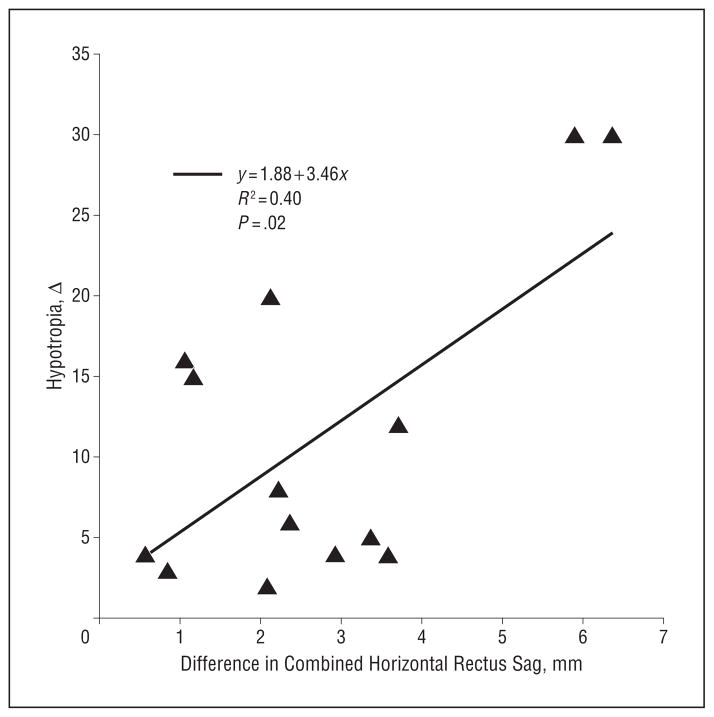
Patients with SES exhibited thinning, elongation, or frequently rupture of the LR-SR band (Figure 2). Magnetic resonance imaging resolution was adequate to appreciate superotemporal bowing of the band in milder cases and abrupt termination of an attenuated LR-SR band remnant in the superolateral orbit in more severe cases (Figure 2). In DPE, the LR-SR band was ruptured in 14 of 22 orbits, and in CVS, it was ruptured in 31 of 34 orbits; these proportions were not significantly different (P > .50, χ2 test). The LR-SR band was visualized more posteriorly in patients with SES than in controls, 8.0 (1.8) mm posterior to the globe–optic nerve junction in DPE (n = 8) and 6.0 (2.0) mm in CVS (n = 3). In those patients with DPE who had intact LR-SR bands, LR-SR band length (P = .04), posterior position (P < .001), and LR angulation (P<.001) were significantly greater than in young controls. Whereas the LR-SR band length in DPE was similar to that of older controls (P = .44), the LR-SR band was significantly more posterior (P<.001) and LR angulation was greater (P = .007) in DPE. Similar parameters for CVS could not be statistically compared because only 3 orbits in patients with CVS had intact LR-SR bands. Thus, it would appear that LR-SR band ligament rupture is associated with asymmetrical LR sag and hypotropia in SES. There was a significant correlation (R2 = 0.4; P = .02) between the difference in combined LR plus MR sag in the hypotropic and hypertropic eye in CVS vs the magnitude of vertical strabismus (Figure 5).
Figure 5.
Linear regression showing significant correlation between binocular difference in horizontal rectus sag (combined lateral rectus and medial rectus sag) and magnitude of the hypotropia in cyclovertical strabismus.
SO MUSCLE
The maximum SO cross-sectional area in CVS was measured bilaterally using published methods13 to rule out neurogenic atrophy typical of SO palsy. The mean (SD) maximum SO cross section measured 19.4 (2.9)mm2 in the hypertropic eye, not significantly different from the comparable value of 20.9 (5.6) mm2 in the hypotropic eye (P = .18). All of the observed SO cross sections were within published normal 95% CIs of 18.6 to 21.8mm2.13
COMMENT
The LR-SR band ligament interconnects the SR and LR pulleys, suspending the LR vertically within the orbit2,6 against inferior tension exerted by the inferior oblique muscle, whose orbital layer inserts on the LR pulley.14,15 Microscopy has demonstrated progressive elongation and rupture of the LR-SR band ligament with age, associated with LR pulley sag. In a 93-year-old orbit evaluated histologically, the LR-SR band was so attenuated that it was indistinguishable from the lateral extension of the levator aponeurosis.6 It was therefore proposed in an earlier study6 that the 3 elderly, nonmyopic, strabismic patients who sought care because of unilateral hypotropia and DPE exhibited a previously unrecognized entity called SES. Sagging eye syndrome was supposed to be associated with age-related orbital connective tissue degeneration, external manifestations of which were baggy eyelids, superior sulcus deformity, aponeurotic blepharoptosis, and the likelihood of previous blepharoplasty or similar cosmetic surgery.6 Sagging eye syndrome was proposed to differ from the heavy eye syndrome, an association of axial high myopia, esotropia, and hypotropia.16
Our study confirms the predicted mechanisms and clinical associations of SES. All patients with SES, whether pure esotropia as in DPE, combined esotropia and CVS, or pure CVS, exhibited the predicted highly significant inferior displacement of the LR pulley. All patients with CVS, with or without associated esotropia, exhibited highly significant asymmetrical inferior displacement of the LR pulley. In each case, the eye with the greater inferior LR pulley displacement exhibited more hypotropia and greater excyclotropia than its hypertropic fellow.
Our study extends confirmation of postulated mechanisms of SES with striking new pathophysiologic observations. Every case of SES, whether pure esotropia as in DPE, combined esotropia and CVS, or pure CVS, also exhibited marked elongation of the rectus EOMs. Although even nonstrabismic older patients exhibited a roughly 50% lengthening of the otherwise intact LR-SR band, the ligament was ruptured in 64% of orbits with DPE and 91% of orbits with CVS. This high prevalence for LR-SR band rupture supports anecdotal clinical reports of acute, painful onset of both horizontal and vertical diplopia in SES and suggests a mechanical mechanism for the associated oblique angulation of the LR orientation relative to the vertical in SES. The magnitude of CVS correlated with the magnitude of horizontal rectus pulley sag.
An unexpected emergent finding from the MRI study was the highly significant displacement of all 4 rectus pulleys away from the orbital center in SES. These displacements ranged from 2 to 14 mm and were much greater than observed in nonstrabismic participants of comparable age. The IR pulley was also displaced approximately 6 mm more laterally in both SES and nonstrabismic older patients. Lateral displacement of the IR pulley could be due to age-related degeneration of the insertion upon it of the orbital layer of the inferior oblique muscle, which has been demonstrated histologically.1,2 The LR was also observed to be obliquely angulated in SES, perhaps owing to pressure of superotemporally migrating orbital fat unconstrained by the degenerated LR-SR band and associated connective tissues.
Large centrifugal displacements of rectus pulleys in SES geometrically elongate EOM paths from origin to scleral insertion. This is consistent with direct MRI measurements of horizontal EOM lengths, which were approximately 40%, or 14 mm, longer than the EOMs of nonstrabismic younger and older controls. Relative to the resections and recessions of rectus EOMs performed in typical strabismus surgery, the elongations of rectus EOMs in SES are striking and would suggest increased resting length rather than merely passive stretching from normal path length. It is possible that the marked rectus EOM elongation in DPE explains the observed requirement for unusually large dosages of MR recession surgical correction.7 Wide-spread anatomical changes in the orbits in SES probably also influence the effects of other forms of strabismus surgery. These changes may result, in part, from thinning of orbital connective tissues that permit both shifts in EOMs and migration of orbital fat that could produce a host of adaptive and pathologic effects on EOMs and perhaps their patterns of innervation. It is indeed remarkable that the extreme EOM elongations and pulley shifts observed in patients with SES do not produce more severe angles of strabismus than are clinically observed. To our knowledge, the potential impact of EOM elongation in other forms of strabismus has not been considered before and deserves further study in other settings.
Although the current study did not attempt to determine the relative prevalence of SES in relationship to all causes of acquired adult strabismus, an effort was made to exclude well-understood causes of strabismus, such as orbital trauma, thyroid ophthalmopathy, restriction, and EOM paralysis. After these clinical exclusions, the remaining cases proved to be SES. The physician can thus infer that the majority of similar acquired adult DPE and CVS are likely to be caused by SES and are likely to exhibit the characteristic adnexal signs, including blepharoptosis and superior sulcus defect. However, many patients with SES had previously undergone blepharoplasty or face-lift, so some of the orbital findings may have been partially iatrogenic.
This study has broad clinical implications that can benefit patients without requiring clinical orbital imaging. Patients with SES are recognizable from their external appearances and motility patterns. Typical clinical findings of SES include generalized sag of the levator aponeurosis, resulting in a superior sulcus deformity, aponeurotic ptosis, or high eyelid creases (Figure 1A-C). Many patients with SES were first seen with diplopia long after undergoing surgical correction of blepharoptosis. Most patients with SES exhibit symmetrically limited supraduction, although all exhibit full horizontal ductions and clinically normal horizontal saccadic eye velocities. If such patients seek care for acute or chronic horizontal binocular diplopia for distant but not near targets, as is characteristic of DPE, in the absence of associated neurologic complaints, further etiologic investigations are probably unnecessary because the diagnosis of SES can be presumed. Patients manifesting acute or chronic onset of vertical binocular diplopia in clinical circumstances suggestive of SES may be spared neurologic investigations if there is no other clinical evidence of a cranial neuropathy or other acute neurologic event. All patients in the current series with CVS exhibited normal SO size by MRI, a finding that excludes SO palsy secondary to SO denervation. Another relevant clinical finding that distinguished SES from SO palsy is that whereas in SES the hypotropic eye is excyclotropic, in SO palsy the hypertropic eye is excyclotropic.
In conclusion, SES is a manifestation of age-related, orbital connective tissue degeneration. Although most strabismus in SES is associated with severe elongation of the LR-SR band ligament leading to its rupture, all rectus EOM pulleys become centrifugally displaced in SES, a presumably gradual process that markedly elongates the resting lengths of the EOMs. Bilaterally symmetrical downward displacement, termed sag, of the LR pulleys in SES may asymptomatically and symmetrically reduce supraduction but symptomatically cause “divergence paralysis” esotropia for distant targets. Bilaterally asymmetrical LR pulley sag results in hypotropia and excyclotropia of the eye with the greater sag, causing symptomatic cyclovertical diplopia. Thus, SES represents a mechanical cause of acquired, adult horizontal and vertical strabismus. Because SES is typically associated with clinically obvious adnexal changes, recognition of this SES as the cause of chronic or acute acquired diplopia may avert neurologic evaluation and imaging in most cases.
Acknowledgments
Funding/Support: This study was supported by grant EY08313 from the National Eye Institute of the US Public Health Service, The Shaw Family Endowment Fund, and the BOYSCAST Fellowship of the Department of Science and Technology, Government of India (Dr Chaudhuri).
Role of the Sponsor: The National Eye Institute and Shaw Family Endowment Fund funded the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Footnotes
Author Contributions: Both authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: None reported.
Disclaimer: Magnetic resonance imaging surface coils used in this project are not approved by the US Food and Drug Administration for this purpose. The authors have no proprietary interests in the surface coils.
References
- 1.Demer JL. More respect for connective tissues. J AAPOS. 2008;12(1):5–6. doi: 10.1016/j.jaapos.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43 (9):2923–2932. [PubMed] [Google Scholar]
- 3.Frueh BR. The mechanistic classification of ptosis. Ophthalmology. 1980;87(10):1019–1021. doi: 10.1016/s0161-6420(80)35135-x. [DOI] [PubMed] [Google Scholar]
- 4.Clark RA, Isenberg SJ. The range of ocular movements decreases with aging. J AAPOS. 2001;5(1):26–30. doi: 10.1067/mpa.2001.111016. [DOI] [PubMed] [Google Scholar]
- 5.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134 (6):872–878. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 6.Rutar T, Demer JL. “Heavy eye” syndrome in the absence of high myopia: a connective tissue degeneration in elderly strabismic patients. J AAPOS. 2009;13 (1):36–44. doi: 10.1016/j.jaapos.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri Z, Demer JL. Medial rectus recession is as effective as lateral rectus resection in divergence paralysis esotropia. Arch Ophthalmol. 2012;130(10):1280–1284. doi: 10.1001/archophthalmol.2012.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson DM. Divergence insufficiency revisited: natural history of idiopathic cases and neurologic associations. Arch Ophthalmol. 2000;118(9):1237–1241. doi: 10.1001/archopht.118.9.1237. [DOI] [PubMed] [Google Scholar]
- 9.Lim L, Rosenbaum AL, Demer JL. Saccadic velocity analysis in patients with divergence paralysis. J Pediatr Ophthalmol Strabismus. 1995;32(2):76–81. doi: 10.3928/0191-3913-19950301-04. [DOI] [PubMed] [Google Scholar]
- 10.Kothari MT, Venkatesan G, Shah JP, Kothari K, Nirmalan PK. Can ocular torsion be measured using the slitlamp biomicroscope? Indian J Ophthalmol. 2005;53(1):43–47. doi: 10.4103/0301-4738.15284. [DOI] [PubMed] [Google Scholar]
- 11.Lefèvre F, Leroy K, Delrieu B, Lassale D, Péchereau A. Study of the optic nerve head-fovea angle with retinophotography in healthy patients. J Fr Ophtalmol. 2007;30(6):598–606. doi: 10.1016/s0181-5512(07)89664-1. [DOI] [PubMed] [Google Scholar]
- 12.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41(12):3787–3797. [PubMed] [Google Scholar]
- 13.Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011;129(7):904–908. doi: 10.1001/archophthalmol.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44(9):3856–3865. doi: 10.1167/iovs.03-0160. [DOI] [PubMed] [Google Scholar]
- 15.Demer J. The anatomy of strabismus. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology and Strabismus. Edinburgh, UK: Elsevier Saunders; 2005. pp. 849–868. [Google Scholar]
- 16.Demer JL, Von Noorden GK. High myopia as an unusual cause of restrictive motility disturbance. Surv Ophthalmol. 1989;33(4):281–284. doi: 10.1016/0039-6257(82)90154-0. [DOI] [PubMed] [Google Scholar]