Abstract
The hepatitis B virus continues to be a major pathogen worldwide despite the availability of an effective parenteral vaccine for over 20 years. Orally-delivered subunit vaccines produced in maize may help to alleviate the disease burden by providing a low-cost, heat stable alternative to the parenteral vaccine. Oral subunit vaccination has been an elusive goal due to the large amounts of antigen required to induce an immunologic response when administered through the digestive tract. Here we show that high levels of HBsAg were obtained in maize grain, the grain was formed into edible wafers, and wafers were fed to mice at a concentration of approximately 300µg/g. When these wafers were made with supercritical fluid extraction (SFE)-treated maize material, robust IgG and IgA responses in sera were observed that were comparable to the injected commercial vaccine Recombivax®). In addition, all mice administered SFE wafers showed high secretory IgA titers in fecal material whereas Recombivax® treated mice showed no detectable titer. Increased salivary IgA titers were also detected in SFE-fed mice but not in Recombivax® treated mice. Wafers made from hexane-treated, or full fat, maize material induced immunologic responses, but fecal titers were attenuated relative to those produced by SFE-treated wafers. These responses demonstrate the feasibility of using a two-dose oral vaccine booster in the absence of an adjuvant to induce immunologic responses in both sera and at mucosal surfaces, and highlight the potential limitations of using an exclusively parenteral dosing regime.
Keywords: Hepatitis B, mucosal, oral vaccine, plant vaccine, bioencapsulation, immunogenicity, HBsAg, supercritical fluid extraction
Introduction
Despite the availability of an effective parenteral vaccine for over 20 years, hepatitis B virus (HBV) remains an important problem, with 240 million chronically infected patients worldwide [1]. The present recommendation for the vaccine consists of injecting a 10 or 20µg dose of the HBV small surface antigen, HBsAg, as a primary dose followed by two boosting doses. Although seroconversion occurs in greater than 90% of the general population using commercialized vaccines [2], there are specific segments of the population that are poor responders or non-responders. Among them are the elderly, obese individuals, HIV-positive patients, and individuals with celiac disease, irritable bowel disease, Down syndrome or chronic kidney disease [3–13].
An oral vaccine may improve seroconversion in the general population by stimulating the immune system at mucosal sites, tissues that are traditionally primary sites of infection. In addition, an oral vaccine would be easier to administer and could increase compliance in populations that historically forego one or all of the HBsAg doses such as hemophiliacs [14], at-risk youth [15], transient populations [16], persons engaging in high-risk sexual activity [17–18], and healthcare workers [19]. On a global scale, a maize-produced oral alternative could provide a low-cost, heat-stable alternative to parenteral vaccines [2, 20] and therefore improve coverage in remote areas or resource-poor areas that cannot afford the infrastructure for reliable cold storage, needle administration, and waste disposal.
Many attempts have been made to develop a viable oral vaccine system with some success. Encouragingly, when HBsAg was expressed in potato tissue and fed tohuman volunteers as a booster dose, an increase in antibody titer was observed in 63% of participants [21]. The authors speculated that an increased concentration of antigen would be needed to improve seroconversion rates but, unfortunately, highly concentrated HBsAg in potato tissue has not been forthcoming. Increased antigen concentrations have been recently achieved in maize and have shown improved responses in mice relative to the potato material [22].
A key advantage of cereal grains is their ability for long-term stable storage of recombinant proteins [23–24]. If the raw material is to be used for oral vaccine formulations, shelf life can be extended by removing lipids from the grain, leading to reduced rancidity, oil degradation and radical formation [25]. Several methods can be used for lipid extraction, including hexane extraction and supercritical fluid extraction (SFE) with carbon dioxide (CO2). Hexane is a solvent that is routinely used for the extraction of oil from plant products, resulting in marketable vegetable and essential oils. Unfortunately hexane is a neurotoxin and therefore requires extensive safety precautions during extraction and disposal. Supercricital CO2, formed at pressures above 74 bar and 31°C, is emerging as an alternative commercial extraction solvent due to its relatively benign properties [26]. Using hexane extracted maize material as an oral vaccine can elicit a strong immunological response in mice [22], but SFE-treated material has not been tested for its immunogenicity.
In the present study, more highly concentrated maize material was assessed as a booster in mice relative to the commercial vaccine. Different maize lipid removal techniques were also implemented and assessed for their effect on immunologic responses in mice.
Materials and Methods
Maize material
All maize material used for the mouse study was derived from seed containing the HBG construct, as previously described [20]. All maize material used in the mouse studies was hybrid grain derived from heterozygous plants that contained genetic background from elite parent inbred lines 16038 and MBS5411. Control germ was G909 germ from the Grain Processing Corporation (Muscatine, IA).
Seed processing
HBG hybrid seed was soaked for 5 days in water (4°C) to approximately 50% moisture, germ was extracted by hand, dried overnight at 37°C to a final moisture of 6–15%, and ground to a fine cornmeal consistency. Ground germ was defatted by either hexane extraction or supercritical fluid extraction (SFE). Hexane extractions were conducted as previously described (Hayden et al, 2012, PBJ). SFE treatment consisted of CO2 extraction at 350 bar, 40–53°C vessel temperature, using a 5L vessel in an SFT-250 (Supercritical Fluid Technologies, Newark, DE).
Wafer processing
Germ expressing HBsAg was treated with hexane or SFE to remove lipids. During this process, HBsAg concentration can either increase due to loss of lipids in the biomass or decrease due to loss of recombinant protein during the extraction method. To compensate for these changes in concentration, HBsAg germ materials were blended with control germ to obtain approximately equivalent concentrations of HBsAg in all germ flour used to make wafers. Control wafers were made with SFE-treated control germ. Each wafer contained 2.5g germ flour and 0.65g of ultrafine baker’s sugar (C&H). Water was added to give a moldable consistency that was 15% of the germ weight for full fat material, and 25% for SFE- and hexane-defatted material. Wafers were hand-pressed into circular plastic molds (cat#40116, Decagon/AquaLab, Pullman, WA) and dried in a VWR 1430 vacuum oven (VWR Scientific, Radnor, PA) at 58–62°C, 21–2” Hg until wafers lost >90% of the added water.
Antigen detection
To test HBsAg antigen levels in ground maize material or wafers, 100mg samples were weighed out in duplicate and each sample was extracted in 1mL PBS+1%TritonX-100. Four wafers were tested per treatment. HBsAg was assayed by sandwich ELISA, as described previously [22] with the following modifications: extracts were diluted 1:1000 and assayed using a monoclonal capture antibody (cat#C01246M, Meridian Life Sciences, Memphis, TN), and a polyclonal HRP-conjugated detection antibody (cat#B65811P, Meridian Life Sciences).
Mouse study
BALB/c inbred mice (Harlan) were randomly assigned to treatments 1 through 6 and housed individually. Eleven mice were assigned to treatments 1 through 3 and ten mice were assigned to treatments 4 and 5. All treatments, except treatment 6, were injected with 0.5µg of Recombivax® (Merck, Whitehouse Station, NJ) on day 0, and were boosted with full fat wafers, hexane-defatted wafers, SFE-defatted wafers, Recombivax®, or control wafers (treatments 1, 2, 3, 4, and 5, respectively), with boosting initiated on day 112 and day 126 post-primary injection. For each boost, two wafers were offered per day for three consecutive days or a single intra-muscular Recombivax® injection was administered on the first day. Treatment 6 consisted of 5 mice which were injected with 0.9% sterile saline on day 0 and boosted with control wafers as above.
Anti-HBsAg antibody detection in mice
Blood samples were collected by submandibular venous puncture every 2–4 weeks, centrifuged to remove red blood cells, and stored in 50% glycerol at −20°C. On boosting days, serum was collected a few hours prior to boosting. Fecal material was collected from cages that were cleaned 24 hours prior to sampling, and samples were stored at −20°C. Fecal samples were collected twice a week for the first 5 weeks, and again twice a week starting one day prior to the first boost and ending the week of the terminal bleed. Serum anti-HBsAg IgG and IgA were detected using a sandwich ELISA. Plates were coated with rHBsAg (cat#R86872, Meridian Life Sciences), serum samples diluted 1:250, and HRP-conjugated anti-mouse IgG (cat#ab6789, Abcam, Cambridge, MA) or AP-conjugated anti-mouse IgA (cat#ab97232, Abcam) were used to detect IgG and IgA, respectively. For secretory IgA, 100mg of fecal pellets were resuspended in 1mL of 1%BSA in PBS containing a protease inhibitor (cat# 11836153001, Roche Diagnostics GmbH, Mannheim, Germany) and diluted an additional 1:50 and used in the same assay as the serum samples. For saliva Ig, saliva was collected on day 141 post-primary injection, diluted between 1:5 and 1:25, and detected using the ETI-AB-AUK PLUS assay kit (DiaSorin, Saluggia, Italy) which calculates total anti-HBsAg Ig in mIU/mL based on the WHO 2nd International Standard. Serum Ig was also detected using the DiaSorin kit by diluting serum 1:50 or 1:500 so that titers fell on the linear part of the standard curve.
Immunoblot
One hundred milligrams of wafer material was first extracted three times in PBS + 0.05% Tween 20 to remove native corn proteins that non-specifically bind to the detection antibody (it is estimated that <10% HBsAg was removed in these first extractions). A fourth extraction in PBS + 0.1% TritonX-100 was then performed to extract HBsAg. Ten microlitres of each extract was heated to 100°C for 10 minutes and run on an SDS-PAGE gel under reducing conditions (50mM DTT) and analyzed by an immunoblot, as previously described [22].
Statistics
Data for fecal IgA, serum IgA, serum IgG, and terminal bleed serum mIU/mL were log transformed in order to normalize the data and equalize variability, then analyzed using an ANOVA. For each dataset, statistical differences were assessed at the 5% overall significance level using Tukey’s HSD procedure. Treatments sharing a group letter for a given assay show insufficient evidence of statistically significant differences. Fecal IgA responses were compared using data collected 135 days post-injection. Serum IgG and IgA pre-immune subtracted O.D. values were normalized to bleed 5, which was taken directly preceding the first boosting dose (day 112). Statistical differences were assessed for the normalized terminal bleed, taken at day 147 (O.D. at day 147/ O.D. at day 112). Anti-HBsAg Ig titers (mIU/mL) were analyzed for differences between terminal bleeds (post-boost) and between pre-boost versus post-boost change in titers. Saliva mIU/mL were log transformed and also analyzed using Tukey’s HSD procedure at the 5% significance level after excluding one high outlier in the SFE boosting treatment. Removing the outlier decreased the overall mean of the SFE treatment, but increased the power of the statistical test due to an overall decrease in variability without the outlier.
Results
Concentration of HBsAg in maize material
Historically, it has been difficult to accumulate HBsAg in plant tissue at concentrations that are high enough to elicit a robust immune response via oral administration. Recent breeding efforts for increased HBsAg expression have shown promise [22] therefore maize lines were backcrossed into elite parental inbred lines and used to produce highly expressing hybrid grain. Because the promoter driving expression of HBsAg is primarily active in the germ (embryo), germ was separated from the endosperm and assayed for HBsAg. Germ from hybrid grain showed very high levels of HBsAg, with a mean of 581µg HBsAg/g germ, the highest reported concentration to date in a plant production system.
The germ portion of the seed typically contains 30% oil [27], therefore removal of the oil can significantly concentrate the HBsAg in the germ and extend its shelf life [28]. To determine whether oil removal can impact the immune response, the germ was ground and differentially processed by hexane treatment, supercritical fluid extraction (SFE) treatment, or no treatment (full fat). Previous data have shown that hexane-treated maize material can produce an immune response in mice [22]. In the interest of finding a more food-safe oil extraction method, SFE treatment of the maize material was included.
HBsAg germ meal from all three samples was mixed with control germ meal that was hexane treated, SFE treated, or untreated to approximate equal concentrations of HBsAg in each type of germ. Germ mixes were then combined with sugar and water, formed into wafers, dried, and HBsAg concentrations determined (Table 1). Based on an ELISA assay, hexane-treated wafers appeared to contain higher concentrations of HBsAg than either full fat or SFE-treated wafers and were more variable in their concentration from wafer to wafer.
Table 1.
Concentration of HBsAg in wafers as determined by ELISA.
| Sample | Mean [HBsAg] (µg/g wafer) |
Std. dev. |
|---|---|---|
| Full fat wafers | 290 | 40 |
| Hexane-treated wafers | 481 | 93 |
| SFE-treated wafers | 297 | 23 |
| Control wafers | None detected* |
Limit of detection is 15µg/g
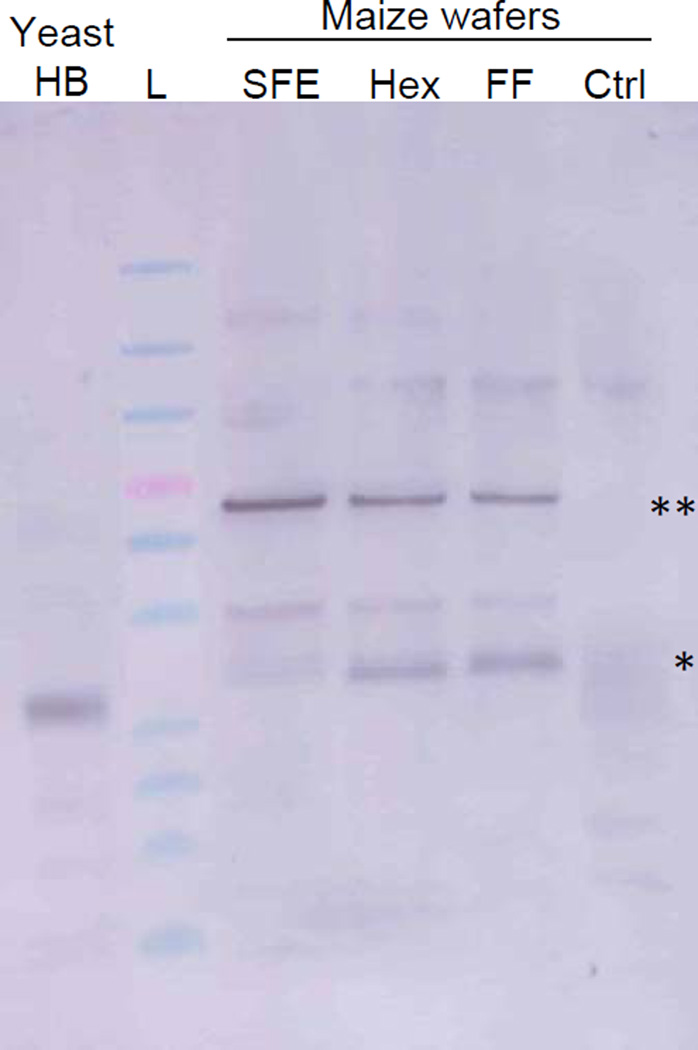
To independently assess the relative concentration of HBsAg in the wafers, protein blots were also performed (Figure 1). The protein blot did not indicate that the hexane treated material contained higher concentrations of HBsAg compared to the other treatments. The hexane-treated and full fat wafers seemed to predominantly form a mix of monomeric and dimeric HBsAg under reducing conditions whereas the SFE-treated wafer primarily formed dimeric structures with very little evidence of monomeric molecules.
Figure 1.
HBsAg monomer and dimer formation as resolved by SDS-PAGE under reducing conditions. Maize wafer samples include SFE-treated (SFE), hexane-treated (Hex), full fat (FF), and control (Ctrl) extracts. Monomers and dimers are indicated by single and double asterisks, respectively. The positive control (Yeast HB) represents 100ng of yeast recombinant HBsAg. Ladder (L) bands mark 181, 115, 82, 64 (red band), 49, 37, 26, 19, 15, and 6 kDa sizes.
Mucosal response to oral administration of HBsAg
To test the immunogenicity of the different germ treatments used in wafer formulations and compare their effect relative to a parenteral boosting regime, BALB/c mice were administered 0.5µg of Recombivax® as a primary injection at day 0. Mice in theRecombivax®-boosted treatment received 0.5µg Recombivax® on days 112 and 126. Boosting with corn wafers was initiated on these same days and spanned three consecutive days. Treatments included wafers made with full fat, hexane-treated, SFE-treated, and control germ. Two 3.1g wafers were administered each day which resulted in approximately 5.5 mg of HBsAg being offered during each boost. Mice consumed approximately 75% of the wafers, approximating a 4 mg dose per boost. A sixth treatment consisted of mice receiving saline as a primary injection and control wafers on boosting days. Mice receiving this last treatment showed no immunological response in any of the assays (data not shown).
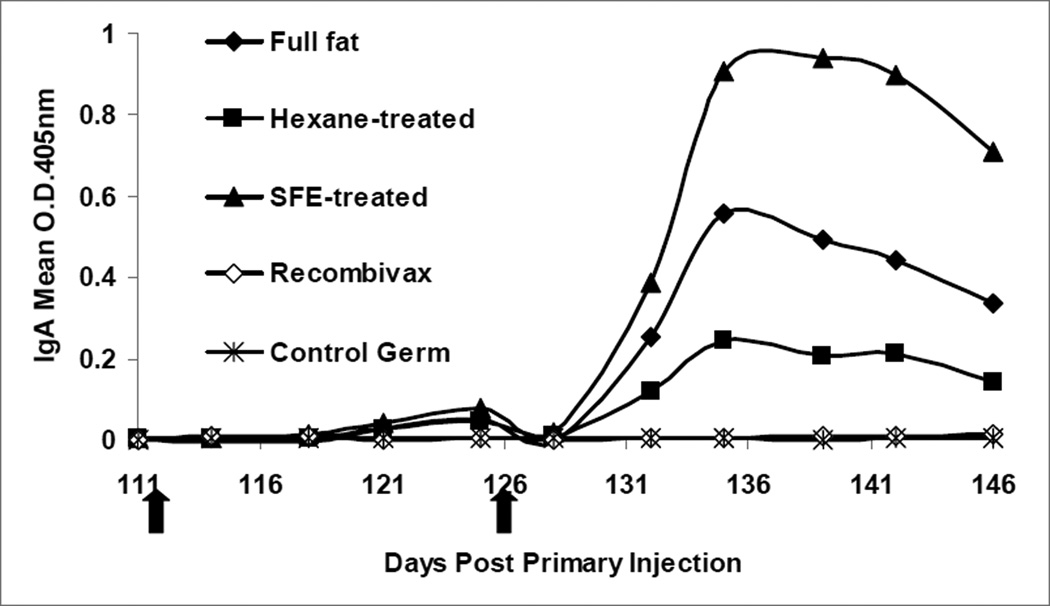
Secretory IgA in fecal material was strongly induced in all treatments of orally delivered HBsAg while no response was detectable in Recombivax®-boosted mice or in control wafer fed mice (Figure 2). Pre-boost data revealed that no response was detected in the first five weeks after primary injection for twelve mice (data not shown). All 11 mice in the SFE wafer treatment group displayed increasing IgA titers after boosting relative to pre-immune titers and more than 70% of mice in full fat and hexane treatments showed strong increases after the second boost.
Figure 2.
Fecal IgA anti-HBsAg response as determined by a sandwich ELISA. Black arrows indicate initiation of oral or Recombivax® boosting. Preimmune-subtracted mean O.D. values were determined for each treatment.
The mucosal response to oral boosting was further assessed via secretory IgA in mouse saliva. All SFE-boosted mice that were tested (n=5) showed detectable total Ig levels (O.D. values greater than 4-fold above the highest value recorded for mice fed control wafers), ranging from 2 to 142 mIU/mL. Additionally, O.D. values from Recombivax®-boosted mice were indistinguishable from control-boosted mice that received either a Recombivax® primary or a saline primary.
Systemic response to oral administration of HBsAg
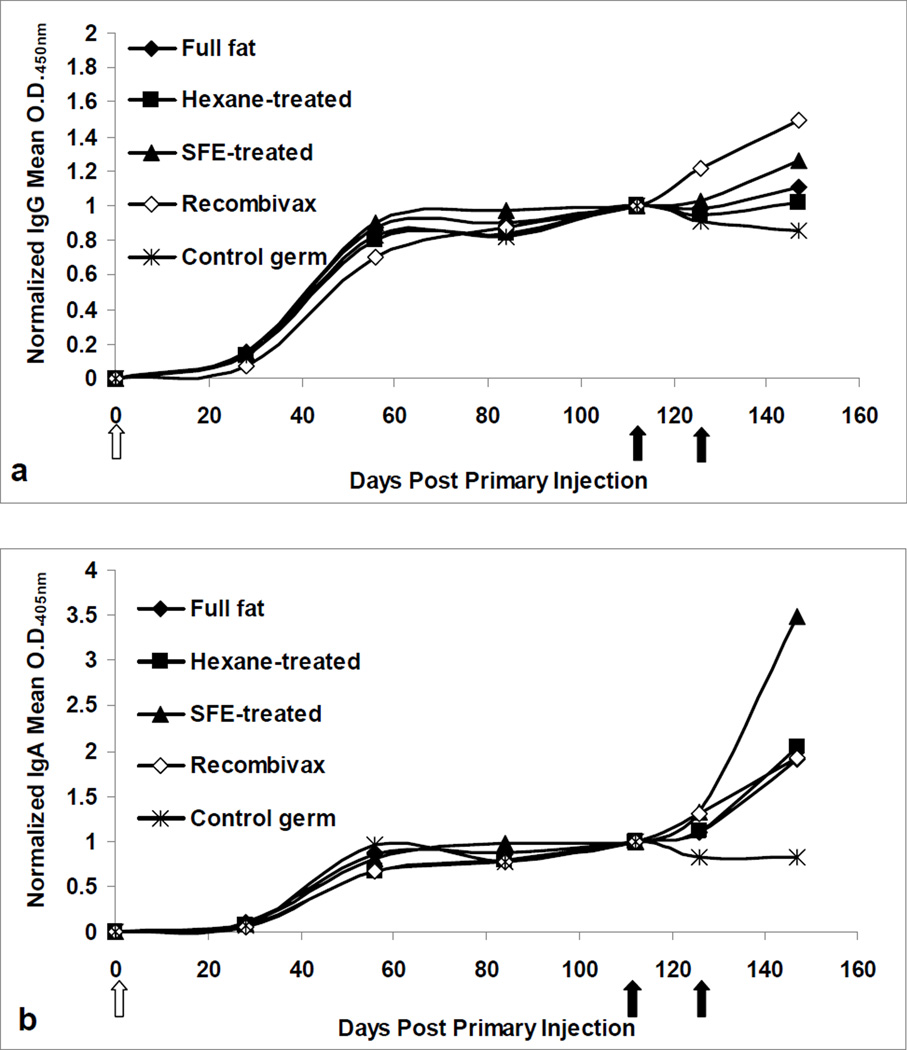
Mice were also tested for their systemic response to the oral and injected HBsAg boosting doses. Mouse titers were normalized to their pre-boost levels and post-boost normalized titers were compared (Figure 3). After boosting, Recombivax® and SFE-boosted mice showed the highest increase in IgG titers (Figure 3a). Induction of IgA titers after boosting was more pronounced for mice fed SFE-treated wafers, increasing 3.5-fold above pre-boost levels (Figure 3b). Mice fed full fat or hexane-treated material showed comparable mean responses to the Recombivax® boosted mice, increasing approximately 2-fold above pre-boost levels. Mice fed control germ wafers showed decreasing IgG and IgA titers after boosting, as expected.
Figure 3.
Serum anti-HBsAg in mice. (a) IgG and (b) IgA response in mice as determined by a sandwich ELISA. Preimmune-subtracted O.D. values were normalized to pre-boost values for each mouse (O.D. at day n/O.D. at day 112) and means were determined for all mice in a given treatment. A white arrow indicates primary injection of 0.5µg Recombivax® on day 0. Black arrows indicate initiation of oral boosting or Recombivax® injection.
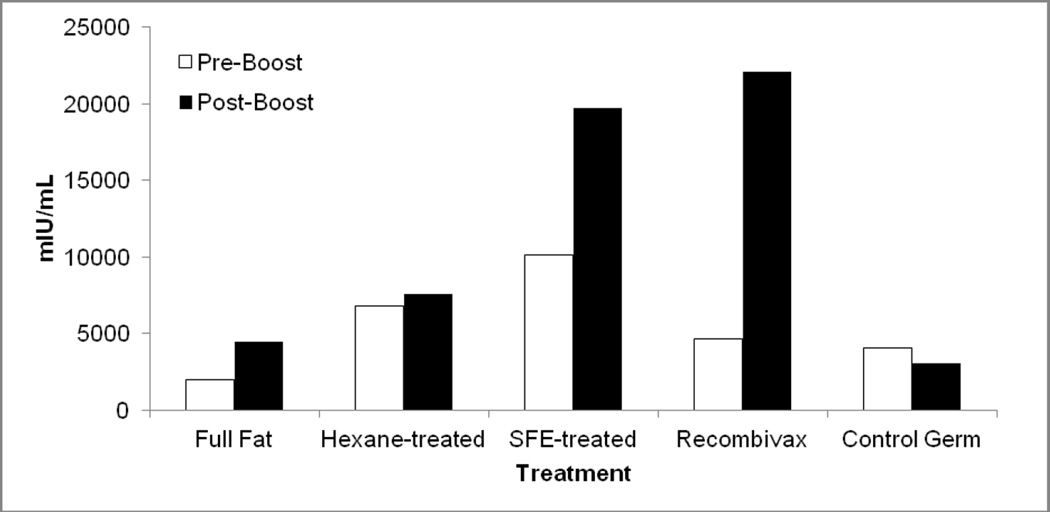
Another metric for detecting serum antibody responses, mIU/mL, was assessed using the ETI-AB-AUK PLUS DiaSorin kit. Post-boost titers were highest for SFE wafer-fed and Recombivax® boosted mice, while full fat and hexane-treated wafers produced smaller increases and lower overall titers. As expected, the control germ wafers produced no response in mice and resulted in a decrease in titer after boosting. Although all the mice presented in Figure 4 were injected with 0.5µg Recombivax® on day 0, there was substantial variability in the pre-boost titers across all treatments.
Figure 4.
Geometric mean titers of serum anti-HBsAg Ig in mice boosted parenterally (Recombivax®) or boosted orally with HBsAg germ or control germ. Pre-boost samples and post-boost samples were collected at 112 days and 147 days (terminal bleed) after primary injection, respectively.
Statistical analysis
Statistical analyses were undertaken for all assays to establish statistical differences between treatments at the 5% overall confidence level (Table 2). Differences in fecal IgA were statistically significant between the oral HBsAg boosting treatments and the Recombivax® boosting treatment. In addition, the SFE wafers produced a response that was significantly higher than Recombivax®, hexane, and control booster treatments.
Table 2.
Statistically significant differences between treatments detected at the 5% overall confidence level. Treatments with the same letter in a given assay do not show statistically significant differences. “A” represents the group with the highest response; “B” represents the group the second highest response, etc.
| Assay | ||||||
|---|---|---|---|---|---|---|
| Treatment (primary:boost) |
Fecal response |
Saliva response |
Serum IgG |
Serum IgA |
Serum mIU/mL | |
| (pre- to post- boost change) |
(final titer) |
|||||
| RBX:SFE | A | A | A B | A | A B | A |
| RBX:FF | A B | -* | B C | A | B | A B |
| RBX:Hex | B | - | B C | A | B | A B |
| RBX:RBX | C | B | A | A | A | A |
| RBX:Ctrl | C | B | C | B | B | B |
| Saline:Ctrl | - | B | - | - | - | - |
RBX = Recombivax, SFE = supercritical fluid extraction, FF = full fat, Hex = hexane, Ctrl = control wafer
statistical differences not determined for these treatments
Fecal IgA titers were significantly higher for mice fed SFE wafers relative to Recombivax®-treated mice, and injection of the mice with HBsAg was not statistically different than mice fed control wafers.
In terms of the systemic response, there was no statistical difference between Recombivax® boosted mice and SFE-boosted mice for either IgG or IgA normalized titers and both treatments were different from the control wafer treatment, as expected. Mice fed full fat and hexane-treated wafers also showed a significant difference in IgA titers when compared to control wafers, but the same differences were not detected in IgG titers. Serum titers in mIU/mL were analyzed using two different comparisons, due to the large variability in pre-boost titers. Firstly, the pre- to post-boost change in titer was compared across all treatments. SFE and Recombivax® treatments were not found to be statistically different, but SFE treatment was also not different from the control wafer treatment, despite obvious differences in pre- and post-boost mean changes in titer (Figure 4). This could be explained by the highly variable titers across mice in a given treatment. Secondly, mIU/mL terminal bleed titers (post-boost) were assessed and revealed that SFE and Recombivax® treatments were not statistically different from one another but were higher than the control wafer, full fat, and hexane treatments.
Discussion
Despite mounting interest for developing an oral vaccine against hepatitis B, few studies have directly compared oral candidate vaccines against commercially available parenteral vaccines. In this study, HBsAg oral vaccine booster doses were fed to mice and compared to Recombivax® boosting by injection. A boosting dose of approximately 4 mg per mouse, 2-fold greater than maize material fed to mice in a previous mouse study [22], demonstrated the potential of the maize seed production system for increased HBsAg concentration. Ongoing efforts project continued increase in HBsAg concentration over the next several years. High HBsAg accumulation in the maize system is also ensured by the use of an embryo (germ)-specific promoter and the ability to mechanically separate the germ from the rest of the seed [29]. Commercially, germ is routinely separated from endosperm and pericarp tissues [30–31] and therefore does not present an obstacle to increased production. High concentrations and ease of scaled-up processing are features of the maize system that may be key determinants for the delivery of an oral HBsAg vaccine in human populations.
In addition to increasing the concentration of HBsAg in maize material, the present study set out to compare the effect of different germ flour preparation techniques on mouse immunological responses. The germ portion of the seed was left untreated (full fat), or lipids were removed using hexane treatment or SFE treatment with CO2. CO2 is a very non-polar solvent and can be used to extract lipids from various materials. It can remove lipids without compromising enzyme activity [32] and can even increase the activity of some proteins, such as bovine pancreatic lipase [33]. Both hexane and SFE treatment preferentially extract non-polar lipids such as triglycerides, yet they differ in their extraction of phospholipids. SFE treatment extracts 10 to 100-fold fewer phospholipids than hexane treatment, as evidenced by oil fractions collected from corn germ and soybean [34–36], and has been shown to concentrate phospholipids in the solid fraction of egg yolks and buttermilk [37–38]. Since HBsAg is an integral membrane protein, is known to be associated with phospholipids in human serum, and has improved immunogenicity when incorporated into lipid micelles [39], the phospholipid content in the wafers may alter the immunogenic and structural properties of the protein. Alternatively or concomitantly, the SFE process itself may increase oligomerization of the HBsAg protein, a structural change shown to occur with SFE-treated ribonuclease [40]. The increased oligomerization may then impact the immunogenicity of the wafers. This hypothesis is supported by protein blot analysis of the three germ treatments, in which SFE-treated germ showed preferential dimer formation whereas hexane-treated and full fat germ showed preferential monomer formation (Figure 1). This difference may in turn, explain differences observed in the immunologic response in the subsequent mouse trials (see below). It may also explain the discrepancy between relative germ concentrations of HBsAg as determined by ELISA versus protein blot. Higher concentration in hexane-treated material seen by ELISA may result from an HBsAg molecule that is more fully exposed for lack of phospholipid components and an enrichment of monomeric subunits, therefore leading to increased binding of the polyclonal antibody used for detection. It is also worth noting that dimer formation in the germ samples is evident in the immunoblot, even under reducing conditions. This may indicate that HBsAg secondary structure is more resistant to denaturation in the maize material than in the purified yeast rHBsAg sample, which is fully reduced to the monomeric form under the same conditions. A more extensive investigation of the physical properties of these different samples is ongoing as is a characterization of optimal SFE conditions for HBsAg oligomer formation.
In terms of immunologic responses, differences between the germ treatments were most striking in the fecal secretory IgA response to oral boosting. SFE-treated wafers produced the strongest response, while hexane-treated wafers produced the weakest response to the orally-administered HBsAg (Figure 2). Interestingly, no response was detected from the Recombivax®-injected boost, suggesting that mucosal immunologic responses against HBsAg are largely induced by antigen delivered via mucosal routes. This may have important implications for protection against pathogens that primarily invade mucosal surfaces. These data suggest that oral boosting can provide a more balanced systemic/mucosal response than injection alone and may warrant a review of present vaccination practices for providing optimal protection against pathogen assault.
Increased mucosal antibody titers for orally boosted mice were also evident in saliva, albeit at low titers, and were absent in Recombivax®-injected and control mice. Smaller increases in saliva titers may be explained by the high turnover of fluid in the oral cavity, or perhaps a suboptimal response due to a timepoint collection with a non-maximal titer. Because saliva collection is physically demanding for the mice, a more thorough characterization of the salivary IgA response over time will need to be conducted in human volunteers or a larger model organism.
Another aim of the study was to compare the systemic response for injected boosting and oral boosting regimes. In serum, oral boosting with SFE material was comparable to injected boosting, as assessed by IgG and IgA pre-boost normalized titers (Figure 3). The fold increase in IgG titer from SFE boosting showed no statistically significant difference when compared to Recombivax® boosting, although Recombivax®did have an overall greater increase in these samples. To test whether these sample differences are reproducible, additional mouse studies will need to be undertaken. Serum IgA seemed to show a much greater response in mice fed SFE wafers, with titers more than 3-fold above pre-boost levels, while the Recombivax® injections resulted in a 2-fold increase above pre-boost levels; however, this difference was not statistically significant. It is not clear whether these fold-changes were influenced by pre-boost titers, since SFE-treated pre-boost titers were two-fold lower than Recombivax® pre-boost titers. Nonetheless, it can be argued that the boosting effect is at least comparable in both treatments, as final post-boost titers were similar. Again, additional mouse studies will need to be conducted to verify these two scenarios.
Serum titers were also assessed by assaying mIU/mL of total Ig anti-HBsAg antibody (Figure 4). As seen with the serum IgA titers, mouse treatments showed very different pre-boost titers, despite all mice receiving equivalent primary injections at the initiation of the study. The high variability complicates analysis of the post-boost titers since it decreases the power of the statistical analyses, but tentative conclusions can be made. When comparing SFE and Recombivax® treatments, there was no statistical difference when analyzing either the total mIU/mL titers in the post-boost (terminal) bleed, or the change in titer from pre- to post-boost bleeds. In addition, terminal bleed titers were lower in hexane-treated and full fat fed mice and significantly different than Recombivax®-boosted mice. SFE treatment, therefore, seems to be the preferred oral boosting option of the three wafer treatments.
A final aim of the study was to confirm the viability of a 2-dose oral boosting regime. This study clearly shows that two oral doses can induce a robust immune response, and demonstrates that oral boosting could be used as an alternative to parenteral boosting.
Conclusions
These data establish the potential for a hepatitis B oral vaccine in the absence of an adjuvant. They indicate that serum titers in mice boosted orally with SFE material or parenterally with Recombivax® injection are comparable, and that SFE material is far more effective than Recombivax® for inducing a mucosal response. Hexane-treated and full fat wafers also induced immunologic responses, but with reduced efficacy relative to the SFE-treated wafers and the Recombivax® injected booster doses.
Highlights.
Oral HBsAg boosting induces strong mucosal responses whereas Recombivax® does not
SFE-treated wafers enhance the immune response over full fat or hexane-treated wafers
SFE treatment of maize favors HBsAg dimer formation
Two boosting doses of oral or parenteral vaccine produce comparable responses
Oral HBsAg boosting does not require an adjuvant for a robust response
Acknowledgments
This project was supported by NIH grant 2R44AI068239-03A1. The authors would like to thank Erin Fanning and Jenna Kranz for their assistance during the animal studies, and Alessa Moscoso and Maria Fischer for their assistance with the processing of fecal and serum samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Hayden CA. In: An oral vaccine for hepatitis B: challenges, setbacks, and breakthroughs, in Commercial plant-produced recombinant protein products: Case studies. Howard JA, Hood EE, editors. Accepted, Springer; [Google Scholar]
- 3.Ahishali E, Boztas G, Akyuz F, Ibrisim D, Poturoglu S, Pinarbasi B, Ozdil S, Mungan Z. Response to hepatitis B vaccination in patients with celiac disease. Digestive diseases and sciences. 2008;53(8):2156–2159. doi: 10.1007/s10620-007-0128-3. [DOI] [PubMed] [Google Scholar]
- 4.Landrum ML, Hullsiek KH, O'Connell RJ, Chun HM, Ganesan A, Okulicz JF, Lalani T, Weintrob AC, Crum-Cianflone NF, Agan BK. Hepatitis B Vaccine Antibody Response and the Risk of Clinical AIDS or Death. PLOS One. 2012;7(3):e33488. doi: 10.1371/journal.pone.0033488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurence JC. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. The American journal of medicine. 2005;118(10):75–83. doi: 10.1016/j.amjmed.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi S, Spina M, Spicuzza L, Rotolo N, La Rosa M. Hepatitis B vaccination failure in celiac disease: Is there a need to reassess current immunization strategies? Vaccine. 2009;27(43):6030–6033. doi: 10.1016/j.vaccine.2009.07.099. [DOI] [PubMed] [Google Scholar]
- 7.Roome AJ, Walsh SJ, Cartter ML, Hadler JL. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA: the Journal of the American Medical Association. 1993;270(24):2931–2934. [PubMed] [Google Scholar]
- 8.Tohme RA, Awosika-Olumo D, Nielsen C, Khuwaja S, Scott J, Xing J, Drobeniuc J, Hu DJ, Turner C, Wafeeg T. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine. 2011;29:9316–9320. doi: 10.1016/j.vaccine.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vida Pérez L, Gómez Camacho F, García Sánchez V, Iglesias Flores EM, Castillo Molina L, Cerezo Ruiz A, Casáis Juanena L, De Dios Vega JF. Eficacia de la vacuna contra el virus de la hepatitis B en pacientes con enfermedad inflamatoria intestinal. Medicina clínica. 2009;132(9):331–335. doi: 10.1016/j.medcli.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Williams RE, Sena AC, Moorman AC, Moore ZS, Sharapov UM, Drobenuic J, Hu DJ, Wood HW, Xing J, Spradling PR. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine. 2012;30(21):3147–3150. doi: 10.1016/j.vaccine.2012.02.078. [DOI] [PubMed] [Google Scholar]
- 11.Zitt E, Sprenger-Mähr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine. 2011;30:931–935. doi: 10.1016/j.vaccine.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 12.Nisihara R, De Bem R, Negreiros P, Utiyama S, Oliveira N, Amarante H. Low hepatitis B vaccine response in children with Down syndrome from Brazil. Child: Care, Health and Development. 2013 doi: 10.1111/cch.12099. [DOI] [PubMed] [Google Scholar]
- 13.Vermeiren A, Hoebe CJ, Dukers-Muijrers NH. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. Journal of Clinical Virology. 2013;58(1):262–264. doi: 10.1016/j.jcv.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Lu P-j, Byrd KK, Murphy TV, Weinbaum C. Hepatitis B vaccination coverage among high-risk adults 18–49 years, US, 2009. Vaccine. 2011;29(40):7049–7057. doi: 10.1016/j.vaccine.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Lum PJ, Ochoa KC, Hahn JA, Shafer KP, Evans JL, Moss AR. Hepatitis B virus immunization among young injection drug users in San Francisco, Calif: the UFO Study. American Journal of Public Health. 2003;93(6):919–923. doi: 10.2105/ajph.93.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyamathi AM, Marlow E, Branson C, Marfisee M, Nandy K. Hepatitis A/B vaccine completion among homeless adults with history of incarceration. Journal of Forensic Nursing. 2012:13–22. doi: 10.1111/j.1939-3938.2011.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linkins RW, Chonwattana W, Holtz TH, Wasinrapee P, Chaikummao S, Varangrat A, Tongtoyai J, Mock PA, Curlin ME, Sirivongrangson P. Hepatitis A and hepatitis B infection prevalence and associated risk factors in men who have sex with men, Bangkok, 2006–2008. Journal of Medical Virology. 2013;85(9):1499–1505. doi: 10.1002/jmv.23637. [DOI] [PubMed] [Google Scholar]
- 18.MacKellar DA, Valleroy LA, Secura GM, McFarland W, Shehan D, Ford W, LaLota M, Celentano DD, Koblin BA, Torian LV. Two decades after vaccine license: hepatitis B immunization and infection among young men who have sex with men. American Journal of Public Health. 2001;91(6):965–971. doi: 10.2105/ajph.91.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simard EP, Miller JT, George PA, Wasley A, Alter MJ, Bell BP, Finelli L. Hepatitis B Vaccination Coverage Levels Among Healthcare Workers in the United States, 2002–2003. Infection Control and Hospital Epidemiology. 2007;28(7):783–790. doi: 10.1086/518730. [DOI] [PubMed] [Google Scholar]
- 20.Hayden CA, Egelkrout EM, Moscoso AM, Enrique C, Keener TK, Jimenez-Flores R, Wong JC, Howard JA. Production of highly concentrated, heat-stable hepatitis B surface antigen in maize. Plant Biotechnology Journal. 2012;10(8):979–984. doi: 10.1111/j.1467-7652.2012.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, Natarajan N, Goodwin P, Arntzen CJ, Mason HS. Immunogenicity in humans of an edible vaccine for hepatitis B. Proceedings of the National Academy of Sciences, USA. 2005;102(9):3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayden CA, Streatfield SJ, Lamphear BJ, Fake GM, Keener TK, Walker JH, Clements JD, Turner DD, Tizard IR, Howard JA. Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen. Vaccine. 2012;30(19):2937–2942. doi: 10.1016/j.vaccine.2012.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM. Plant-based production of biopharmaceuticals. Current Opinion in Plant Biology. 2004;7(2):152–158. doi: 10.1016/j.pbi.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Lamphear BJ, Streatfield SJ, Jilka JM, Brooks CA, Barker DK, Turner DD, Delaney DE, Garcia M, Wiggins B, Woodard SL, Hood EE, Tizard IR, Lawhorn B, Howard JA. Delivery of subunit vaccines in maize seed. J Control Release. 2002;85(1–3):169–180. doi: 10.1016/S0168-3659(02)00282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pignitter M, Somoza V. Critical Evaluation of Methods for the Measurement of Oxidative Rancidity in Vegetable Oils. Journal of Food and Drug Analysis. 2012;20(4):772–777. [Google Scholar]
- 26.Beckman EJ. Supercritical and near-critical CO2 in green chemical synthesis and processing. The Journal of Supercritical Fluids. 2004;28(2):121–191. [Google Scholar]
- 27.Bunge Typical Composition of Yellow Dent Corn. 2013 Sep 20; Available from: http://www.bungenorthamerica.com/news/pubs/03_bunge_milling_process_diagram.pdf. [Google Scholar]
- 28.Eskin N, Przybylski R. Antioxidants and Shelf Life of Foods, in Food Shelf Life Stability: Chemical, Biochemical, and Microbiological Changes. CRC Press; 2010. pp. 175–210. [Google Scholar]
- 29.Gent JF. Apparatus for degermination and decortication of Indian corn. US Patent No. 735664. US Patent Office. 1903
- 30.Beall Beall Degerminator Co. 2013 Nov 26; Available from: http://www.satake-512 group.com/about/scope.html. [Google Scholar]
- 31.Satake Satake Group. 2013 Nov 26; Available from: http://www.satake512group.com/about/scope.html. [Google Scholar]
- 32.Sellers SP, Clark GS, Sievers RE, Carpenter JF. Dry powders of stable protein formulations from aqueous solutions prepared using supercritical CO2-assisted aerosolization. Journal of Pharmaceutical Sciences. 2001;90(6):785–797. doi: 10.1002/jps.1032. [DOI] [PubMed] [Google Scholar]
- 33.Gieβauf A, Gamse T. A simple process for increasing the specific activity of porcine pancreatic lipase by supercritical carbon dioxide treatment. Journal of Molecular Catalysis B: Enzymatic. 2000;9(1):57–64. [Google Scholar]
- 34.Friedrich JP, List GR. Characterization of soybean oil extracted by supercritical carbon dioxide and hexane. Journal of Agricultural and Food Chemistry. 1982;30(1):192–193. [Google Scholar]
- 35.List G, Friedrich J. Oxidative stability of seed oils extracted with supercritical carbon dioxide 1. Journal of the American Oil Chemists Society. 1989;66(1):98–101. [Google Scholar]
- 36.Rónyai E, Simandi B, Tömösközi S, Deák A, Vigh L, Weinbrenner Z. Supercritical fluid extraction of corn germ with carbon dioxide–ethyl alcohol mixture. The Journal of Supercritical Fluids. 1998;14(1):75–81. [Google Scholar]
- 37.Astaire J, Ward R, German J, Jimenez-Flores R. Concentration of polar MFGM lipids from buttermilk by microfiltration and supercritical fluid extraction. Journal of Dairy Science. 2003;86(7):2297–2307. doi: 10.3168/jds.S0022-0302(03)73822-3. [DOI] [PubMed] [Google Scholar]
- 38.Froning G, Wehling R, Cuppett S, Pierce M, Niemann L, Siekman D. Extraction of cholesterol and other lipids from dried egg yolk using supercritical carbon dioxide. Journal of Food Science. 1990;55(1):95–98. [Google Scholar]
- 39.Gavilanes F, Gonzalez-Ros JM, Peterson DL. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. Journal of Biological Chemistry. 1982;257(13):7770–7777. [PubMed] [Google Scholar]
- 40.Weder JK. Effect of supercritical carbon dioxide on proteins. Zeitschrift für Lebensmittel-Untersuchung und Forschung. 1980;171(2):95–100. [Google Scholar]