Mutations in PNPO are a known cause of neonatal onset seizures that are resistant to pyridoxine but responsive to pyridoxal phosphate (PLP). Mills et al. show that PNPO mutations can also cause neonatal onset seizures that respond to pyridoxine but worsen with PLP, as well as PLP-responsive infantile spasms.
Keywords: pyridoxal 5’-phosphate (PLP), pyridoxine, pyridox(am)ine 5’-phosphate oxidase (PNPO), seizures, epilepsy
Abstract
The first described patients with pyridox(am)ine 5’-phosphate oxidase deficiency all had neonatal onset seizures that did not respond to treatment with pyridoxine but responded to treatment with pyridoxal 5’-phosphate. Our data suggest, however, that the clinical spectrum of pyridox(am)ine 5’-phosphate oxidase deficiency is much broader than has been reported in the literature. Sequencing of the PNPO gene was undertaken for a cohort of 82 individuals who had shown a reduction in frequency and severity of seizures in response to pyridoxine or pyridoxal 5’-phosphate. Novel sequence changes were studied using a new cell-free expression system and a mass spectrometry-based assay for pyridoxamine phosphate oxidase. Three groups of patients with PNPO mutations that had reduced enzyme activity were identified: (i) patients with neonatal onset seizures responding to pyridoxal 5’-phosphate (n = 6); (ii) a patient with infantile spasms (onset 5 months) responsive to pyridoxal 5’-phosphate (n = 1); and (iii) patients with seizures starting under 3 months of age responding to pyridoxine (n = 8). Data suggest that certain genotypes (R225H/C and D33V) are more likely to result in seizures that to respond to treatment with pyridoxine. Other mutations seem to be associated with infertility, miscarriage and prematurity. However, the situation is clearly complex with the same combination of mutations being seen in patients who responded and did not respond to pyridoxine. It is possible that pyridoxine responsiveness in PNPO deficiency is affected by prematurity and age at the time of the therapeutic trial. Other additional factors that are likely to influence treatment response and outcome include riboflavin status and how well the foetus has been supplied with vitamin B6 by the mother. For some patients there was a worsening of symptoms on changing from pyridoxine to pyridoxal 5’-phosphate. Many of the mutations in PNPO affected residues involved in binding flavin mononucleotide or pyridoxal 5’-phosphate and many of them showed residual enzyme activity. One sequence change (R116Q), predicted to affect flavin mononucleotide binding and binding of the two PNPO dimers, and with high residual activity was found in Groups (ii) and (iii). This sequence change has been reported in the 1000 Genomes project suggesting it could be a polymorphism but alternatively it could be a common mutation, perhaps responsible for the susceptibility locus for genetic generalized epilepsy on 17q21.32 (close to rs72823592). We believe the reduction in PNPO activity and B6-responsive epilepsy in the patients reported here indicates that it contributes to the pathogenesis of epilepsy.
Introduction
Pyridox(am)ine phosphate oxidase (PNPO, EC 1.4.3.5) is a flavin mononucleotide (FMN)-dependent oxidase required for synthesis of pyridoxal 5’-phosphate (PLP) from pyridoxine (and its phosphate, PNP, and glucoside) and from pyridoxamine (and its phosphate, PMP) in the diet. It is also required for recycling PMP to regenerate PLP, the active cofactor. PLP is the cofactor for >140 enzyme-catalyzed reactions in man, including many involved in synthesis or degradation of amino acids or amines that serve as neurotransmitters or neuromodulators in the brain (Garcia-Cazorla et al., 2012; Mills et al., 2012).
Neonatal epileptic encephalopathy is characterized by the onset, shortly after birth, of drug-resistant seizures associated with severe neurological dysfunction that can be fatal. PNPO deficiency (OMIM 6032870) is an autosomal recessive inborn error of metabolism that leads to a seizure disorder, presenting in the newborn period (neonatal epileptic encephalopathy) or early infancy, that can be treated with pyridoxal 5’-phosphate but (classically) not pyridoxine. Mills et al. (2005) showed that mutations in PNPO led to reduced enzyme activity when expressed in Chinese hamster ovary cells.
A review by Garcia-Cazorla et al. (2012) of 16 cases with PNPO deficiency (eight families) revealed that clinical features included in utero seizures, 3/16; foetal distress before delivery, 5/16; premature birth, mean 32 weeks gestation; low Apgar scores/requiring intubation at delivery, 5/16; onset of seizures—in first 24 h, 11/14; between 24 h and 72 h, 2/14; between 72 h and 2 weeks 1/14; burst suppression pattern on EEG, 10/11; seizures completely resistant to antiepileptic drugs, 13/16; completely resistant to pyridoxine, 7/10; metabolic acidosis 6/16; raised blood lactate 8/16; distressing spasms (dystonia), 3/16; anaemia, 3/16; hepatomegaly, 3/16; abdominal distension, 2/16; and hypoglycaemia 2/16. In the small number of patients tested, CSF PLP concentration was low (Ormazabal et al., 2008). However, other disorders can also lead to low CSF PLP (Footitt et al., 2011).
In addition to the small number of infants in the literature whose PLP-responsive neonatal epileptic encephalopathy has been shown to be a result of PNPO deficiency, a larger number of infants has been described for whom empirical clinical trials demonstrated that their severe epilepsy was better controlled with PLP than with pyridoxine (Wang et al., 2005). This suggests that PNPO deficiency might be a cause, not only of neonatal epileptic encephalopathy, but also of other later-onset seizure disorders.
A genome-wide association study of patients of European ancestry identified an important susceptibility locus for genetic generalized epilepsies as a whole at 17q21.32 (rs72823592), the closest gene to which is PNPO (EPICURE Consortium et al., 2012). This data suggests that mild PNPO deficiency could be a susceptibility factor for genetic generalized epilepsies presenting at various ages.
Mutations in PNPO known to be associated with neonatal epileptic encephalopathy for which expression studies have shown reduced enzyme activity are: IVS3-1g>a, X262Q, R229W (Mills et al., 2005) and R95H (Khayat et al., 2008). Other probable neonatal epileptic encephalopathy-causing PNPO mutations include R95C, D33V, c.246delT (Hoffmann et al., 2007), A174X (Ruiz et al., 2008), R225C (Veerapandiyan et al., 2011) and G118R (Pearl et al., 2012).
The effects of R229W on PNPO catalytic function and crystal structure have been studied by Musayev et al. (2009). This variant is 850-fold less efficient than the wild-type enzyme because of a 192-fold decrease in pyridoxine 5'-phosphate affinity and a 4.5-fold decrease in catalytic activity. There is also a 50-fold reduction in affinity for the FMN cofactor. The decrease in affinity for pyridoxine 5’-phosphate suggests that, for this mutation, significantly increased synthesis of PLP might be achieved by high dose pyridoxine. In fact, in the premature infants affected by the R229W mutation, administration of pyridoxine did lead to partial improvement in clonic contractions and lip-smacking automatisms; in contrast, patients harbouring mutations with no residual activity showed no response to pyridoxine. Musayev et al. (2009) suggested that, for the R229W mutation at least, another treatment likely to be beneficial would be riboflavin, which would supply extra FMN and partially overcome the effect of reduced FMN binding.
Thus, a picture is emerging of PNPO sequence variations that produce epilepsy that only responds to treatment with PLP, through variations that may respond to pyridoxine and/or riboflavin to PNPO variations that might be insufficient to produce epilepsy alone but might do so in concert with other factors determining brain PLP levels such as genes influencing blood PLP levels [e.g. the TNSALP gene (now known as ALPL) encoding tissue non-specific alkaline phosphatase (Hazra et al., 2009; Tanaka et al., 2009)] and dietary intakes of B6 vitamers and riboflavin.
In this study we looked for PNPO sequence variations in children with epilepsy that responded to treatment with pyridoxine or PLP. We characterized the phenotypic spectrum of PNPO deficiency further and demonstrated that some children with PNPO deficiency respond to treatment with pyridoxine. To determine the effect of sequence variations on PNPO enzyme activity we set up a new, cell-free system for expression of the mutant proteins and a new mass spectrometry-based enzyme assay for measurement of PNPO activity.
Materials and methods
Patients
This study was approved by the Ethics Committee of UCL Institute of Child Health and Great Ormond Street Hospital (04/Q0508/81). Patients included satisfied the following criteria: (i) improved seizure control following administration of pyridoxine or PLP; and (ii) exclusion of antiquitin deficiency by measurement of urinary α-aminoadipic semialdehyde and/or sequencing of ALDH7A1 (Mills et al., 2010). Detailed histories and the questionnaire sent to clinicians are available in the Supplementary material.
Chemical reagents
All chemicals unless mentioned specifically were from Sigma Aldrich. D2 PLP was kindly supplied by Professor Coburn, Department of Chemistry, Indiana University-Purdue University, Fort Wayne, Indiana, USA.
PNPO sequencing
Mutation analysis of genomic DNA and complementary DNA was as described previously (Mills et al., 2005).
Site-directed mutagenesis of PNPO and expression studies
Wild-type PNPO complementary DNA had been cloned into pSP72 previously (Mills et al., 2005). Site-directed mutagenesis was carried out using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene), according to manufacturer’s instructions. The complementary DNA was then amplified using ProofStart™ DNA polymerase (Qiagen) using primers detailed in Supplementary Table 1. EcoRI digestion products were subcloned into the pT7CFE1-CHis expression vector (Thermo Scientific). PNPO was expressed using the Thermo Scientific 1-Step Human Coupled IVT Kit according to manufacturer’s instructions; this expression system uses a HeLa cell lysate. The reaction was incubated for 6 h at 30°C. PNPO enzyme activity was measured with PMP as substrate. Expressed protein (8 µl) was incubated at 37°C in the dark in 20 mM potassium phosphate (pH 7.6) containing 2.5 µM PMP and 1.5 µM FMN in a final volume of 120 µl. The reaction was stopped by addition of 120 µl 0.3 N trichloroacetic acid containing d2-PLP and incubated on ice for 30 min before centrifugation (10 000 rpm, 10 min at 4°C). The supernatant containing the B6 vitamers was analysed by HPLC-MS/MS (Footitt et al., 2013). Enzyme activity was calculated as pmol PLP synthesized/mg protein/min and expressed as a percentage of the activity obtained with the wild-type enzyme.
Results
Mutations
Sequencing of the PNPO gene was undertaken for 82 individuals that had shown some reduction in frequency and severity of seizures in response to pyridoxine or PLP. Mutations were identified in PNPO for 15 patients (Table 1) from 14 families. Five of these were novel; three missense mutations and two deletions/insertions. The novel missense mutations E120K, P213S and R225H have not been reported as polymorphisms in Ensembl (http://www.ensembl.org). All novel missense mutations were tested for pathogenicity using both SIFT (Sim et al., 2012) and the PolyPhen web server (Ramensky et al. 2002) and were predicted to be damaging or probably damaging, respectively (Supplementary Table 2).
Table 1.
Summary of mutations/sequence variants found in PNPO that have an effect on PNPO enzyme activity
| Group | Patient | Current age | Mutation/sequence variant | Presumed effect | Age of seizure onset | References |
|---|---|---|---|---|---|---|
| (i) | 1 | 4 y | c.[284G>A] (M) + c.[148G> A];c.[364-1G>A]a (P) | p.R95H (M) + p.E50K;Splice errors a (P) | 30 min | Mills et al., 2005; Khayat et al., 2008 |
| 2 | 5 y 2 m | c.[98A>T] + c.[98A>T] | p.D33V + p.D33V | 6 h | Hoffmann et al., 2007 | |
| 3 | 2 y 7 m | c.[637C>T] + c.[637C>T] | p.P213S + p.P213S | 90 min | Novel | |
| 4* | 4 m | c.[637C>T] + c.[637C>T] | p.P213S + p.P213S | None* | Novel | |
| 5 | 1 y | c.[283C>T] (M) + c.[283C>T] (P) | p.R95C (M) + p.R95C (P) | 2 h | Khayat et al., 2008 | |
| 6 | 4 y | c.[641dupA] +?** | p.Q214fs +? ** | 5 h | Novel | |
| (ii) | 7 | 6 y 5 m | c.[347G>A] (M) + c.[347G>A] (P) | p.R116Q (M) + p.R116Q (P) | 5 m | Novel |
| (iii) | 8 | 9 y | c.[347G>A] + c.[347G>A] | p.R116Q + p.R116Q | 3 h | Novel |
| 9 | 2 y | c.[98A>T] + c.[998A>T] | p.D33V + p.D33V | 3 w | Hoffmann et al., 2007 | |
| 10 | 23 y | c.[98A>T] + c.[358G>A] | p.D33V + p.E120K | 2 m | Hoffmann et al., 2007 + novel | |
| 11 | 21 y | c.[98A>T] (P) + c.264-21_ 264-1delinsC (M) | p.D33V (P) + Splice errors (M) | 3 h | Hoffmann et al., 2007 + novel | |
| 12 | 41 y | c.[98A>T] + c.[347G>A] + c. [673C>T] | p.D33V + p.R116Q + p.R225Cb | 14 d | Hoffmann et al., 2007 + novel + Veerapandiyan et al., 2011 | |
| 13 | 9 y | c.[674G>A] + c.[674G>A] | p.R225H + p.R225H | 24 h | Novel | |
| 14 | 3 y | c.[347G>A];c.[674G>A] + c.[347G >A];c.[674G>A] | p.R116Q;p.R225H + p.R116Q;p.R225H c | 30 min | Novel | |
| 15 | 9 y | c.[347G>A];c.[674G>A] + c.[347G >A];c.[674G>A] | p.R116Q;p.R225H + p.R116Q;p.R225H c | 10 h | Novel |
(i) Neonatal onset seizures responding to pyridoxal 5’-phosphate;
(ii) Infantile spasms (onset 5 months) responsive to pyridoxal 5’-phosphate;
(iii) Seizures starting under 3 months of age responding to pyridoxine.
Where parent DNA available inheritance was investigated and the allele carrying the mutation is indicated as P (paternal) or M (maternal).
*Sibling of Patient 3, no seizures as treated prophylactically;
**Second mutation not found, complementary DNA and genomic DNA sequenced;
ac.[148G>A] and c.[364-1G>A] were inherited in cis, this was confirmed by analysing parental DNA;
b No parental DNA was available to ascertain which muation R116Q was in cis with;
cAssume that R116Q has been inherited in cis with R225H, no parental DNA was available to confirm this.
Previously reported mutations included D33V, R95H, R95C and R225C. E50K, which has been reported as a single nucleotide polymorphism (Mills et al., 2005), was detected in Patient 1. The sequence variant R116Q (rs 17679445) was detected in 5 of 15 patients. This is reported on Ensembl as a single nucleotide polymorphism in the majority of populations with the exception of individuals of Asian ancestry (Supplementary Table 3) with a prevalence of 4–10% depending on the subpopulation investigated. R116Q was found in some patients in combination with other mutations that we believe are pathogenic and which have not been reported previously in the general population. However, R116Q was the only sequence change detected in Patients 7 and 8. Comparison of the frequency of R116Q in our PNPO-deficient patients relative to 1000 Genomes allele frequencies (http://www.ensembl.org) suggests that R116Q is over-represented in our patients compared to the general population (Supplementary Table 3).
Clinical phenotypes
Our series of PNPO patients has identified some new clinical presentations, including seizures with onset under 3 months responsive to treatment with pyridoxine and seizures with onset after 3 months responsive to PLP, suggesting that the phenotype of this disorder is much broader than reported previously. Patient clinical features are summarized in Table 2 and individual histories are given in the Supplementary material.
Table 2.
Demographic, clinical and electroencephalographic features observed in the present series with PNPO deficiency
| Clinical features and demographics | Incidence |
|---|---|
| Gender | Male n = 9; female n = 6 |
| Ethnicity | Caucasian n = 10; Turkish n = 1; Asian (British) n = 1; Kosovan n = 1; Pakastani n = 1; Unknown n = 1 |
| Parental consanguinity | 3/11 (27%) |
| Family history of infertility / foetal loss | 4/12 (25%) |
| Gestational age ≤ 37/40 | 6/13 (46%) |
| Abnormal intrauterine movements | 3/11 (27%) |
| Foetal distress | 4/11 (36%) |
| Apgar score <7 at 1 min | 2/11 (18%) |
| Acidosis | 5/10 (50%) |
| Respiratory distress | 4/11 (36%) |
| Hypotonia (neonatal) | 6/10 (60%) |
| Abdominal distension / vomiting | 1/7 (14%) |
| Irritability | 4/10 (40%) |
| Seizure onset within first 28 days | 12/14 (86%) |
| Resistance to antiepileptic drugs | Complete: 8/14 (57%); partial: 6/14 (43%) |
| EEG | Burst suppression: 6/11 (55%); Hypsarrhythmia: 1/11 (9%); Otherc: 4/11 (36%) |
| Seizure type: clonic | 5/11 (45%) |
| Seizure type: tonic | 3/10 (30%) |
| Seizure type: generalized tonic-clonic | 11/14 (79%) |
| Seizure type: myoclonic jerks | 6/11 (55%) |
| Seizure type: focal | 5/11 (45%) |
| Response to pyridoxine | 8/13 (62%) |
| Worsening of seizures upon change from PN to PLP | 3/8 (38%) |
| Trial of PLPa | 10/15 (67%) |
| Response to PLP trial | Immediate: 6/10 (60%); within 12 h to 3 days: 2/10 (20%) |
| Trial of PLP withdrawalb | 5/7 (71%) |
| Speech delay | 8/13 (62%) |
| Motor delay | Marked: 3/12 (33%); Minimal: 3/12 (17%); None: 6/12 (50)% |
| Breakthrough seizures with fever | 6/12 (50%) |
| Observed in the present series but not previously described in clinically diagnosed PNPO deficiency | |
| Severe neuropathy with persistent loss of ankle jerks (75 mg TDS PN) | 1 patient |
| Gut perforation and septicaemia | 1 patient |
| Liver function abnormalities | 2 patients |
aOne patient treated prophylactically.
bEither planned, accidental or reduced dose/kg due to age.
cIncluded suppression rhythms over right mid-temporal zone and focal seizures (bilateral, independent) for one patient and generalized suppressions and sharp wave complexes over both hemispheres for another. Where the incidence denominator is <15 the information was not completed/available for the patient in the proforma returned by the treating clinician.
Five patients were identified with neonatal onset seizures responsive to PLP and one affected sibling was treated prophylactically, in utero and from birth (Patient 4). Immediate seizure control was seen upon administration of PLP in three of five of these patients and seizure control was achieved in Patients 6 and 3 after treatment with PLP for 12 h and 3 days, respectively. Patient 7 did not present with seizures until 5 months of age. Initial doses of PLP ranged from 10–85 mg/kg/day and maintenance doses range from 10–72 mg/kg/day. Individual treatment regimes are detailed in the Supplementary material.
To our surprise, 62% of patients in this series that had an onset of seizures under 3 months showed a dramatic response to treatment with pyridoxine (Patients 8–15); initial and maintenance doses ranging from 18–55 and 6–26 mg/kg/day, respectively.
Reported seizure types (Table 2) included clonic (45%), myoclonic jerks (55%), tonic (30%), generalized tonic-clonic (79%) and focal (45%). EEG abnormalities included burst suppression and hypsarrhythmia (Table 2, Supplementary material). A cranial ultrasound at 5 h showed bilateral grade 3 intraventricular haemorrhages with associated moderate ventricular dilatation in one patient. The MRI findings (Supplementary material) varied from normal to extensive white matter oedema and a possible haemorrhage and changes reported as consistent with hypoxic ischaemic encephalopathy. Two patients on PLP treatment have persistently deranged liver function tests.
Expression studies
In vitro expression studies using the HeLa cell lysate system showed that wild-type PNPO activity was readily measurable. Several PNPO sequence variants that had been expressed previously using the Chinese hamster ovary cell system (R229W, X262Q and E50K) (Mills et al., 2005) were investigated using this more rapid approach so that we could compare the two expression systems. Using this new system we investigated the effects of R116Q on PNPO activity to determine if the single nucleotide polymorphism is ‘possibly damaging’ as predicted by PolyPhen, or ‘tolerated’ as predicted by SIFT (Supplementary Table 2). The effects of R225H, R225C and D33V were also studied in more detail as these sequence variants had been found in various combinations with R116Q.
Characterization of wild-type PNPO activity and the various mutant constructs revealed a pre-steady state period where the rate of PLP synthesis was slower than that seen between 10–20 min. This was true for all constructs investigated and was also apparent when PNPO was overexpressed in Chinese hamster ovary cells previously (unpublished data).
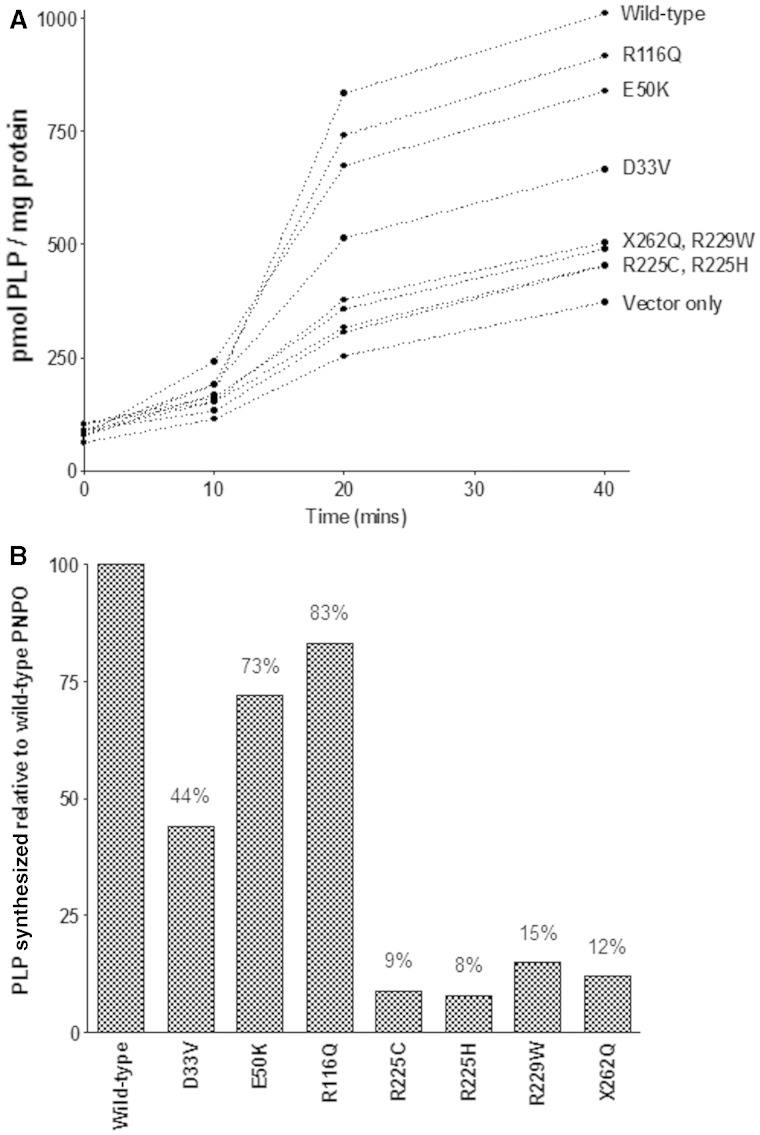
Figure 1 shows that the presence of R229W, X262Q, R225H and R225C led to a reduction in PLP synthesis to 8–15% of the rate catalyzed by wild-type PNPO. The effect of D33V on PNPO activity was less dramatic with ∼45% PNPO activity being retained. E50K, which had no effect on PNPO activity when overexpressed in Chinese hamster ovary cells, resulted in decreased PNPO activity by ∼25% when compared to wild-type activity whereas the presence of R116Q decreased PNPO activity by 17%.
Figure 1.
(A) PNPO activity (conversion of PMP to PLP) was measured by LC MS/MS and activity expressed as pmol PLP/mg protein. (B) Amount of PLP synthesized over a 40-min period by PNPO containing various mutations/sequence variants was compared with that of wild-type PNPO.
Discussion
Mutations
This article takes the number of published disease-causing mutations in PNPO to 14. However, if we include sequence variants reported previously to be single nucleotide polymorphisms and shown here to result in reduced PNPO activity i.e. E50K and R116Q, this number rises to 16. Although some mutations are ‘private’, D33V, R95H/C and R225H/C appear common; being responsible for 15%, 23% and 14% of mutated alleles, respectively. Whilst the novel missense mutations P213S and E120K affect residues conserved across mammalian species, as do the sequence variants E50K and R116Q (Supplementary Fig. 1), R225H and R225C affect a residue conserved not only across mammalian species but also across fish and lower organisms (Supplementary Fig. 2). The novel 21-base deletion/single base insertion (c.264-21_264-1delinsC) is predicted to result in skipping of Exon 3. If translated this would cause a frameshift in the protein sequence after T87, altering the next 25 amino acids before introducing an early stop codon. Unfortunately no complementary DNA was available for confirmation. The insertion of a single nucleotide in exon 7 (c.641dupA) is predicted to cause a change in the amino acid sequence and an early stop codon.
Expression studies
Characterization of the PNPO wild-type and mutant proteins revealed a slower pre-steady state period (Fig. 1A). Ordinarily transient periods in enzyme catalyzed reactions only occur for a few seconds whilst the enzyme and substrate form a complex, however, the lag period here persisted for the first 5 min. PNPO has been shown to be a sluggish enzyme with a turnover number of only 0.19/s and 0.20/s for PNP and PMP, respectively and it is self-regulated by its product PLP (Musayev et al., 2003). PNPO is a dimeric enzyme that contains a non-catalytic site on each of the monomers that binds PLP tightly (Safo et al., 2005). Crystallographic studies suggested that a tunnel may exist between the active site and this secondary non-catalytic site acting to protect PLP from nucleophiles and channelling it to vitamin B6 apoenzymes (Safo et al., 2005). If the endogenous PLP in the cell free expression system (Time 0; Fig. 1A) is bound at the non-catalytic site, the active site would be open and the enzyme reaction would proceed with steady-state kinetics. However, if the endogenous PLP is tightly bound at the active site in the closed position, the substrate PMP would have to bind at an allosteric site to enable a conformational change to occur. This would allow the tightly bound PLP at the active site to be released and catalysis to proceed (Safo et al., 2005). This may explain the slower rate of reaction observed initially.
Comparison of PNPO activity for two mutations that have been over-expressed previously in Chinese hamster ovary cells, i.e. R229W and X262Q, to that obtained using the new more rapid cell-free expression system confirmed that these sequence variants are pathogenic. Previously these mutations produced ∼30% and undetectable PNPO activity, respectively (Mills et al., 2005) whereas in the new system these mutations resulted in 15% and 12% residual activity, respectively. The discrepancy between these two systems is perhaps not surprising; the LC-MS/MS based system is more sensitive than the spectrophotometric assay and also allows for PLP product inhibition. E50K which had previously been shown to have no effect on PNPO activity when over-expressed in Chinese hamster ovary cells (Mills et al., 2005), did have 30% lower activity than wild-type. E50K has been identified (previously and in Patient 1) in cis with the splice site mutation c.364-1G>A (IVS3-1g>a). Previous expression studies concluded that c.364-1G>A is a pathogenic mutation (Mills et al., 2005) and that E50K was a polymorphism. E50 is conserved across mammalian species (Supplementary Fig. 2) and is predicted by SIFT and PolyPhen to have a ‘damaging effect’ and to be ‘probably damaging’ to protein activity, respectively (Supplementary Table 2). Whether this sequence variant—on its own or in combination with environmental factors—would prove sufficient to result in B6-responsive epilepsy remains to be seen.
The effects of R225H, R225C and D33V on PNPO activity were also investigated as these sequence variants were found in various combinations with R116Q. Expression of R225H and R225C dramatically reduced PNPO activity to 9% and 8% of wild-type activity, respectively (Fig. 1). The effect of D33V on PNPO activity was investigated as Kang et al., (2004) reported that deletion of the first 56 N-terminal amino acid residues of human PNPO affected neither binding of coenzyme nor catalytic activity. In our expression system D33V dramatically reduced enzyme activity by ∼60%. This was not surprising as D33V accounts for 15% of sequence changes found in PNPO patients to date. We conclude therefore that D33V is pathogenic.
The presence of R116Q decreased PNPO activity by ∼20% when compared with wild-type (Fig. 1). R116 is not only conserved in mammals but also in Escherichia coli (Supplementary Fig. 2). This residue contributes to one of two pairs of intersubunit salt-bridges binding the two monomeric PNPO subunits to constitute the functional dimer (Musayev et al., 2003). R116Q is also predicted to affect FMN binding. The prevalence of this sequence change within populations suggests that this polymorphism might be a common contributor or susceptibility allele to epilepsy that responds to treatment with PLP. R225H, R225C and D33V were present in tandem with R116Q in Patients 12, 14 and 15. We have yet to study these combinations. When two mutations occur in cis in the same allele in cystic fibrosis the combination may either improve or exacerbate the phenotype (Polizzi et al., 2011).
Clinical phenotype
Our data suggest that the clinical spectrum of PNPO deficiency is much broader than has been reported. The first described patients did not respond to treatment with pyridoxine; cessation of seizures only occurred with PLP. Recently, however, Pearl et al. (2012) reported a patient with PNPO mutations who showed a transient response to pyridoxine. Three groups of patients could be identified in this study with regard to their response to treatment: (i) patients that had neonatal onset seizures responding to treatment with PLP (n = 6); (ii) one child with infantile spasms (onset 5 months) responsive to PLP; and (iii) patients with seizures that responded to pyridoxine (n = 8). Patient 11, from Group (iii), had improved seizure control with a switch from pyridoxine to PLP treatment at the age of 19 years. However, for three patients in this group symptoms deteriorated with this change in treatment. The worsening of seizure control with attempting to switch to PLP therapy is noteworthy. High doses of PLP can cause seizures in experimental animals and in infants (Ishioka et al., 1995; Hammen et al., 1998). PNPO plays a role in controlling intracellular PLP levels through inhibition of the enzyme activity by high concentrations of PLP. It is possible that a mutant enzyme with residual activity could show impaired inhibition by PLP thus increasing the risk of development of toxic levels. Alternatively, it may be the build-up of another metabolite e.g. PMP that has an adverse effect on some patients on PLP treatment.
Patient 12 in Group (iii), at the age of 19 years, found that a regular morning aura could be prevented by taking a multivitamin preparation in addition to pyridoxine. The tablet contains 100 mg riboflavin (precursor of FMN, the cofactor for PNPO) and it is possible that this boosted residual PNPO activity. Patients 12 and 14 in Group (iii) had autistic features.
Patient 10, being treated with pyridoxine, developed a severe neuropathy with persistent loss of ankle jerks. This is the first patient with PNPO deficiency that we have encountered to develop a severe neuropathy. Neuropathy is well recognized in normal adults taking doses >200 mg/day of pyridoxine and we see it in some patients with antiquitin deficiency on similar doses. Analysis of plasma B6 vitamer levels in two antiquitin-deficient patients treated with pyridoxine and showing mild peripheral neuropathy, revealed that plasma PLP remained low whereas pyridoxal, pyridoxamine, PMP and pyridoxine were increased (Footitt et al., 2013). Animal experiments have suggested that pyridoxine may be more toxic to the PNS than other B6 vitamers (Levine et al., 2004).
Two patients in this series that are treated with PLP have persistently deranged liver function tests. Similar findings have been documented in a child with PNPO deficiency receiving a high dose (100 mg/kg/day) PLP treatment (Mills et al., 2012). A liver biopsy showed early cirrhosis, which was attributed to the PLP therapy. The PLP dose has now been reduced to 60 mg/kg/day and although the liver function tests have improved, they have not normalized and there is persistent evidence of hepatic fibrosis and portal hypertension. Liver toxicity secondary to high dose PLP (1000 mg/day) has also been reported for a child with homocystinuria (Yoshida et al., 1985). It is uncertain whether the cause of this toxicity is directly due to the high dose PLP or to degradation products of PLP that may form by photochemical reactions. Liver function should be monitored in all children treated with high doses of PLP.
Premature delivery (<37/40) has been documented in the majority of genetically confirmed PNPO-deficient cases reported in the literature with many patients also having low Apgar scores at delivery (often requiring intubation). In this series 54% of the patients were born at term and, although two patients required assistance with breathing (continuous positive airway pressure/mechanical ventilation) and one patient required resuscitation, most of the patients were in good condition at birth.
In the majority of reported cases, seizures commenced within the first hours of life. While seizures commenced in 10/14 of the individuals reported here at <24 h, three patients did not present with seizures until later (2, 3 and 8 weeks old, respectively) and Patient 7 did not present until 5 months of age (Table 3). Patient 7 remains seizure-free on a dose of PLP of 10 mg/kg/day; most of the other patients treated with PLP have required 2–10 times this dose.
Table 3.
Possible effects of genotype and environmental factors on patient response to pyridoxine, prematurity and developmental outcome
| Group | Patient | Genotype | Born prematurely | Ethnicity | Mother given B6 | Neonatal feeding | Seizure onset | Time taken to control seizures | Response to PN | Current age | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (i) | 1 | R95H + E50K;Splice errorsa | + | Asian | ? | 30 min | 5 d | - | 2 y 8 m | Global delay | |
| 2 | D33V/D33V | - | Caucasian | B | 6 h | 8 w | - | 4 y | Normal | ||
| 3 | P213S/P213S | - | Caucasian | B/F* | 90 min | 3–6 d | - | 2 y 7 m | Normal | ||
| 4** | P213S/P213S | + | Caucasian | + | F | None | No seizures | Not tried | 4 m | Normal | |
| 5 | R95C/R95C | + | Turkish | F | 2 h | 7 d | Not tried | 1 y | IQ 66 at 9 m. Mild truncal hypotonia and speech delay | ||
| 6d | Q214fs/? | + | Caucasian | PN/F | 5 h | 15 d | - | 4 y | Normal | ||
| (ii) | 7 | R116Q/R116Q | ? | Caucasian | B | 5 m | 24 h | - | 4 y | Normal/advanced | |
| (iii) | 8 | R116Q/R116Q | - | Pakastani | ? | 3 h | 2.5 m | + | 7 y | Mild learning disability | |
| 9 | D33V/D33V | - | Caucasian | F | 3 w | 1 w | + | 2 y | Minimal delay | ||
| 10 | D33V/E120K | ? | Caucasian | ? | 2 m | 4 m | + | 21 y | Mild intellectual disability | ||
| 11 | D33V/Splice errors | + | Caucasian | F | 3 h | 6 m | + | 21 y | Severe delay/no language | ||
| 12 | D33V + R225C + R116Qb | - | Caucasian | F | 14 d | 3.5 m | + | 41 | IQ 93. Dyslexia and Aspergers | ||
| 13 | R225H/R225H | ? | ? | ? | 24 h | 6 m | + | 7 y | Spastic quadriplegia with good social contact | ||
| 14 | R225H;R116Q + R225H;R116Qc | - | Caucasian | ? | 30 min | 2 w | + | 2 y 7 m | Minimal delay | ||
| 15 | R225H;R116Q + R225H;R116Qc | + | Kosovan | ? | 10 h | 5 d | + | 8 y | DQ 65 |
(i) Neonatal onset seizures responding to pyridoxal 5’-phosphate.
(ii) Infantile spasms (onset 5 months) responsive to pyridoxal 5’-phosphate.
(iii) Seizures starting under 3 months of age responding to pyridoxine.
B = breast-fed; F = formula fed; PN = parenteral nutrition; – = no; + = yes
*Formula from 2 weeks
**Sibling of Patient 3.
ac.[148G>A] and c.[364-1G>A] were inherited in cis, this was confrimed by analysing parental DNA.
bNo parental DNA was available to ascertain which muation R116Q was in cis with.
cAssume that R116Q has been inherited in cis with R225H, no parental DNA was available to confirm this.
dSecond mutation not found.
A history of infertility should alert the clinician to a possible diagnosis of PNPO deficiency as heterozygous couples seem to have reduced rates of conception. Four of eight families reported previously have undergone several attempts at in vitro fertilization treatment and/or suffered early pregnancy losses. In this study four families reported miscarriages, treatment for infertility and/or a molar pregnancy.
As with many rare diseases, it is difficult to draw conclusions about the long-term outcome. If untreated, PNPO deficiency is usually fatal with a single child surviving with severe epilepsy and psychomotor delay to 3 years of age (Hoffmann et al., 2007). In the seven cases where treatment with PLP was initiated, only one child died and this was as a result of fungal sepsis (Ruiz et al., 2008). The other six survived with varying degrees of disability. Here 3 of 12 patients have marked motor delay; for Patients 11 and 13 this may relate to the duration of poor seizure control. Six of twelve patients show no evidence of any disability. Three patients reported here are now older than 21 years and the eldest patient is 41 and his neurological abnormalities are limited to dyslexia and Asperger’s syndrome.
Influence of environmental factors on phenotypes of individuals with identical or similar genotypes
D33V: a predominantly pyridoxine responsive genotype?
The response of Patients 8–15 to pyridoxine suggests that they have sufficient enzyme activity to allow synthesis of PLP from pyridoxine. Within this group, the missense mutation D33V was common. Expression studies showed that D33V did not totally abolish PNPO activity (44% residual activity). The response to treatment with pyridoxine is therefore perhaps not surprising. However, D33V was also found in Patient 2 whose seizures showed no response to pyridoxine. The situation is obviously complex. It is possible that pyridoxine responsiveness in PNPO deficiency is affected by prematurity and age and riboflavin status at the time of the therapeutic trial. Patient 9 (homozygous for D33V) responded to treatment with pyridoxine and was born at term, formula fed and tested for pyridoxine responsiveness at 3 weeks of age. Patient 10 (compound heterozygote for D33V and E120K) who showed a good response to pyridoxine (started at 6 months) in terms of both seizure control and psychomotor development and did not develop seizures until after the neonatal period (2 months). Patient 11 (compound heterozygote for D33V and c.264-21_264-1delinsC) resembled Patient 10 with regard to treatment response but differed in terms of neuropsychological outcome. Perhaps the poorer outcome in Patient 11 relates to the poorly controlled seizures in the first 2 months of life (Jonas et al., 2005). Seizures in Patient 12 (D33V in combination with R116Q and R225C) also responded well to treatment with pyridoxine. Conversely seizures in Patient 2 (homozygous D33V) failed to respond to a 10-day trial of 100 mg pyridoxine/day and previously reported patients [Patient 4; homozygous D33V (Goyal et al., 2013) and Patient 3; heterozygous D33V/heterozygous c.246delT (Hoffmann et al., 2007)] failed to respond to shorter trials of pyridoxine. Perhaps Patient 2 did not respond to pyridoxine because she was breast fed; breast feeding can be associated with a relative deficiency of riboflavin (Hovi et al., 1979) or perhaps because she has a second genetic factor compromising PLP homeostasis or neurotransmitter metabolism.
R225H/C genotype: a pyridoxine responsive phenotype?
Patients 14 and 15 had the same sequence variants (homozygous for R225H + R116Q) strongly suggesting that this is a pyridoxine-responsive genotype. Patient 13 (homozygous for R225H) also responded to treatment with pyridoxine, as did Patient 12 who was heterozygous for R225C. Expression of R225H and R225C revealed 8% residual activity—presumably sufficient to allow synthesis of PLP from pyridoxine. Further work is required to determine whether, in the R225H/C variants, pyridoxine phosphate oxidase activity is better preserved than pyridoxamine phosphate oxidase activity. These enzyme activities can be differentially affected by inhibitors (Takeuchi et al., 1985).
R95H, R95C and E50K with c.[364-1G>A]: alleles associated with infertility, miscarriage, premature birth and PLP-responsive seizures
Patient 1 was compound heterozygous for E50K plus c.[364-1G>A] on the paternal allele and R95H on the maternal allele. Homozygosity for E50K plus c.[364-1G>A] produced a similar clinical picture (parental infertility, premature birth and PLP-responsiveness) in two siblings (Mills et al., 2005). Patient 5 (homozygous for R95C) was premature and PLP-responsive and his parents had a history of miscarriage. An infant born at 36 weeks gestation with R95H/R95H in another series had severe neonatal epileptic encephalopathy with a brief, incomplete response to pyridoxine but still a fatal outcome (Khayat et al., 2008). In the Chinese hamster ovary cell expression system, PNPO enzyme activity for E50K plus c.[364-1G>A] and R95H was undetectable and 18% of wild-type, respectively. Why with one or both mutations showing 18% residual activity, should there be no or minimal response to pyridoxine? One possibility is that all of these patients were premature and tested for pyridoxine responsiveness in the first week of life. PNPO transcription is low in the foetus compared to adults (30% adult levels in liver; 2% adult levels in brain) (Ngo et al., 1998; Kang et al., 2004). An analysis of B6 vitamers in very preterm infants has shown that CSF may contain significant amounts of pyridoxine alongside a low PLP concentration (Albersen et al., 2012). Thus PNPO activity is already critically low in infants born before 30 weeks. PNPO mutations can therefore be expected to lead to more severe effects in a preterm than in a term infant. Further, this may suggest a therapeutic role for PLP in seizures in preterm infants without PNPO mutations. In addition newborns can develop riboflavin deficiency in the first days of life (Hovi et al., 1979) and this could further reduce PNPO activity.
Does the maternal supply of B6 to the developing foetus affect phenotype?
Patient 8, who was in poor condition at birth and started having seizures 3 h after birth, is homozygous for the sequence variant R116Q—the same genotype as Patient 7 who was normal until the age of 5 months. Therefore, whilst a predisposition to B6-responsive seizures may be determined by the R116Q/R116Q phenotype, other factors determine whether a child is in poor condition at birth and develops seizures in the first 24 h. The comparison of Patients 3 and 4 (siblings) indicates that the most likely additional factor is how well the foetus has been supplied with vitamin B6 by the mother (Schenker et al., 1992). In the case of Patient 4, the mother took a multivitamin preparation during pregnancy (2.6 mg/day of pyridoxine and 1.8 mg/day of riboflavin) and an additional PLP supplement just before delivery, which appeared to prevent intrauterine seizures and early post-natal seizures. A recent study suggests that PLP levels in Pakistani adults are lower than in other ethnic groups (Iqbal et al., 2009). If we look at the backgrounds of patients born prematurely, and/or those that had low Apgar scores and/or those that needed resuscitation, 17/21 are from a non-Caucasian background whereas for patients not meeting these criteria, the figure is only 1 in 8 (Table 4). Differences in maternal plasma PLP and pyridoxal levels may explain the phenotypic differences seen in Patients 7 and 8, both of whom are homozygous for R116Q.
Table 4.
Ethnicity of PNPO-deficient patients born at or before 36 weeks gestation and/or required resuscitation at birth and/or had low Apgar scores
| Premature/required resuscitation/ low Apgar score | Term and good condition at birth | Patient identifier(s) | Reference |
|---|---|---|---|
| Asian (n = 2) | J1, J2 | Mills et al., 2005 | |
| Turkish (n = 4) | G1, G4, G5, G6 | Mills et al., 2005 | |
| Pakistani (n = 2) | K1, K2 | Mills et al., 2005 | |
| Lebanese / Iraqi (n = 1) | Lebanese / Iraqi (n = 1) | 1, 2 | Hoffmann et al., 2007 |
| Caucasian (n = 1) | 3 | Hoffmann et al., 2007 | |
| Lebanese (n = 3) | 4, 5, 6 | Hoffmann et al., 2007; Bagci et al., 2008 | |
| Arab Muslim (n = 1) | IV.3 | Khayat et al., 2008 | |
| Asian (n = 2) | 1, 8 | This study | |
| Caucasian (n = 6) | 2, 7, 9, 10, 12, 14 | This study | |
| Caucasian (n = 4) | 3, 4, 6, 11 | This study | |
| Kosovan Muslim (n = 1) | 15 | This study | |
| Turkish (n = 1) | 5 | This study | |
| Non-Caucasian: Caucasian = 17:4 | Non-Caucasian: Caucasian = 1:7 |
Prevalence of R116Q variant
The presence of R116Q-decreased PNPO activity by 20% when compared with wild-type; however, R116Q is listed as a single nucleotide polymorphism in Ensembl (Supplementary Table 3). It has been suggested that low PNPO activity in erythrocytes may confer resistance to malaria (Anderson et al., 1993). A second enzyme important in maintaining high levels of PLP in the red cell is pyridoxal kinase. Mean red blood cell pyridoxal kinase activity in black Americans is 40% that of white Americans (Chern and Beutler, 1976) and it has been suggested that this lower activity was favoured by natural selection because it conferred resistance to malaria (Martin et al., 1978; Flanagan and Beutler, 2006). Plasmodium produces enzymes for de novo synthesis of PLP and for phosphorylation of pyridoxal, but it is possible that erythrocyte schizogony requires some PLP to be provided by the host. A gene encoding PNPO, and therefore capable of regenerating PLP from PMP, has not been convincingly demonstrated in plasmodium (Müller and Kappes, 2007). Furthermore, at least one parasite enzyme that is important in erythrocyte schizogony (ornithine decarboxylase) gradually loses activity that can be restored by the addition of PLP (Bitonti et al., 1987).
Conclusion
Three groups of patients with PNPO mutations that reduced enzyme activity have been identified: (i) patients with neonatal onset seizures responding to PLP; (ii) a patient with infantile spasms (onset 5 months) responsive to PLP; and (iii) patients with seizures starting before 3 months of age responding to pyridoxine. One sequence variant, R116Q, a single nucleotide polymorphism that has been reported in the general population, was found to have an effect on PNPO activity. We believe the reduction in PNPO activity and B6-responsive epilepsy in the patients reported here indicates that it is a variant that contributes to the pathogenesis of epilepsy. It is possible that R116Q is responsible for the susceptibility locus for genetic generalized epilepsy on 17q21.32 (close to rs72823592). Although additional studies will be necessary to delineate this further, our findings support the use of DNA tests for PNPO deficiency in a wide range of infants with epilepsy.
Supplementary Material
Acknowledgements
The authors would like to thank Prof. Joe T.R. Clarke for his involvement in this study.
Glossary
Abbreviations
- FMN
flavin mononucleotide
- PLP
pyridoxal 5’-phosphate
- PMP
pyridoxamine phosphate
Funding
P.T.C. and P.B.M. are supported by Great Ormond Street Children’s Charity (GOSHCC). This project was funded by grants from the NIHR and GOSHCC Neuroscience Initiative.
Supplementary material
Supplementary material is available at Brain online.
References
- Albersen M, Groenendaal F, van der Ham M, de Koning TJ, Bosma M, Visser WF, et al. Vitamin B6 vitamer concentrations in cerebrospinal fluid differ between preterm and term newborn infants. Pediatrics. 2012;130:e191–8. doi: 10.1542/peds.2011-3751. [DOI] [PubMed] [Google Scholar]
- Anderson BB, Giuberti M, Perry GM, Salsini G, Casadio I, Vullo C. Low red blood cell glutathione reductase and pyridoxine phosphate oxidase activities not related to dietary riboflavin: selection by malaria? Am J Clin Nutr. 1993;57:666–72. doi: 10.1093/ajcn/57.5.666. [DOI] [PubMed] [Google Scholar]
- Bagci S, Zschocke J, Hoffmann GF, Bast T, Klepper J, Müller A, et al. Pyridoxal phosphate-dependent neonatal epileptic encephalopathy. Arch Dis Child. 2008;93:F151–2. doi: 10.1136/adc.2006.115162. [DOI] [PubMed] [Google Scholar]
- Bitonti AJ, McCann PP, Sjoerdsma A. Plasmodium falciparum and Plasmodium berghei: effects of ornithine decarboxylase inhibitors on erythrocytic schizogony. Exp Parasitol. 1987;64:237–43. doi: 10.1016/0014-4894(87)90148-2. [DOI] [PubMed] [Google Scholar]
- Chern CJ, Beutler E. Biochemical and electrophoretic studies of erythrocyte pyridoxine kinase in white and black Americans. Am J Hum Genet. 1976;28:9–17. [PMC free article] [PubMed] [Google Scholar]
- EPICURE Consortium; EMINet Consortium. Steffens M, Leu C, Ruppert AK, Zara F, et al. Genome-wide association analysis of genetic generalised epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum Mol Genet. 2012;21:5359–72. doi: 10.1093/hmg/dds373. [DOI] [PubMed] [Google Scholar]
- Flanagan JM, Beutler E. The genetic basis of human erythrocyte pyridoxal kinase activity variation. Haematologica. 2006;91:801–4. [PubMed] [Google Scholar]
- Footitt EJ, Clayton PT, Mills K, Heales SJ, Neergheen V, Oppenheim M, et al. Measurement of plasma B(6) vitamer profiles in children with inborn errors of vitamin B(6) metabolism using an LC-MS/MS method. J Inherit Metab Dis. 2013;36:139–45. doi: 10.1007/s10545-012-9493-y. [DOI] [PubMed] [Google Scholar]
- Footitt EJ, Heales SJ, Mills PB, Allen GF, Oppenheim M, Clayton PT. Pyridoxal 5'-phosphate in cerebrospinal fluid; factors affecting concentration. J Inherit Metab Dis. 2011;34:529–38. doi: 10.1007/s10545-011-9279-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Cazorla A, Gibson KM, Clayton PT. Disorders of neurotransmission. In: Saudubray JM, van den Berghe G, Walter JH, editors. In born metabolic diseases. 5th edn. Berlin, Heidelberg: Springer-Verlag; 2012. pp. 410–17. [Google Scholar]
- Goyal M, Fequiere PR, McGrath TM, Hyland K. Seizures with decreased levels of pyridoxal phosphate in cerebrospinal fluid. Pediatr Neurol. 2013;48:227–31. doi: 10.1016/j.pediatrneurol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Hammen A, Wagner B, Berkhoff M, Donati F. A paradoxical rise of neonatal seizures after treatment with vitamin B6. Eur J Paediatr Neurol. 1998;2:319–22. doi: 10.1016/s1090-3798(98)80007-x. [DOI] [PubMed] [Google Scholar]
- Hazra A, Kraft P, Lazarus R, Chen C, Chanock SJ, Jacques P, et al. Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum Mol Genet. 2009;18:4677–87. doi: 10.1093/hmg/ddp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann GF, Schmitt B, Windfuhr M, Wagner N, Strehl H, Bagci S, et al. Pyridoxal 5 ‘-phosphate may be curative in early-onset epileptic encephalopathy. J Inherit Metab Dis. 2007;30:96–9. doi: 10.1007/s10545-006-0508-4. [DOI] [PubMed] [Google Scholar]
- Hovi L, Hekali R, Siimes MA. Evidence of riboflavin depletion in breast-fed newborns and its further acceleration during treatment of hyperbilirubinemia by phototherapy. Acta Paediatr Scand. 1979;68:567–70. doi: 10.1111/j.1651-2227.1979.tb05056.x. [DOI] [PubMed] [Google Scholar]
- Iqbal MP, Lindblad BS, Mehboobali N, Yusuf FA, Khan AH, Iqbal SP, et al. Folic acid and vitamin B6 deficiencies related hyperhomocysteinemia in apparently healthy Pakistani adults; is mass micronutrient supplementation indicated in this population? J Coll Physicians Surg Pak. 2009;19:308–12. [PubMed] [Google Scholar]
- Ishioka N, Sato J, Nakamura J, Ohkubo T, Takeda A, Kurioka S. In vivo modification of GABAA receptor with a high dose of pyridoxal phosphate induces tonic-clonic convulsion in immature mice. Neurochem Int. 1995;26:369–73. doi: 10.1016/0197-0186(94)00145-k. [DOI] [PubMed] [Google Scholar]
- Jonas R, Asarnow RF, LoPresti C, Yudovin S, Koh S, Wu JY, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology. 2005;64:746–50. doi: 10.1212/01.WNL.0000151970.29205.70. [DOI] [PubMed] [Google Scholar]
- Kang JH, Hong ML, Kim DW, Park J, Kang TC, Won MH, et al. Genomic organization, tissue distribution and deletion mutation of human pyridoxine 5′-phosphate oxidase. Eur J Biochem. 2004;271:2452–61. doi: 10.1111/j.1432-1033.2004.04175.x. [DOI] [PubMed] [Google Scholar]
- Khayat M, Korman SH, Frankel P, Weintraub Z, Hershckowitz S, Sheffer VF, et al. PNPO deficiency: an under diagnosed inborn error of pyridoxine metabolism. Mol Genet Metab. 2008;94:431–4. doi: 10.1016/j.ymgme.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Levine S, Saltzman A. Pyridoxine (Vitamin B6) Neurotoxicity: enhancement by protein-deficient diet. J Appl Toxicol. 2004;24:497–500. doi: 10.1002/jat.1007. [DOI] [PubMed] [Google Scholar]
- Martin SK, Miller LH, Kark JA, Hicks CU, Haut MJ, Okoye VC, et al. Low erythrocyte pyridoxal-kinase activity in Blacks: its possible relation to falciparum malaria. Lancet. 1978;1:466–8. doi: 10.1016/s0140-6736(78)90133-2. [DOI] [PubMed] [Google Scholar]
- Mills PB, Footitt EJ, Clayton PT. Chapter 156.1: Vitamin B6 metabolism and inborn errors. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, et al., editors. The online metabolic and molecular bases of inherited disease. 2012. http://www.ommbid.com/OMMBID/a/c.html/vitamins/vitamin_b6_metabolism_inborn_errors. [Google Scholar]
- Mills PB, Footitt EJ, Mills KA, Tuschl K, Aylett S, Varadkar S, et al. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency) Brain. 2010;133:2148–59. doi: 10.1093/brain/awq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PB, Surtees RAH, Champion MP, Beesley CE, Dalton N, Scambler PJ, et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5′-phosphate oxidase. Hum Mol Genet. 2005;14:1077–86. doi: 10.1093/hmg/ddi120. [DOI] [PubMed] [Google Scholar]
- Müller S, Kappes B. Vitamin and cofactor biosynthesis pathways in Plasmodium and other apicomplexan parasites. Trends Parasitol. 2007;23:112–21. doi: 10.1016/j.pt.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musayev FN, Di Salvo ML, Ko T-P, Schirch V, Safo MK. Structure and properties of recombinant human pyridoxine 5′-phosphate oxidase. Prot Sci. 2003;12:1455–63. doi: 10.1110/ps.0356203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musayev FN, Di Salvo ML, Saavedra MA, Contestabile R, Ghatge MS, Haynes A, et al. Molecular basis of reduced pyridoxine 5′-phosphate oxidase catalytic activity in neonatal epileptic encephalopathy disorder. J Biol Chem. 2009;284:30949–56. doi: 10.1074/jbc.M109.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo EO, LePage GR, Thanassi JW, Meisler N, Nutter LM. Absence of pyridoxine-5′-phosphate oxidase (PNPO) activity in neoplastic cells: isolation, characterization, and expression of PNPO cDNA. Biochemistry. 1998;37:7741–8. doi: 10.1021/bi972983r. [DOI] [PubMed] [Google Scholar]
- Ormazabal A, Oppenheim M, Serrano M, García-Cazorla A, Campistol J, Ribes A, et al. Pyridoxal 5′-phosphate values in cerebrospinal fluid: reference values and diagnosis of PNPO deficiency in paediatric patients. Mol Genet Metab. 2008;94:173–7. doi: 10.1016/j.ymgme.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Pearl PL, Hyland K, Chiles J, McGavin CL, Yu Y, Taylor D. Partial pyridoxine responsiveness in PNPO deficiency. JIMD. 2012;9:139–42. doi: 10.1007/8904_2012_194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzi A, Tesse R, Santostasi T, Diana A, Manca A, Logrillo VP, et al. Genotype-phenotype correlation in cystic fibrosis patients bearing [H939R;H949L] allele. Genet Mol Biol. 2011;34:416–20. doi: 10.1590/S1415-47572011000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Garcia-Villoria J, Ormazabal A, Zschocke J, Fiol M, Navarro-Sastre A, et al. A new fatal case of pyridox(am)ine 5′-phosphate oxidase (PNPO) deficiency. Mol Genet Metab. 2008;93:216–18. doi: 10.1016/j.ymgme.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Safo MK, Musayev FN, Schirch V. Structure of Escherichia coli pyridoxine 5'-phosphate oxidase in a tetragonal crystal form: insights into the mechanistic pathway of the enzyme. Acta Crystallogr D Biol Crystallogr. 2005;61:599–604. doi: 10.1107/S0907444905005512. [DOI] [PubMed] [Google Scholar]
- Schenker S, Johnson RF, Mahuren JD, Henderson GI, Coburn SP. Human placental vitamin B6 (pyridoxal) transport: normal characteristics and effects of ethanol. Am J Physiol. 1992;262:R966–74. doi: 10.1152/ajpregu.1992.262.6.R966. [DOI] [PubMed] [Google Scholar]
- Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–7. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi F, Tsubouchi R, Shibata Y. Effect of tryptophan metabolites on the activities of rat liver pyridoxal kinase and pyridoxamine 5-phosphate oxidase in vitro. Biochem J. 1985;227:537–44. doi: 10.1042/bj2270537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Scheet P, Giusti B, Bandinelli S, Piras MG, Usala G, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. 2009;84:477–82. doi: 10.1016/j.ajhg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerapandiyan A, Winchester SA, Gallentine WB, Smith EC, Kansagra S, Hyland K, et al. Electroencephalographic and seizure manifestations of pyridoxal 5'-phosphate-dependent epilepsy. Epilepsy Behav. 2011;20:494–501. doi: 10.1016/j.yebeh.2010.12.046. [DOI] [PubMed] [Google Scholar]
- Wang HS, Kuo MF, Chou ML, Hung PC, Lin KL, Hsieh MY, et al. Pyridoxal phosphate is better than pyridoxine for controlling idiopathic intractable epilepsy. Arch Dis Child. 2005;90:512–15. doi: 10.1136/adc.2003.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida I, Sakaguchi Y, Nakano M, Yamashita F, Hitoshi T. Pyridoxal phosphate-induced liver injury in a patient with homocystinuria. J Inherit Metab Dis. 1985;8:91. doi: 10.1007/BF01801674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.