Abstract
The coagulation cascade is activated during viral infections. This response may be part of the host defense system to limit spread of the pathogen. However, excessive activation of the coagulation cascade can be deleterious. In fact, inhibition of the tissue factor/factor VIIa complex reduced mortality in a monkey model of Ebola hemorrhagic fever. Other studies showed that incorporation of tissue factor into the envelope of herpes simplex virus increases infection of endothelial cells and mice. Furthermore, binding of factor X to adenovirus serotype 5 enhances infection of hepatocytes but also increases the activation of the innate immune response to the virus. Coagulation proteases activate protease-activated receptors (PARs). Interestingly, we and others found that PAR1 and PAR2 modulate the immune response to viral infection. For instance, PAR1 positively regulates TLR3-dependent expression of the antiviral protein interferon β, whereas PAR2 negatively regulates expression during coxsackievirus group B infection. These studies indicate that the coagulation cascade plays multiple roles during viral infections.
Virus life cycle
The life cycle of a virus can be divided into 3 stages: (1) virus infection, (2) virus replication and assembly, and (3) virus release. After entry to the cell, the virus is unpacked, viral genomes are replicated, and components are assembled to form new viral particles. The last phase of the viral life cycle is the release of infectious particles, which may be achieved by either cell lysis or budding of particles from the membrane. Viruses can be divided into 2 groups: enveloped and nonenveloped. Influenza A virus (IAV) and herpes simplex virus (HSV) are examples of enveloped viruses. Part of the envelope is derived from the host cell membrane and covers the capsid. It fuses with cell membranes during infection of cells and may help the virus to evade the immune system. Adenovirus (Adv) and coxsackievirus group B (CVB) are examples of nonenveloped viruses.
The innate immune response to virus infection
The innate and adaptive immune systems have evolved to protect humans from pathogens, such as viruses. The presence of a virus is detected by specialized cells, such as natural killer (NK) cells, macrophages, monocytes, and dendritic cells, as well as nonprofessional immune cells, such as fibroblasts and cardiomyocytes.1,2 Pattern-recognition receptors (PRRs) on these cells recognize pathogen-associated molecular patterns (PAMPs) produced by the pathogen, such as lipopolysaccharide (LPS), double-stranded (ds) or single-stranded (ss) RNA, or viral DNA.1-3 Toll-like receptors (TLRs) are a family of membrane receptors that recognize PAMPs.2 They are widely expressed by immune cells and other nonimmune cells, such as endothelial cells, epithelial cells, fibroblasts, cardiomyocytes, and platelets.1,2,4 Viral infections are mainly detected by endosomal TLRs that recognize nucleic acid ligands from the virus.2,5 TLR3 recognizes the dsRNA of dsRNA viruses and dsRNA that is made during replication of ssRNA viruses. TLR7 and TLR8 recognize ssRNA, whereas TLR9 recognizes dsDNA from DNA viruses. In addition, TLR2 and TLR4 recognize PAMPs derived from the viral capsid or envelop. dsRNA present in the cytosol is also detected by cytosolic PRRs, such as retinoic acid–inducible gene I and melanoma differentiation-associated gene 5.1,6 TLR3 can be activated experimentally by the dsRNA mimetic polyinosinic-polycytidylic acid (poly I:C).
Activation of these PRRs leads to the expression of the type I interferons (IFNs) IFN-α and IFN-β, type II IFNs (IFN-γ), chemokines, and cytokines.7 TLR3 uses the adaptor protein Toll/IL-1 receptor/resistance domain–containing adaptor-inducing IFN-β (TRIF) to activate mitogen-activated protein kinases, AP-1, nuclear factor κB (NF-κB), and IFN regulatory factor 3 (IRF3), which induce IFN-β expression.8 In contrast, TLR7, TLR8, and TLR9 all use MyD88 to activate mitogen-activated protein kinases, NF-κB, and IRF7 signaling, which leads to IFN-α expression.1 IFNs coordinate the antiviral response by inducing the expression of IFN-stimulated genes (ISGs) that reduce virus replication and infection.7 Furthermore, chemokines, such as CXCL10 (also known as IP10), and cytokines recruit NK cells and phagocytes to kill virally infected cells.7,9 Finally, activation of dendritic cells, NK cells, and macrophages by type I IFNs leads to the production of IFN-γ, which stimulates the adaptive immune response to the virus.7
Activation of the coagulation protease cascade during viral infection
The coagulation system is activated in response to infection by a variety of different viruses, such as HIV, CVB3, Dengue virus, and Ebola virus.10-15 This response likely evolved as a host defense system to limit the spread of the virus. However, acute viremia can lead to disseminated intravascular coagulation and subsequent hemorrhage that contributes to multiorgan failure and mortality.16 Tissue factor (TF) appears to be the major activator of the coagulation cascade during viral infection. TF expression is increased in endothelial cells infected with HSV and Dengue virus.10,13 In addition, Ebola virus infection induces TF expression in lymphoid macrophages and circulating blood cells, which is associated with Ebola hemorrhagic fever.11,16 Furthermore, the TLR3 agonist poly I:C induces TF expression in cultured endothelial cells and activates the coagulation system in mice.17 Importantly, inhibition of the TF/factor VIIa (FVIIa) complex reduced the cytokine storm and mortality in a rhesus monkey model of Ebola hemorrhagic fever.18 This study demonstrates the deleterious effects of overactivation of the coagulation cascade during viral infection.
PARs
Protease-activated receptors (PARs) are 7-membrane–spanning, G-protein–coupled receptors.19 There are 4 members in the family: PAR1 to PAR4. They are widely expressed by both immune and nonprofessional immune cells.20-23 Platelets exhibit a species-specific pattern of PAR expression; human platelets express PAR1 and PAR4, whereas mouse platelets express PAR3 and PAR4. PARs can be activated by a variety of proteases, including serine proteases generated during activation of blood coagulation. Proteolytic cleavage of the N-terminal exodomain exposes a tethered ligand that binds to the receptors and induces intracellular signaling. Thrombin is the primary activator of PAR1, PAR3, and PAR4, whereas PAR2 is activated by trypsin and tryptase as well as FVIIa and FXa.19,20,24 PAR signaling can be either proinflammatory or anti-inflammatory depending of the particular PAR, cell type, and disease model.21,25
The coagulation system and PARs enhance infection of HSV in endothelial cells and in mice
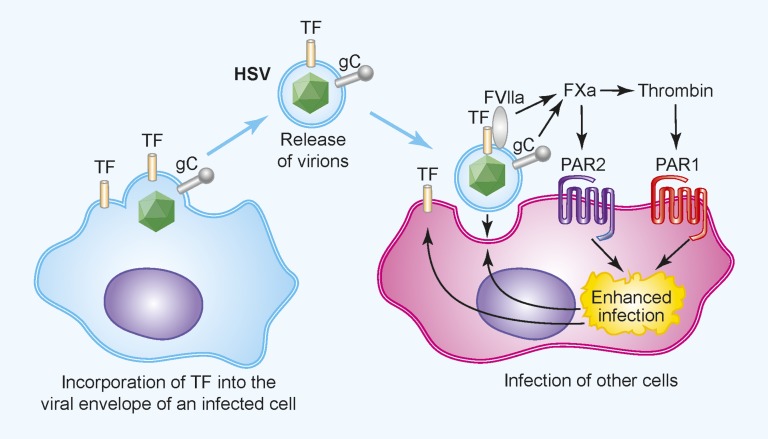
Enveloped viruses use components from the host’s cell membrane to cover the viral capsid. These membranes can be derived from the outer cell layer of an infected host cell or from internal membranes, such as the endoplasmic reticulum. In addition to proteins from the host, the outer lipid bilayer contains virally encoded proteins. Selected incorporation of host proteins, such as TF, into the HSV envelop leads to activation of coagulation when the virus enters the bloodstream (Figure 1).26 In addition, HSV1 expresses a protein called glycoprotein C that enhances the activation of coagulation.27,28 HSV may activate the clotting system to coat virally infected cells with fibrin to reduce their recognition and killing by NK cells and phagocytes.15,29 Interestingly, Sutherland and colleagues found that activation of PAR1 and PAR2 increases HSV infection of endothelial cells in vitro (Figure 1).26,30 Recently, this group analyzed the role of TF in HSV1 infection in mice.31 They observed higher levels of virus in various organs of mice infected with HSV1 virus produced in TF-expressing cells compared with mice infected with HSV1 virus produced in TF-negative cells. Furthermore, inhibition of TF coagulant activity reduced virus levels in all organs, whereas inhibition of TF signaling activity only reduced levels of virus in the blood and spleen.31 These results indicate that TF-dependent activation of the coagulation cascade increases HSV1 infection in both cultured endothelial cells and in mice.
Figure 1.
The coagulation cascade and PAR1 and PAR2 enhance HSV infection of endothelial cells. TF is incorporated into the envelope of HSV after infection of TF+ cells. Upon binding to a target cell, TF in the envelope activates the coagulation cascade. In addition, the virally encoded glycoprotein C enhances activation of FX. The local generation of FXa and thrombin activate PAR1 and PAR2 on the cells, which increases infection, possibly by modifying receptors that mediate virus binding and entry. Professional illustration by Paulette Dennis.
Role of factor X and the clotting cascade in Adv and Adv-associated virus infection
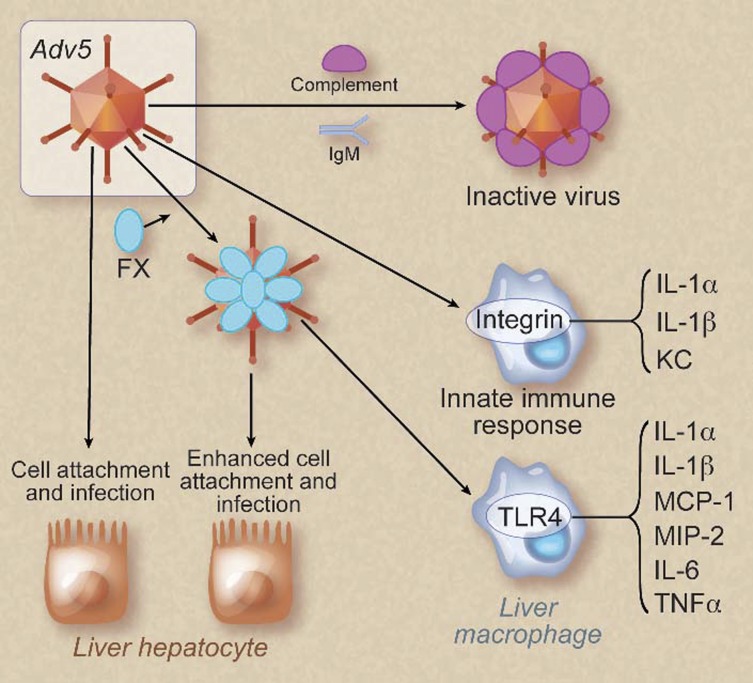
Adv and adenovirus-associated viruses (AAVs) are nonenveloped viruses that are commonly used as gene therapy vectors. Intriguingly, a variety of coagulation proteins, including FX, were shown to promote Adv-mediated gene delivery to hepatocytes.32 Specifically, FX binding to the hexon of Adv serotype 5 (Adv5) increased cell transduction (Figure 2).33,34 FX was also shown to shield Adv5 from attack from complement and immunoglobulin M antibodies, which increased viral attachment and infection of hepatocytes in vivo (Figure 2).35 In contrast to the studies showing a beneficial role of FX binding for Adv5 infection, binding of FX to Adv5 increased macrophage recognition of the virus and enhanced the innate immune response by activation of the TLR4-MyD88-NF-κB pathway (Figure 2).36 However, the net effect of FX binding to Adv5 appears to be positive, because blocking of this interaction reduces infection in mice.
Figure 2.
Binding of FX to Adv5 increases infection of hepatocytes and enhances activation of macrophages. Adv5 without FX bound is recognized by immunoglobulin M (IgM) and complement and inactivated. This form of virus has a low infectivity and mildly activates the innate immune system by binding to integrins on liver macrophages. Binding of FX to Adv5 enhances infection of liver hepatocytes by increasing the binding of virus to the cells. In addition, Adv5 coated with FX induces a more robust innate immune response in macrophages by activating the TLR4-NF-κB pathway. KC, CXCL1. Professional illustration by Debra T. Dartez.
A recent study analyzed the effect of different anticoagulants on AAV infection in mice because they are used during administration of AAV vectors.37 Unfractionated heparin as well as FXa and thrombin inhibitors significantly reduced AAV-2, but not AAV-5 and AAV-8, infections in mice.37 Surprisingly, low molecular weight heparin (LMWH) had no effect on AAV-5 infection, which may be due to a lower anticoagulant potency. Adv5 infection was also reduced by the different anticoagulants. The differential effect of anticoagulants on Adv5 and AAV-2 infection vs AAV-5 infection suggests that FXa and thrombin modulate virus attachment and internalization through changes in receptors, possibly through PARs. Heparins may also interfere with the binding of viruses to cells. Taken together, these studies indicate that activation of the coagulation system enhances Adv and AAV infection.
PAR1 and PAR2 modulate TLR signaling in neutrophils, epithelial cells, macrophages, and cardiac fibroblasts (CFs)
PARs and TLRs have been proposed to act as a dual-sensor system in the innate immune response to infection.38 Interestingly, Moretti and colleagues found that PAR signaling requires the presence of TLRs.38 For instance, PAR1 and PAR2 activation of p38, but not extracellular signal-regulated kinase 1/2 and NF-κB, was abolished in TLR4-deficient murine neutrophils.38 In contrast, PAR1 and PAR2 activation of extracellular signal-regulated kinase 1/2 and NF-κB was reduced in cells deficient in TLR2 and MyD88.38 However, the more likely scenario during invasion of a pathogen is that TLRs are the major receptors that respond to infection and that PARs act by modulating TLR signaling.15,39-42
PAR2 modulation of TLR2, TLR3, and TLR4 signaling in epithelial cells and CFs
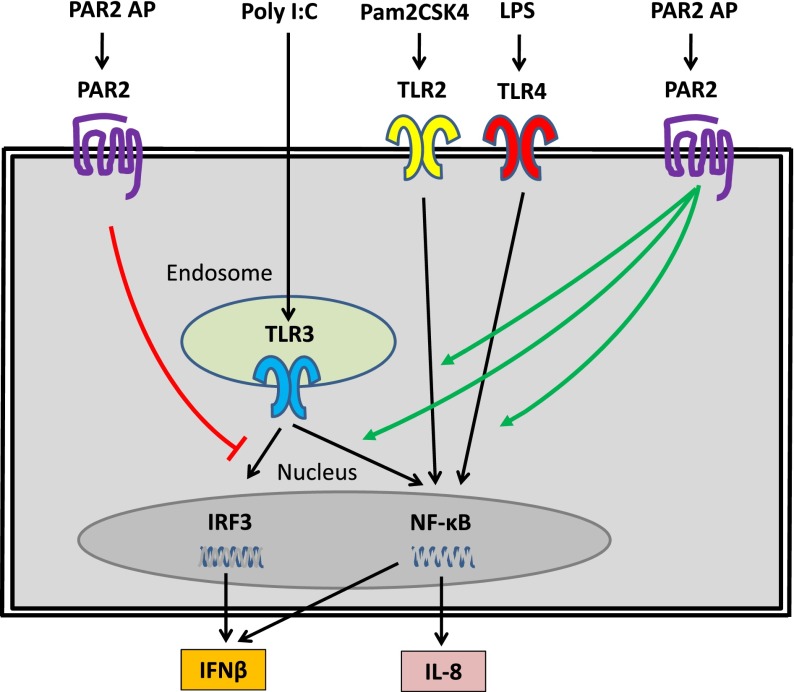
Many of the studies on PAR2 modulation of TLR signaling in different cells types have been performed by the Vogel group. In an early study, they showed that TLR4-dependent activation of human epithelial cell lines was enhanced by PAR2 stimulation, possibly through direct interaction between PAR2 and TLR4 and its adaptor proteins (Figure 3).41 They extended this study by investigating the effect of PAR2 activation on TLR2-, TLR3-, and TLR4-dependent signaling in human mucosal epithelial cell lines.40 PAR2 activation enhanced TLR2- and TLR3-dependent activation of NF-κB and interleukin (IL)-8 expression (Figure 3).40 These results indicate that in human epithelial cells, PAR2 positively regulates TLR2-, TLR3-, and TLR4-dependent expression of inflammatory pathway via increased activation of NF-κB.
Figure 3.
PAR2 modulates TLR signaling in human epithelial cell lines. PAR2 positively regulated TLR2, TLR3, and TLR4 activation of NF-κB and IL-8 expression but negatively regulated TLR3 activation of IRF3 and IFN-β expression. The TLR-specific ligands are shown together with PAR2 agonist peptide (AP), which was used to activate PAR2.
In contrast to studies showing a positive effect of PAR2 on TLR4 signaling, the Vogel group found that activation of PAR2 on primary murine macrophages suppressed LPS induction of tumor necrosis factor α (TNF-α), IL-6, and IL-12 expression.25 Similarly, a recent study found that FXa stimulation of human mononuclear cells and primary murine macrophages suppressed LPS induction of TNF-α in a PAR2-dependent manner.43 However, we did not find any effect of PAR2 deficiency on the expression of TNF-α or IL-6 in a mouse endotoxemia model.44 These studies indicate that PAR2 can positively and negatively regulate TLR4 signaling in different cell types.
The effect of PAR2 activation in human epithelial cells on the TLR3-dependent antiviral pathway driven by IRF3 was also analyzed by the Vogel group.40 Surprisingly, PAR2 activation inhibited TLR3-dependent activation of this antiviral pathway (Figure 3). Specifically, PAR2 activation reduced TLR3-dependent activation of IRF3 and IFN-β expression and the downstream ISGs CXCL10 and CCL5.40 Similarly, the Riteau group found that PAR2 activation suppressed CCL5 expression by A549 human epithelial cells after influenza A infection.45 We found that murine embryonic CFs lacking PAR2 expressed higher levels of IFN-β and CCL5 expression after poly I:C stimulation compared with wild-type (WT) fibroblasts (Figure 4).42 Taken together, these results indicate that PAR2 suppresses TLR3-dependent expression of the antiviral pathway in both epithelial cells and CFs.
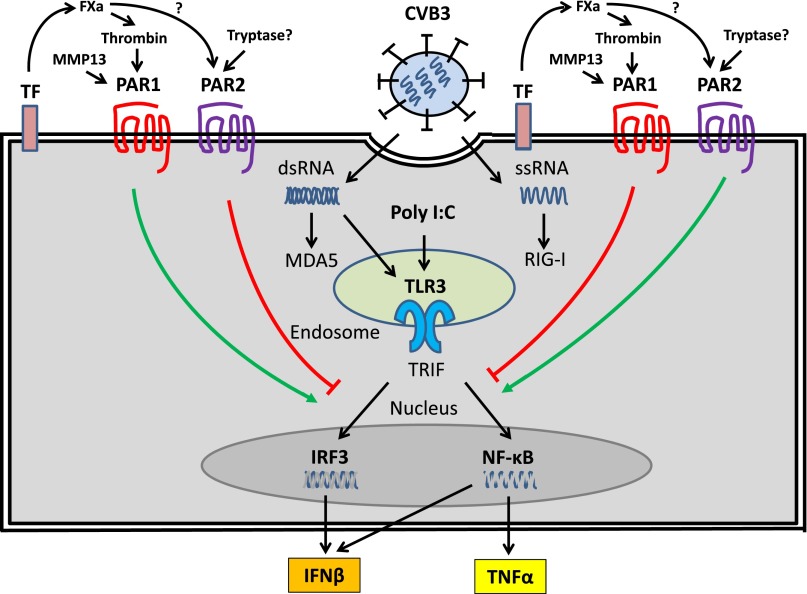
Figure 4.
PAR1 and PAR2 modulate TLR3-dependent expression of IFN-β in CFs. CVB3 infection of CFs leads to the release of ssRNA and the replication intermediate dsRNA into the cytosol. These foreign nucleic acids are recognized by TLR3 in endosomes and the cytoplasmic PRRs retinoic acid–inducible gene I (RIG-I) and melanoma differentiated gene 5 (MDA5). The TLR3-IFN-β pathway plays a central role in the innate immune response to CVB3 infection. Activation of PAR1 by TF-dependent generation of thrombin and MMP13 enhances TLR3-dependent activation of IRF3 and induction of IFN-β but suppresses TLR3-dependent activation of NF-κB and TNF-α expression. In contrast, activation of PAR2, possibly by FXa and/or tryptase, inhibits TLR3-dependent induction of IFN-β expression but enhances TLR3-dependent induction of TNF-α.
PAR1 modulation of TLR3 and TLR4 signaling in CFs
We analyzed the effect of PAR1 activation on TLR3-dependent signaling in murine CFs. Interestingly, we discovered that PAR1 activation enhanced TLR3-dependent expression of IFN-β and CXCL10 expression (Figure 4).15 This enhancement was abolished in PAR1 knockout (ko) cells and appears to be mediated, in part, by a PAR1-dependent increase in the activation of p38.15 We also observed a PAR1-dependent enhancement of poly I:C induction of IFN-β and CXCL10 expression in murine bone marrow–derived macrophages.46 Recently, we found that activation of PAR1 inhibited poly I:C induction of TNF-α expression by CFs and inhibited LPS induction of TNF-α expression by mouse embryonic fibroblasts (Michael F. J. Bode, S.A., and N.M., unpublished data). This effect appears to be via an inhibition of NF-κB activation (Figure 4). Taken together, these results demonstrate that PAR1 and PAR2 modulate TLR signaling and the innate immune response to viral infections.
Roles of PAR1 and PAR2 in mouse models of coxsackievirus B3-induced myocarditis
CVB is a nonenveloped enterovirus of the Picornaviridae family and a (+) ssRNA genome. CVB infection of cells is mediated by virus binding to the coxsackievirus-Adv receptor and the decay-accelerating factor on the cell surface followed by endocytosis.47 Mouse studies have shown that a deficiency of either TLR3 or its adaptor, TRIF, is associated with an increase in levels of virus and mortality after CVB3 or CVB4 infection.48-50 Interestingly, type I IFNs (IFN-α and IFN-β), but not type II IFN (IFN-γ), are required for the innate immune response to CVB3.51 IFN-α and IFN-β are expressed at low levels by various cells but are induced in virally infected cells, such as CFs and cardiomyocytes in the heart.52 These studies indicate that the TLR3-TRIF-IFN-β pathway plays a central role in the innate immune response to CVB3 infection and myocarditis.
PAR2ko mice have decreased CVB3-induced myocarditis
We recently investigated the role of PAR2 in a mouse model of CVB3-induced myocarditis. We found that PAR2ko mice exhibited strikingly less CVB3-induced myocarditis compared with WT mice (Table 1).42 Interestingly, infected PAR2ko mice expressed increased levels of cardiac IFN-β compared with infected WT mice, which may explain the reduced levels of CVB3 in the heart.42 In vitro studies demonstrated that PAR2ko CFs either infected with CVB3 or stimulated with poly I:C expressed more IFN-β and CCL5 compared with WT cells.42 Furthermore, activation of PAR2 inhibited poly I:C–induced phosphorylation of Stat1, which is activated downstream of IFN-β binding to its receptors.42 These data suggest that is PAR2 suppresses the TLR3-TRIF-IFN-β pathway. These results are consistent with previous studies by the Vogel and Riteau groups showing that PAR2 activation in human epithelial cells inhibits TLR3-dependent activation of IRF3 and IFN-β expression as well as the downstream ISGs CXCL10 and CCL5.40,45
Table 1.
Roles of PAR1 and PAR2 in a mouse model of CVB3-induced myocarditis
| Mouse | Results | Conclusion | Laboratory | Reference |
|---|---|---|---|---|
| PAR2ko mice | Increased IFN-β and CCL5 expression; decreased CVB3 genomes; decreased cardiac injury | PAR2 negatively regulates TLR3-dependent IFN-β expression. | Rauch | 42 |
| PAR1ko mice | Decreased IFN-β and CXCL10 expression; increased CVB3 genomes; increased cardiac injury | PAR1 positively regulates the TLR3-IFN-β pathway. | Mackman | 15 |
At present, the proteases that activate PAR2 during CVB3 infection have not been elucidated. Possible candidates include mast cell tryptase and chymase.53 Mast cells in the heart rapidly degranulate after CVB3 infection.54 Moreover, mast cell–deficient mice are protected against encephalomyocarditis viral myocarditis,55 which is a similar phenotype to PAR2ko mice. We are currently studying the role of mast cells and tryptase in CVB3-induced myocarditis. Virally encoded proteases may also activate PAR2 during infection. For instance, papain-like protease 2 expressed by the porcine epidemic diarrhea virus inhibited poly I:C induction of the IFN-β promoter by reducing levels of ubiquitinated proteins.56 It would be interesting to determine the role of PAR2 in this response. Further studies are needed to determine the proteases that activate PAR2 during CVB3 infection.
PAR1ko mice have increased CVB3-induced myocarditis
In contrast to the results with PAR2ko mice, we found that PAR1ko mice had increased CVB3-induced myocarditis compared with WT controls (Table 1).15 PAR1ko mice expressed lower levels of IFN-β and CXCL10 after infection and had higher levels of CVB3 in their hearts compared with WT mice.15 The reduced IFN-β expression was associated with reduced NK cell infiltration into the heart in PAR1ko mice compared with WT controls.15 In addition, we found that thrombin stimulation of WT murine splenocytes, but not PAR1ko cells, increased NK cell activity.15 This is consistent with a previous study using human lymphocytes.57 We also investigated the effect of overexpression of PAR1 on cardiomyocytes on CVB3-induced myocarditis using a transgenic mouse model. Mice overexpressing PAR1 on cardiomyocytes expressed higher levels of IFN-β after CVB3 infection and had reduced myocarditis compared with WT mice.15
In vitro studies with murine CFs demonstrated that PAR1 activation enhanced poly I:C induction of IFN-β and CXCL10 expression (Figure 4).15 Interestingly, we observed lower levels of CXCL10 expression in PAR1ko CFs stimulated with poly I:C alone compared with WT cells.15 We recently discovered that isoproterenol stimulation of CFs induces the expression and release of matrix metalloproteinase (MMPs) that activate PAR1.58 Importantly, a pan-MMP inhibitor and an MMP13–specific inhibitor reduced poly I:C induction of CXCL10 expression in WT CFs to levels observed with PAR1ko CFs, indicating that MMP13 and possibly other MMPs were contributing to CXCL10 expression by activating PAR1 (Figure 4).15
Role of the coagulation cascade in CVB3-induced myocarditis
Based on the known activators of PAR1 and our in vitro studies with CFs, we hypothesized that both thrombin and MMP-13 were the primary activators of PAR1 during CVB3 infection (Figure 4). Consistent with this hypothesis, we found that inhibition of either TF or thrombin increased levels of CVB3 in the heart and cardiac injury, suggesting that the TF-thrombin-PAR1 pathway contributes to the innate immune response to CVB3 infection.15 In addition, inhibition of MMP13 in CVB3-infected mice increased myocarditis.15
In contrast to our study, inhibition of coagulation with LMWH reduced cardiac fibrosis and mortality by 20%.59 It was proposed that the reduction of fibrosis was due to inhibition of fibrin deposition in the myocardium. Another study observed reduced fibrosis and improved heart function 8 days after CVB3 infection in mice treated with the FXa inhibitor fondaparinux.60 Others have proposed that administration of a FXIII inhibitor or a fibrinolytic agent would be beneficial by reducing the deposition of crosslinked fibrin and fibrosis in the myocardium that occurs in viral myocarditis.61 Activation of PAR1 also increases fibrosis62 and may contribute to cardiac fibrosis during CVB3 infection. The differences in the results with various anticoagulants may be due to the degree of anticoagulation or additional activities of the anticoagulants that may include inhibition of viral attachment by LMWH. For instance, a large variety of viruses, including CVB3, use cell-surface heparan sulfate proteoglycans for attachment and infection, which can be blocked by heparin and heparin-like molecules.63,64 Further studies are required to determine the role of the coagulation cascade in CVB3-induced myocarditis.
Roles of PAR1 and PAR2 in mouse models of IAV infection
IAV is a major cause of mortality in the elderly population. It is an enveloped ssRNA virus and binds to receptors containing sialic acids. Proteolytic cleavage of hemagglutinin on the surface of the viral particle is required for fusion of the viral envelope with the endosome and release into the cytosol.65 Several PRRs, including TLR3 and TLR4, are activated following IAV infection.40,66 TLR3 deficiency results in unrestricted IAV replication.67 Interestingly, PAR2 enhanced IFN-γ–induced suppression of IAV replication in human monocytes.68 In addition, the expression of all 4 PARs is increased after IAV infection in the airway and lung.69 IAV infection also leads to the expression of various PAR activating proteases, such as trypsin and MMPs, which may contribute to viral replication and inflammation.70 In IAV infection, neutrophils are essential for early virus clearance, but excessive infiltration increases mortality and hemorrhages.71 Activated immune cells, such as neutrophils and macrophages, secrete cathepsin G and other proteases that can activate PAR1 and PAR4.72 Furthermore, neutrophil cathepsin G and elastase can disarm PAR2 on human lung epithelial cells but activate PAR2 on nonepithelial cells.72,73 Therefore, there are a variety of proteases present during IAV infection that could activate PARs and alter IAV infection and the immune response.
Role of PAR2 in IAV infection in mice
In vitro studies using human epithelial cell lines and murine CFs showed that activation of PAR2 inhibited the TLR3-IFN-β antiviral pathway.40,42,45 These results suggested that PAR2ko mice would be protected from IAV infection because they would express higher levels of IFN-β. Indeed, the Vogel group found that PAR2ko mice exhibited reduced mortality after infection with the mouse adapted influenza A strain H1N1 (Table 2).40 In addition, PAR2 inhibition was found to reduce the replication of IAV in cardiomyocytes in vitro.70 In contrast to these studies, the Riteau group reported that H1N1 infected PAR2ko mice had a small increase in mortality compared with WT mice (Table 2).45 The authors concluded that PAR2 protected mice by increasing IFN-γ expression. The Riteau group also showed that administration of SLIGRL, a PAR2 agonist peptide, to WT mice increased survival after H1N1 infection.45 However, a recent study showed that this peptide mediated PAR2-independent protection against IAV.74
Table 2.
Roles of PAR1 and PAR2 in a mouse model of H1N1 infection
| Mouse | Results | Conclusion | Laboratory | Reference |
|---|---|---|---|---|
| PAR2ko mice | Increased survival | PAR2 negatively regulates antiviral responses. | Vogel | 40 |
| PAR1ko mice | Decreased CXCL10 expression; increased H1N1 genomes | PAR1 positively regulates the TLR3-IFN-β pathway. | Mackman | 15 |
| PAR2ko mice | Increased IFN-γ expression; decreased survival | PAR2 positively regulates IFN-γ expression. | Riteau | 45 |
| PAR1ko mice | Decreased inflammation; increased survival | PAR1 increases lung inflammation. | Riteau | 77 |
It is difficult to explain the different results found with IAV infection in PAR2ko mice (Table 2). This could be due to differences in virus dose and virus preparation. Importantly, the Vogel group propagated H1N1 in the allantoic fluid of eggs, whereas the Riteau group used Madin-Darby canine kidney cells.40,45 This would introduce different host proteins into the envelope that can alter the host response to the virus. For instance, IAV propagated in eggs may exhibit a higher virulence due to hemagglutinin cleavage by ectopic expressed FX-like proteases, which are not expressed by Madin-Darby canine kidney cells.75,76
Role of PAR1 in influenza infection in mice
Our in vitro studies with CFs indicated that PAR1 enhanced TLR3-dependent IFN-β expression.15 These data suggest that PAR1ko mice may be more susceptible to IAV infection because they would have reduced levels of the TLR3-IFN-β antiviral pathway. Indeed, we found that PAR1ko mice expressed lower levels of CXCL10 and had higher levels of virus in their lungs compared with WT mice 3 days after H1N1 infection (Table 2).15 In contrast to our results, the Riteau group reported that PAR1ko mice had reduced mortality compared with WT controls after H1N1 infection (Table 2).77 Intransal administration of a PAR1 agonist peptide into WT mice also increased mortality after H1N1 infection.77 Similarly, activation of PAR1 with an agonist peptide increased the susceptibility of mice to human metapneumovirus.78 It was concluded that PAR1 was proinflammatory and increased the cytokine storm and lethality after infection with either H1N1 or human metapneumovirus.77,78 Again, it is difficult to explain the different results observed with PAR1ko mice. We propagated IAV in eggs in a similar manner to the Vogel group. Riteau and colleagues recently proposed that PAR1 may play a protective role in “non-severe IAV infection” where it cooperates with PRRs to eliminate the pathogen.79 However, during “severe IAV infection,” PAR1 may enhance inflammation. This model suggests that PAR1 can be protective or deleterious depending on the dose of IAV.
Roles of the coagulation cascade and platelets in mouse models of IAV infection
Lethal H1N1 infection in mice is associated with both pulmonary and systemic activation of coagulation.80-82 Mice containing the prothrombotic variant of factor V called Leiden exhibited increased levels of IAV in the lung 48 hours after infection compared with WT mice.81 However, there were no differences in the activation of coagulation, early immune responses, or survival between factor V Leiden and WT mice.81 Inhibition of activated protein C (APC) worsened lung histopathology but lowered neutrophil influx and delayed mortality during IAV infection in mice.83 Administration of APC reduced the virus load in the lungs 48 hours after infection but did not affect lung histopathology, neutrophil influx, or survival.80 APC is an anticoagulant and a known activator of PAR1. Therefore, it is possible that part of the beneficial effect of APC is mediated by a reduction in coagulation and/or activation of PAR1 and enhancement of antiviral responses.
Based on their findings, the Riteau group suggested “that PAR1 antagonism might be explored as a treatment for influenza.”77 In humans, thrombin activation of platelets is primarily mediated by PAR1, and therefore PAR1 antagonists would inhibit platelet activation. Importantly, viral infections of the lung cause alveolar hemorrhage that increases morbidity and mortality and morbidity in humans.84 It has been hypothesized that aspirin contributed to the incidence and severity of viral pathology in the 1918-1919 influenza pandemic.85 Moreover, treatment with antipyretics increased mortality in animal models of influenza infection.86 Consistent with this notion, we observed that impaired hemostasis due to either a genetic deficiency of TF or an absence of PAR4 dramatically increased lung hemorrhage and increased mortality in mice infected with H1N1.87 Pharmacologic inhibition of platelets and the clotting cascade also increased mortality of WT mice infected with H1N1 (S.A., Kohei Tatsumi, and N.M., unpublished data). In line with our observations, a reduction of fibrinogen levels with the snake venom ancrod also increased the severity of IAV infection in mice.88 Taken together, these results suggest that antithrombotic therapy may have an adverse effect during IAV infection by increasing lung hemorrhages and mortality.
Dengue virus
Dengue virus is the most important arthropod-transmitted viral disease in the world.89 Major complications are Dengue shock syndrome and Dengue hemorrhagic fever. In Dengue shock syndrome, there is increased cytokine expression and activation of coagulation similar to bacterial sepsis.90 In vitro studies showed that Dengue virus infection of endothelial cells induced expression of TF and PAR1.13 Interestingly, Dengue virus proteins show sequence homologies to coagulation proteases.91 This suggests that Dengue hemorrhagic fever may be caused by an autoimmune reaction against thrombin and other components of the coagulation cascade leading to an antithrombotic and profibrinolytic state.86,87 APC-dependent activation of PAR1 reduced Dengue virus–mediated endothelial permeability and inflammatory response,92 which suggests that APC may be protective during Dengue virus infection.
HIV
HIV infection increases inflammation and activates the coagulation cascade.93 Higher levels of IL-6 and d-dimer were associated with mortality in HIV patients.94 Antiretroviral therapy reduces inflammation and ongoing coagulation.93 It was shown that HIV can infect the brain and dorsal root ganglia, which leads to increased levels of thrombin and PAR1 as well as the development of encephalitis and distal sensory polyneuropathy.95,96 PAR1 signaling may contribute to neuronal inflammation and damage during HIV infection.95,96 Furthermore, activation of PAR1 and PAR2 may lead to increased HIV infection of certain cell types due to increased expression of the HIV coreceptor CCR5.97 However, PAR1 stimulation can also increase IL-10 and suppressor of cytokine signaling-3 expression, which would promote anti-inflammatory signals in microglia cells.98
Activation of TLR3 inhibits HIV replication in macrophages.99 We observed that costimulation of PAR1 and TLR3 led to increased IFN-β–dependent responses in bone marrow–derived macrophages and dendritic cells.46 It is therefore possible that PAR1 may also contribute to antiviral responses during HIV infection. Another study suggested that the thrombin-PAR1 axis contributes to adaptive immune responses through increased T-cell motility and production of proinflammatory cytokines during HIV infection.100
Conclusion
Activation of the coagulation cascade during viral infection is probably a protective mechanism to limit the spread of the infection. However, excessive clotting can lead to disseminated intravascular coagulation and subsequent hemorrhage, such as during Ebola hemorrhagic fever and Dengue hemorrhagic fever. FX binding to Adv5 not only enhances its infection of hepatocytes but also increases its recognition by macrophages as part of the innate immune system. PAR1 and PAR2 also modulate infection and the innate immune response to viral infection. PARs modulate TLR signaling in epithelial cells, CFs, and primary murine macrophages. Anticoagulants increased lung hemorrhage in an IAV mouse model but produced contradictory results in a mouse model of CVB3-induced myocarditis. Overall, there is a need for additional studies to understand the role of coagulation, proteases, and PARs in different virus infections. Long-term antithrombotic therapy may affect viral infection and replication as well as the immune response to the virus.
Acknowledgments
The authors thank Dr Barbara Sherry (North Carolina State University) for comments on the manuscript.
Financial support was received from the Myocarditis Foundation (S.A.) and the National Institutes of Health, Heart, Lung and Blood Institute (HL119523) (N.M.).
Authorship
Contribution: S.A. and N.M. wrote the manuscript and read and approved the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, University of North Carolina at Chapel Hill, 98 Manning Dr, Campus Box 7035, Chapel Hill, NC 27599; e-mail: nigel_mackman@med.unc.edu.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Beutler BA. TLRs and innate immunity. Blood. 2009;113(7):1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Li J, Li Y, et al. Crosstalk between platelets and the immune system: old systems with new discoveries. Adv Hematol. 2012;2012:384685. [DOI] [PMC free article] [PubMed]
- 5.Gorbea C, Makar KA, Pauschinger M, et al. A role for Toll-like receptor 3 variants in host susceptibility to enteroviral myocarditis and dilated cardiomyopathy. J Biol Chem. 2010;285(30):23208–23223. doi: 10.1074/jbc.M109.047464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 7.Fensterl V, Sen GC. Interferons and viral infections. Biofactors. 2009;35(1):14–20. doi: 10.1002/biof.6. [DOI] [PubMed] [Google Scholar]
- 8.Seya T, Matsumoto M, Ebihara T, Oshiumi H. Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing. Immunol Rev. 2009;227(1):44–53. doi: 10.1111/j.1600-065X.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Liu Z, Lim T, et al. CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ Res. 2009;104(5):628–638. doi: 10.1161/CIRCRESAHA.108.192179. [DOI] [PubMed] [Google Scholar]
- 10.Key NS, Vercellotti GM, Winkelmann JC, et al. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci USA. 1990;87(18):7095–7099. doi: 10.1073/pnas.87.18.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188(11):1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 12.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115(2):161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huerta-Zepeda A, Cabello-Gutiérrez C, Cime-Castillo J, et al. Crosstalk between coagulation and inflammation during Dengue virus infection. Thromb Haemost. 2008;99(5):936–943. doi: 10.1160/TH07-08-0438. [DOI] [PubMed] [Google Scholar]
- 14.Antoniak S, Boltzen U, Riad A, et al. Viral myocarditis and coagulopathy: increased tissue factor expression and plasma thrombogenicity. J Mol Cell Cardiol. 2008;45(1):118–126. doi: 10.1016/j.yjmcc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Antoniak S, Owens AP, III, Baunacke M, et al. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest. 2013;123(3):1310–1322. doi: 10.1172/JCI66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goeijenbier M, van Wissen M, van de Weg C, et al. Review: Viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84(10):1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibamiya A, Hersemeyer K, Schmidt Wöll T, et al. A key role for Toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood. 2009;113(3):714–722. doi: 10.1182/blood-2008-02-137901. [DOI] [PubMed] [Google Scholar]
- 18.Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362(9400):1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 19.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 20.Antoniak S, Pawlinski R, Mackman N. Protease-activated receptors and myocardial infarction. IUBMB Life. 2011;63(6):383–389. doi: 10.1002/iub.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J Leukoc Biol. 2008;83(6):1309–1322. doi: 10.1189/jlb.0108001. [DOI] [PubMed] [Google Scholar]
- 22.Lan RS, Stewart GA, Goldie RG, Henry PJ. Altered expression and in vivo lung function of protease-activated receptors during influenza A virus infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L388–L398. doi: 10.1152/ajplung.00286.2003. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg SF. The cardiovascular actions of protease-activated receptors. Mol Pharmacol. 2005;67(1):2–11. doi: 10.1124/mol.104.003103. [DOI] [PubMed] [Google Scholar]
- 24.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol. 2010;160(2):191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nhu QM, Shirey KA, Pennini ME, Stiltz J, Vogel SN. Proteinase-activated receptor 2 activation promotes an anti-inflammatory and alternatively activated phenotype in LPS-stimulated murine macrophages. Innate Immun. 2012;18(2):193–203. doi: 10.1177/1753425910395044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutherland MR, Ruf W, Pryzdial EL. Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease-activated receptor 2 cofactors that enhance infection. Blood. 2012;119(15):3638–3645. doi: 10.1182/blood-2011-08-376814. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland MR, Friedman HM, Pryzdial EL. Herpes simplex virus type 1-encoded glycoprotein C enhances coagulation factor VIIa activity on the virus. Thromb Haemost. 2004;92(5):947–955. doi: 10.1160/TH04-04-0242. [DOI] [PubMed] [Google Scholar]
- 28.Etingin OR, Silverstein RL, Friedman HM, Hajjar DP. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990;61(4):657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- 29.Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100(5):859–865. doi: 10.1111/j.1349-7006.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland MR, Friedman HM, Pryzdial EL. Thrombin enhances herpes simplex virus infection of cells involving protease-activated receptor 1. J Thromb Haemost. 2007;5(5):1055–1061. doi: 10.1111/j.1538-7836.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland MR, Simon AY, Shanina I, Horwitz MS, Ruf W, Pryzdial EL. Tissue factor on the herpes simplex virus type 1 surface enhances infection in vivo. In: 55th ASH Annual Meeting and Exhibition; December 7-10, 2013. New Orleans, LA. Abstract 2332. [Google Scholar]
- 32.Parker AL, Waddington SN, Nicol CG, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108(8):2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 33.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132(3):397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Coughlan L, Alba R, Parker AL, et al. Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses. 2010;2(10):2290–2355. doi: 10.3390/v2102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Qiu Q, Tian J, et al. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat Med. 2013;19(4):452–457. doi: 10.1038/nm.3107. [DOI] [PubMed] [Google Scholar]
- 36.Doronin K, Flatt JW, Di Paolo NC, et al. Coagulation factor X activates innate immunity to human species C adenovirus. Science. 2012;338(6108):795–798. doi: 10.1126/science.1226625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuettrumpf J, Zou J, Zhang Y, et al. The inhibitory effects of anticoagulation on in vivo gene transfer by adeno-associated viral or adenoviral vectors. Mol Ther. 2006;13(1):88–97. doi: 10.1016/j.ymthe.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Moretti S, Bellocchio S, Bonifazi P, et al. The contribution of PARs to inflammation and immunity to fungi. Mucosal Immunol. 2008;1(2):156–168. doi: 10.1038/mi.2007.13. [DOI] [PubMed] [Google Scholar]
- 39.Antoniak S, Mackman N. Coagulation, protease-activated receptors, and viral myocarditis. J Cardiovasc Transl Res. 2014;7(2):203–211. doi: 10.1007/s12265-013-9515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nhu QM, Shirey K, Teijaro JR, et al. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010;3(1):29–39. doi: 10.1038/mi.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rallabhandi P, Nhu QM, Toshchakov VY, et al. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem. 2008;283(36):24314–24325. doi: 10.1074/jbc.M804800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weithauser A, Bobbert P, Antoniak S, et al. Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis. J Am Coll Cardiol. 2013;62(19):1737–1745. doi: 10.1016/j.jacc.2013.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gleeson EM, O'Donnell JS, Hams E, et al. Activated factor X signaling via protease-activated receptor 2 suppresses pro-inflammatory cytokine production from LPS-stimulated myeloid cells. Haematologica. 2014;99(1):185–193. doi: 10.3324/haematol.2013.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawlinski R, Pedersen B, Schabbauer G, et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103(4):1342–1347. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoufache K, LeBouder F, Morello E, et al. Protective role for protease-activated receptor-2 against influenza virus pathogenesis via an IFN-gamma-dependent pathway. J Immunol. 2009;182(12):7795–7802. doi: 10.4049/jimmunol.0803743. [DOI] [PubMed] [Google Scholar]
- 46.Antoniak S, Milner J, Beck MA, Mackman N. Protease-activated receptor 1 contributes to early immune responses in the lung after influenza A infection. In: XXIV Congress of the International Society on Thrombosis and Haemostasis; June 25-July 4, 2013. Amsterdam, NL. Abstract 849. [Google Scholar]
- 47.Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52(4):274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riad A, Westermann D, Zietsch C, et al. TRIF is a critical survival factor in viral cardiomyopathy. J Immunol. 2011;186(4):2561–2570. doi: 10.4049/jimmunol.1002029. [DOI] [PubMed] [Google Scholar]
- 49.Abston ED, Coronado MJ, Bucek A, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 versus TRIF in progression to chronic disease. Clin Dev Immunol. 2012;2012:129486. [DOI] [PMC free article] [PubMed]
- 50.Richer MJ, Lavallée DJ, Shanina I, Horwitz MS. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS ONE. 2009;4(1):e4127. doi: 10.1371/journal.pone.0004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wessely R, Klingel K, Knowlton KU, Kandolf R. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation. 2001;103(5):756–761. doi: 10.1161/01.cir.103.5.756. [DOI] [PubMed] [Google Scholar]
- 52.Stewart MJ, Smoak K, Blum MA, Sherry B. Basal and reovirus-induced beta interferon (IFN-beta) and IFN-beta-stimulated gene expression are cell type specific in the cardiac protective response. J Virol. 2005;79(5):2979–2987. doi: 10.1128/JVI.79.5.2979-2987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272(7):4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 54.Fairweather D, Frisancho-Kiss S, Gatewood S, et al. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37(2):131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 55.Higuchi H, Hara M, Yamamoto K, et al. Mast cells play a critical role in the pathogenesis of viral myocarditis. Circulation. 2008;118(4):363–372. doi: 10.1161/CIRCULATIONAHA.107.741595. [DOI] [PubMed] [Google Scholar]
- 56.Xing Y, Chen J, Tu J, et al. The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J Gen Virol. 2013;94(Pt 7):1554–1567. doi: 10.1099/vir.0.051169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naldini A, Carney DH. Thrombin modulation of natural killer activity in human peripheral lymphocytes. Cell Immunol. 1996;172(1):35–42. doi: 10.1006/cimm.1996.0212. [DOI] [PubMed] [Google Scholar]
- 58.Jaffré F, Friedman AE, Hu Z, Mackman N, Blaxall BC. β-adrenergic receptor stimulation transactivates protease-activated receptor 1 via matrix metalloproteinase 13 in cardiac cells. Circulation. 2012;125(24):2993–3003. doi: 10.1161/CIRCULATIONAHA.111.066787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frizelle S, Schwarz J, Huber SA, Leslie K. Evaluation of the effects of low molecular weight heparin on inflammation and collagen deposition in chronic coxsackievirus B3-induced myocarditis in A/J mice. Am J Pathol. 1992;141(1):203–209. [PMC free article] [PubMed] [Google Scholar]
- 60.Malz R, Weithaeuser A, Tschöpe C, Schultheiss HP, Rauch U. Inhibition of coagulation factor Xa improves myocardial function during CVB3-induced myocarditis [published online ahead of print February 17, 2014]. Cardiovasc Ther. doi: 10.1111/1755-5922.12069. [DOI] [PubMed] [Google Scholar]
- 61.Schnitt SJ, Stillman IE, Owings DV, Kishimoto C, Dvorak HF, Abelmann WH. Myocardial fibrin deposition in experimental viral myocarditis that progresses to dilated cardiomyopathy. Circ Res. 1993;72(4):914–920. doi: 10.1161/01.res.72.4.914. [DOI] [PubMed] [Google Scholar]
- 62.Howell DC, Johns RH, Lasky JA, et al. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166(5):1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vivès RR, Lortat-Jacob H, Fender P. Heparan sulphate proteoglycans and viral vectors : ally or foe? Curr Gene Ther. 2006;6(1):35–44. doi: 10.2174/156652306775515565. [DOI] [PubMed] [Google Scholar]
- 64.Zautner AE, Körner U, Henke A, Badorff C, Schmidtke M. Heparan sulfates and coxsackievirus-adenovirus receptor: each one mediates coxsackievirus B3 PD infection. J Virol. 2003;77(18):10071–10077. doi: 10.1128/JVI.77.18.10071-10077.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun X, Tse LV, Ferguson AD, Whittaker GR. Modifications to the hemagglutinin cleavage site control the virulence of a neurotropic H1N1 influenza virus. J Virol. 2010;84(17):8683–8690. doi: 10.1128/JVI.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Goffic R, Pothlichet J, Vitour D, et al. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178(6):3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 67.Le Goffic R, Balloy V, Lagranderie M, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2(6):e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feld M, Shpacovitch VM, Ehrhardt C, et al. Agonists of proteinase-activated receptor-2 enhance IFN-gamma-inducible effects on human monocytes: role in influenza A infection. J Immunol. 2008;180(10):6903–6910. doi: 10.4049/jimmunol.180.10.6903. [DOI] [PubMed] [Google Scholar]
- 69.Lan RS, Stewart GA, Henry PJ. Modulation of airway smooth muscle tone by protease activated receptor-1,-2,-3 and -4 in trachea isolated from influenza A virus-infected mice. Br J Pharmacol. 2000;129(1):63–70. doi: 10.1038/sj.bjp.0703007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan HY, Yano M, Kido H. Effects of inhibitors of Toll-like receptors, protease-activated receptor-2 signalings and trypsin on influenza A virus replication and upregulation of cellular factors in cardiomyocytes. J Med Invest. 2011;58(1-2):19–28. doi: 10.2152/jmi.58.19. [DOI] [PubMed] [Google Scholar]
- 71.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154(1):197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shpacovitch V, Feld M, Bunnett NW, Steinhoff M. Protease-activated receptors: novel PARtners in innate immunity. Trends Immunol. 2007;28(12):541–550. doi: 10.1016/j.it.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Chignard M, Pidard D. Neutrophil and pathogen proteinases versus proteinase-activated receptor-2 lung epithelial cells: more terminators than activators. Am J Respir Cell Mol Biol. 2006;34(4):394–398. doi: 10.1165/rcmb.2005-0250TR. [DOI] [PubMed] [Google Scholar]
- 74.Betts RJ, Mann TS, Henry PJ. Inhibitory influence of the hexapeptidic sequence SLIGRL on influenza A virus infection in mice. J Pharmacol Exp Ther. 2012;343(3):725–735. doi: 10.1124/jpet.112.196485. [DOI] [PubMed] [Google Scholar]
- 75.Seitz C, Isken B, Heynisch B, Rettkowski M, Frensing T, Reichl U. Trypsin promotes efficient influenza vaccine production in MDCK cells by interfering with the antiviral host response. Appl Microbiol Biotechnol. 2012;93(2):601–611. doi: 10.1007/s00253-011-3569-8. [DOI] [PubMed] [Google Scholar]
- 76.Gotoh B, Ogasawara T, Toyoda T, Inocencio NM, Hamaguchi M, Nagai Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 1990;9(12):4189–4195. doi: 10.1002/j.1460-2075.1990.tb07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khoufache K, Berri F, Nacken W, et al. PAR1 contributes to influenza A virus pathogenicity in mice. J Clin Invest. 2013;123(1):206–214. doi: 10.1172/JCI61667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aerts L, Hamelin M-È, Rhéaume C, et al. Modulation of protease activated receptor 1 influences human metapneumovirus disease severity in a mouse model. PLoS ONE. 2013;8(8):e72529. doi: 10.1371/journal.pone.0072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berri F, Lê VB, Jandrot-Perrus M, Lina B, Riteau B. Switch from protective to adverse inflammation during influenza: viral determinants and hemostasis are caught as culprits. Cell Mol Life Sci. 2014;71(5):885–898. doi: 10.1007/s00018-013-1479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schouten M, Sluijs KF, Gerlitz B, et al. Activated protein C ameliorates coagulopathy but does not influence outcome in lethal H1N1 influenza: a controlled laboratory study. Crit Care. 2010;14(2):R65. doi: 10.1186/cc8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schouten M, van der Sluijs KF, Roelofs JJ, Levi M, Van’t Veer C, van der Poll T. Factor V Leiden mutation does not affect coagulopathy or outcome in lethal H1N1 influenza. Eur Respir J. 2010;36(6):1346–1354. doi: 10.1183/09031936.00204909. [DOI] [PubMed] [Google Scholar]
- 82.Keller TT, van der Sluijs KF, de Kruif MD, et al. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res. 2006;99(11):1261–1269. doi: 10.1161/01.RES.0000250834.29108.1a. [DOI] [PubMed] [Google Scholar]
- 83.Schouten M, de Boer JD, van der Sluijs KF, et al. Impact of endogenous protein C on pulmonary coagulation and injury during lethal H1N1 influenza in mice. Am J Respir Cell Mol Biol. 2011;45(4):789–794. doi: 10.1165/rcmb.2010-0370OC. [DOI] [PubMed] [Google Scholar]
- 84.Gilbert CR, Vipul K, Baram M. Novel H1N1 influenza A viral infection complicated by alveolar hemorrhage. Respir Care. 2010;55(5):623–625. [PubMed] [Google Scholar]
- 85.Starko KM. Salicylates and pandemic influenza mortality, 1918-1919 pharmacology, pathology, and historic evidence. Clin Infect Dis. 2009;49(9):1405–1410. doi: 10.1086/606060. [DOI] [PubMed] [Google Scholar]
- 86.Eyers S, Weatherall M, Shirtcliffe P, Perrin K, Beasley R. The effect on mortality of antipyretics in the treatment of influenza infection: systematic review and meta-analysis. J R Soc Med. 2010;103(10):403–411. doi: 10.1258/jrsm.2010.090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antoniak S, Tatsumi K, Milner J, Beck MA, Mackman N. Impaired hemostasis leads to alveolar hemorrhages and increased mortality in influenza A infection. In: 55th ASH Annual Meeting and Exhibition; December 7-10, 2013. New Orleans, LA. Abstract 1095. [Google Scholar]
- 88.Berri F, Rimmelzwaan GF, Hanss M, et al. Plasminogen controls inflammation and pathogenesis of influenza virus infections via fibrinolysis. PLoS Pathog. 2013;9(3):e1003229. doi: 10.1371/journal.ppat.1003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gubler DJ. The economic burden of dengue. Am J Trop Med Hyg. 2012;86(5):743–744. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suharti C, van Gorp EC, Setiati TE, et al. The role of cytokines in activation of coagulation and fibrinolysis in dengue shock syndrome. Thromb Haemost. 2002;87(1):42–46. [PubMed] [Google Scholar]
- 91.Lin YS, Yeh TM, Lin CF, et al. Molecular mimicry between virus and host and its implications for dengue disease pathogenesis. Exp Biol Med (Maywood) 2011;236(5):515–523. doi: 10.1258/ebm.2011.010339. [DOI] [PubMed] [Google Scholar]
- 92.Cabello-Gutiérrez C, Manjarrez-Zavala ME, Huerta-Zepeda A, et al. Modification of the cytoprotective protein C pathway during Dengue virus infection of human endothelial vascular cells. Thromb Haemost. 2009;101(5):916–928. [PubMed] [Google Scholar]
- 93.Baker JV, Brummel-Ziedins K, Neuhaus J, et al. INSIGHT SMART Study Team. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc. 2013;2(4):e000264. doi: 10.1161/JAHA.113.000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuller LH, Tracy R, Belloso W, et al. INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Acharjee S, Zhu Y, Maingat F, et al. Proteinase-activated receptor-1 mediates dorsal root ganglion neuronal degeneration in HIV/AIDS. Brain. 2011;134(Pt 11):3209–3221. doi: 10.1093/brain/awr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boven LA, Vergnolle N, Henry SD, et al. Up-regulation of proteinase-activated receptor 1 expression in astrocytes during HIV encephalitis. J Immunol. 2003;170(5):2638–2646. doi: 10.4049/jimmunol.170.5.2638. [DOI] [PubMed] [Google Scholar]
- 97.Giacaman RA, Nobbs AH, Ross KF, Herzberg MC. Porphyromonas gingivalis selectively up-regulates the HIV-1 coreceptor CCR5 in oral keratinocytes. J Immunol. 2007;179(4):2542–2550. doi: 10.4049/jimmunol.179.4.2542. [DOI] [PubMed] [Google Scholar]
- 98.Fabrizi C, Pompili E, Panetta B, Nori SL, Fumagalli L. Protease-activated receptor-1 regulates cytokine production and induces the suppressor of cytokine signaling-3 in microglia. Int J Mol Med. 2009;24(3):367–371. doi: 10.3892/ijmm_00000241. [DOI] [PubMed] [Google Scholar]
- 99.Zhou Y, Wang X, Liu M, et al. A critical function of toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology. 2010;131(1):40–49. doi: 10.1111/j.1365-2567.2010.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hurley A, Smith M, Karpova T, et al. Enhanced effector function of CD8(+) T cells from healthy controls and HIV-infected patients occurs through thrombin activation of protease-activated receptor 1. J Infect Dis. 2013;207(4):638–650. doi: 10.1093/infdis/jis730. [DOI] [PMC free article] [PubMed] [Google Scholar]