Key Points
Sp1 transcription factor (TF) is activated in WM.
Dual inhibition of Sp1 and MYD88 pathways induces synergistic cell death in WM cells.
Abstract
Sp1 transcription factor controls a pleiotropic group of genes and its aberrant activation has been reported in a number of malignancies, including multiple myeloma. In this study, we investigate and report its aberrant activation in Waldenström macroglobulinemia (WM). Both loss of and gain of Sp1 function studies have highlighted a potential oncogenic role of Sp1 in WM. We have further investigated the effect of a small molecule inhibitor, terameprocol (TMP), targeting Sp1 activity in WM. Treatment with TMP inhibited the growth and survival and impaired nuclear factor-κB and signal transducer and activator of transcription activity in WM cells. We next investigated and observed that TMP treatment induced further inhibition of WM cells in MYD88 knockdown WM cells. Moreover, we observed that Bruton’s tyrosine kinase, a downstream target of MYD88 signaling pathway, is transcriptionally regulated by Sp1 in WM cells. The combined use of TMP with Bruton’s tyrosine kinase or interleukin-1 receptor-associated kinase 1 and 4 inhibitors resulted in a significant and synergistic dose-dependent antiproliferative effect in MYD88-L265P–expressing WM cells. In summary, these results demonstrate Sp1 as an important transcription factor that regulates proliferation and survival of WM cells independent of MYD88 pathway activation, and provide preclinical rationale for clinical development of TMP in WM alone or in combination with inhibitors of MYD88 pathway.
Introduction
Gene expression and proteomic studies have advanced our understanding of Waldenström macroglobulinemia (WM) and identified potential therapeutic targets. Whole-genome sequencing identified somatic mutation involving the MYD88 gene in more than 90% of tumor samples from patients with WM and non-IgM lymphoplasmacytic lymphoma.1 MYD88 L265P mutation enables the expansion of WM cells with activation of nuclear factor-κB (NF-κB) signaling through the induction of Bruton’s tyrosine kinase (BTK) and interleukin-1 receptor-associated kinase 1 and 4 (IRAK 1/4).2 Therapeutics targeting BTK and IRAK 1/4 are under investigation in WM in both a preclinical and a clinical setting. Despite these advances, WM remains incurable with a 5-year survival rate of 50%.3 Therefore, the search for novel therapeutic agents targeting deregulated signaling pathways specifically present in WM is ongoing.
Sp1 is a ubiquitous zinc finger transcription factor (TF) that binds guanine-cytosine–rich elements in the promoter region of inducible genes. Lines of evidence demonstrate that overactivation of Sp1 occurs frequently in a wide variety of human tumors and that high Sp1 expression correlates with aggressive biology and poor clinical outcome of these tumors.4-6 Inhibition of Sp1, both by small interfering RNA (siRNA) knockdown and by pharmacologic agent tetra-O-methyl nordihydroguaiaretic acid, terameprocol (TMP) which competitively inhibits Sp1-DNA binding, has demonstrated antiproliferative activity in a number of solid tumors.7-9 Based on our previous observation that Sp1 transactivation plays an important functional role in myeloma,10 we have investigated its impact on WM cells, both directly and in relationship to MYD88 pathway activation. Our findings show that Sp1 is constitutively active in WM cells with an important role in tumor cell growth and survival, independent of MYD88 signaling.
Materials and methods
Cells
The WM cell lines (WSU-WM, BCWM.1, and WMCL-1) and IgM-secreting low-grade lymphoma cell lines (MEC-1 and RL) were cultured in RPMI 1640 containing 10% fetal bovine serum (Sigma Chemicals), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (GIBCO). Bone marrow (BM) mononuclear cells and primary WM cells from BM aspirates from multiple myeloma patients following informed consent and Dana-Farber Cancer Institute’s Institutional Review Board approval were isolated using Ficoll-Hypaque density gradient sedimentation. Primary WM cells were obtained using CD19 micro-bead selection (Miltenyi Biotec) with more than 90% purity, as confirmed by flow cytometric analysis. This study was conducted in accordance with the Declaration of Helsinki.
siRNA
RNA interference was done by using the TranSilent Human Sp1 siRNA (Panomics Inc., Redwood City, CA). Nontargeting scrambled negative control siRNA (Panomics Inc.) was used as negative control. Briefly, MWCL1 and BCWM1 cells were transiently transfected with Sp1 siRNA with the use of Amaxa technology (KIT V, Program T-030).
Transfection of Sp1 plasmid
A plasmid encoding human Sp1, pCAGGS, was kindly provided by Dr Ferruccio Galbiati (University of Pittsburgh, PA). Transfection was performed with the use of Amaxa technology.
Chromatin immunoprecipitation assays
WM cells were left untreated or treated with TMP (10 μM) for 24 hours. Briefly, cells were cross-linked with 1% formaldehyde for 10 minutes at 37°C. The cross-linked chromatin was then extracted, diluted with lysis buffer, and sheared by sonication. The chromatin was divided into equal samples for immunoprecipitation with anti-Sp1, anti-IgG (negative control) polyclonal antibody (Millipore). The immunoprecipitates were pelleted by centrifugation and incubated at 65°C to reverse the protein-DNA cross-linking. The DNA was extracted from the elute by the QIAquick PCR Purification Kit (Qiagen). Purified DNA was subjected to polymerase chain reaction (PCR). The sequences of the PCR primers used were as follows: primers specific for a region (−264 to −31) in the survivin promoter spanning 7 putative Sp1 binding sites: sense, 5′-TTCTTTGAAAAGCAGTCGAGGGG-3′, antisense, 5′-CGCGATTCAAATCTGGCGGTTA-3′; the region from −272 to +18 bp of the vascular endothelial growth factor promoter was amplified using the following primers: sense, 5′-ccgcgggcgcgtgtctctgg-3′, antisense, 5′-tgccccaagcctccgcgatcctc-3′; SIRT1 promoter containing the putative Sp1 binding site: sense, 5′-GTGACCCGTAGTGTTGTGGTC-3′, antisense, 5′-CATCTTCCAACTGCCTCTCTG-3′; and negative control: sense, 5′-ATGGTTGCCACTGGGGATCT-3′, antisense, 5′-TGCCAAAGCCTAGGGGAAGA-3′. Dihydrofolate reductase primers were purchased from Millipore.
Quantitative reverse-transcription polymerase chain reaction analysis
Expression of human survivin transcripts was determined using real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) based on TaqMan fluorescence methodology, following manufacturer protocols (Applied Biosystems, Foster City, CA). Relative expression was calculated using the comparative δ δ (Ct) method.
Enzyme-linked immunosorbent assay (ELISA) for Sp1 and NF-κB transcription activity
Nuclear protein was extracted with a Nuclear Extraction Kit (Panomics Inc.) and quantified using the Bio-Rad Protein Assay Kit. A total of 15 µg of nuclear protein from each treatment were analyzed for Sp1 or NF-κB activity using the Transcription Factor ELISA Kit (Panomics Inc.), according to the manufacturer’s instructions.
Immunoblotting
Western blotting (WB) was performed to delineate expression levels of total protein (Sp1, survivin, caspase-3, poly ADP ribose polymerase (PARP), BTK, NF-κB, and signal transducer and activator of transcription (STAT3), and phospho-specific isoforms of NF-κB and STAT3. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control (Santa Cruz Biotechnology).
TF activity profiling assay
The activity of 48 TFs was analyzed using the TF Activation Profiling Plate Array I (Signosis Inc.) according to the manufacturer’s instructions. Six micrograms of nuclear protein extracts was assayed per sample. TF activities in WM cells were compared with the activities in normal B cells, and selected based on fold-change method (≤−1.5 or ≥1.5).
Reagents
Tumor cells were treated with and without TMP (Erimos Pharmaceuticals), and/or inhibitors of IRAK 1/4 kinase function (407601, EMDJ) and BTK (ibrutinib; Pharmacyclics Inc.). Drug interactions were assessed by CalcuSyn 2.0 software (Biosoft), which is based on the Chou-Talalay method. When combination index (CI) = 1, this equation represents the conservation isobologram and indicates additive effects. CI <1 indicates synergism; CI >1 indicates antagonism.
Cell proliferation, viability, and apoptosis assay
WM cell proliferation was measured by [3H]-thymidine (PerkinElmer, Boston, MA) incorporation assay and bromodeoxyuridine staining, as previously described.10 Cell viability was analyzed by CellTiter-Glo (CTG) (Promega). Study of caspase activity was performed using Caspase-Glo 3/7 Assay (Promega). Apoptosis was evaluated by flow cytometric analysis following Annexin-V and propidium iodide staining.
In vivo study
The in vivo efficacy of TMP was tested in a murine xenograft model using BCWM1 cell line injected subcutaneously (s.c.) in severe combined immunodeficiency (SCID) mice. Following detection of tumor, mice were treated with either vehicle or TMP (50 mg/kg) s.c. for 5 consecutive days/week for 2 weeks. Tumor growth was measured as previously described.10
Statistical analysis
The statistical significance of differences was analyzed using the Student t test; differences were considered significant when P ≤ .05.
Results
Modulation of Sp1 activity affects cell growth and viability in WM
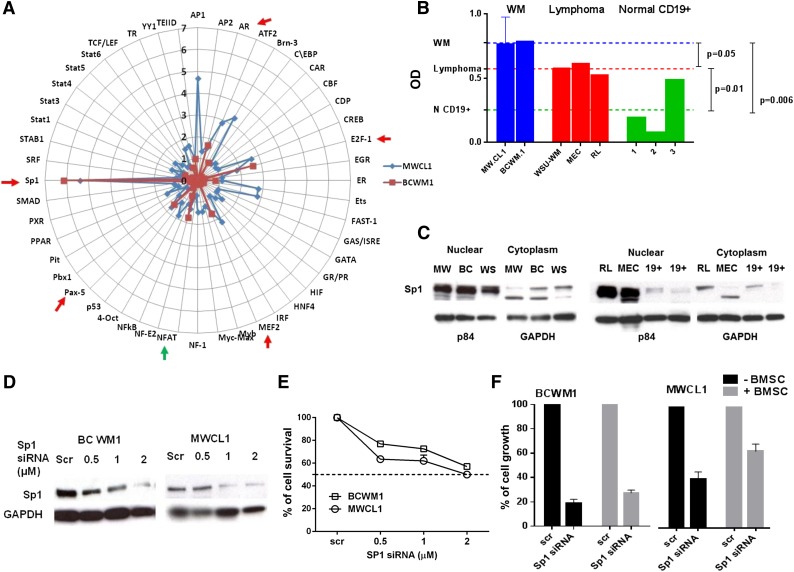
We screened DNA-binding activities of 48 TFs using a TF activation-profiling array (Signosis, Sunnyvale, CA) in order to identify TFs specifically activated in WM cells as compared with normal cells. As shown in Figure 1A, AR, E2F1, MEF2, Pax-5, and Sp1 DNA-binding activities were at least 1.5-fold higher in both MWCL1 and BCWM1 cells compared with normal CD19+ cells from healthy donors (N = 2), with Sp1 being the most significantly activated TF (5.78 ± 0.37-fold increase) in both WM cell lines. This finding, along with our previous observation of a deregulated Sp1 activity in myeloma,10 prompted us to further investigate its role in WM. We observed that WM and low-grade lymphoma cell lines exhibit significantly increased levels of Sp1 DNA-binding activity compared with peripheral blood derived CD19+ cells from normal donors (N = 3) using an ELISA-based assay specific for Sp1 (Figure 1B). We also confirmed that the enhanced Sp1 activity is associated with high nuclear levels of Sp1 protein in tumor cells compared with normal cells by WB analysis (Figure 1C).
Figure 1.
Sp1 DNA-binding activity is high in WM cells and affects tumor growth and viability. (A) Nuclear extracts from MWCL1, BCWM1, and normal CD19+ cells were analyzed using TF activation ELISA array. TF activation profile differences in cancer and normal cells were quantitatively analyzed and compared. TFIID was used as loading control. The radar chart displays fold change values (from 0 to 7) in MWCL1 and BCWM1 cells relative to CD19+ normal cells. (B) Nuclear extracts from WM and low-grade lymphoma cell lines were analyzed for Sp1 activity using the Sp1 TF ELISA kit, which measures Sp1 DNA binding activity. To analyze the specificity of the DNA-binding complexes, cold competition was performed (data not shown). Absorbance was obtained with a spectrophotometer at 450 nm and presented as optical density. Blue, red, and green lines show mean Sp1 binding activity of WM, low-grade lymphoma, and normal CD19+ cells, respectively. (C) Equal amounts of nuclear and cytoplasmic protein extracts from MWCL1 (MW), BCWM.1 (BC), WSU-WM (WS), RL, MEC, and CD19+ normal cells from 2 healthy donors were subjected to WB analysis using anti-Sp1 ab. p84, and GAPDH were used as loading controls for nuclear and cytoplasmic fractions, respectively. (D) BCWM1 and MWCL1 cell lines were transfected using different concentrations of TranSilent Human Sp1 siRNA or scrambled siRNA (Scr). Cell lysates were obtained 48 hours after transfection and subjected to WB analysis to assess decrease in the Sp1 protein expression posttransfection using anti-Sp1 and GAPDH abs. WB analyses confirmed reduction in Sp1 protein level following transient transfection of WM cells with Sp1 siRNA compared with cells transfected with control Scr. (E) The effect of Sp1 knockdown on cell survival in WM cells transfected with Sp1 or control siRNA were assessed by CellTiter Glo assay and presented as change relative to control cells. (F) WM cell lines transfected with 2 µM of Sp1 siRNA or control siRNA were cultured in the absence or presence of BMSC for 24 hours. Tumor cell growth was evaluated by [3H]thymidine uptake and presented as a percentage of cell growth compared with Scr.
To further evaluate the potential oncogenic role of Sp1 in WM, we examined the effect of Sp1 inhibition in two WM cell lines, BCWM1 and MWCL1. These cells were transfected with Sp1 siRNA, and the gene silencing was confirmed at gene expression and protein level by real time PCR (data not shown) and WB (Figure 1D), respectively, after 2 days of transfection. Sp1 knockdown in WM cells led to decreased WM cell viability, as evaluated by CTG (Figure 1E). We have also evaluated the antiproliferative effect of Sp1 silencing in the absence and presence of primary bone marrow stromal cells (BMSCs) isolated from WM patients (Figure 1F). Conversely, overexpression of Sp1 promoted cell growth and increased IgM production in the BCWM1 cell line (data not shown). These results demonstrate the role of Sp1 in WM cell growth and survival, and provide a rationale to therapeutically target Sp1 in WM, using small molecule inhibitors of Sp1.
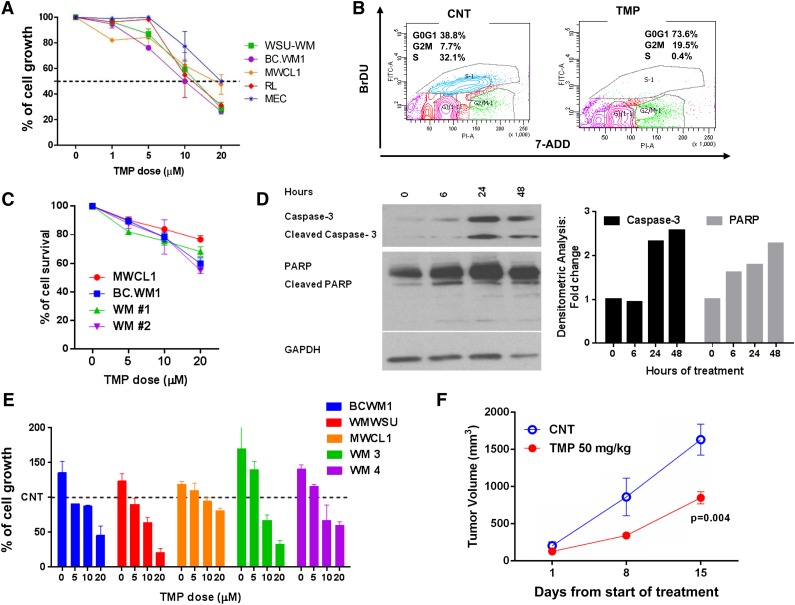
We, therefore, evaluated the antitumor activity of TMP, a previously reported Sp1 inhibitor in WM. TMP exposure led to inhibition of DNA synthesis in WM and IgM-secreting low-grade lymphoma cell lines in a dose- (Figure 2A) and time-dependent (data not shown) fashion, and G1 cell-cycle arrest with concomitant reduction of cells in S phase (Figure 2B). Moreover, we observed inhibition of cell survival in both WM cell lines, as well as purified primary cells from 2 WM patients after 24 hours of treatment with TMP (Figure 2C). A time-dependent induction of PARP and caspase-3 cleavage was also observed upon TMP treatment (Figure 2D). Given the growth promoting effect of interaction between WM cells and BMSCs, we evaluated the antiproliferative effect of TMP in the context of the BM milieu. Treatment with TMP suppressed WM-BMSC interaction-mediated growth of WM cell lines, as well as purified primary cells from 2 WM patients (Figure 2E), whereas it had no effect on the viability of BMSCs (data not shown). Finally, we have evaluated the effect of TMP in vivo in an s.c. xenograft mouse model in which BCWM1 cells were injected s.c. in SCID mice. After development of the tumor (approximately 3 weeks from cell injection), mice were treated either with placebo or TMP for 5 consecutive days/week. As shown in Figure 2F, a reduction in the tumor growth was observed in mice treated with TMP as compared with control mice. The reduced tumor volume observed in treated mice may be a reflection of the effects of TMP on both proliferation, as well as survival.
Figure 2.
Pharmacologic inhibition of Sp1 by TMP decreases WM cell growth. (A) WM and lymphoma cell lines were treated with various concentrations of TMP (1 to 20 µM) for 48 hours, and cell growth was assessed by [3H]thymidine uptake. Data are presented as a percentage of untreated cell proliferation. (B) Flow cytometric analysis of bromodeoxyuridine (BrDU) incorporation was performed after treatment of BCWM1 cells with the inhibitor for 24 hours. Data shown are percentage of cells in the different phases of the cell cycle. (C) BCWM.1, MWCL1, and primary CD19+ WM cells (WM1 and WM2) were cultured with different concentrations of TMP for 24 hours. Cell survival was assessed by CTG, and presented as a percentage of growth of vehicle-treated cells. (D) BCWM.1 cells were left untreated or treated with 10 µM of TMP for different time points. Cells were then subjected to WB analysis using PARP and caspase-3 abs. GAPDH ab was used as loading control for WB analysis (right). Densitometric quantitation of band intensity was performed using ImageJ software. Data were normalized to GAPDH. Fold change compared with time = 0 is shown on graph (left). (E) BCWM.1, WSU-WM, MWCL1, and primary CD19+ WM cells (WM3 and WM4) were cultured in the absence (-) or presence (+) of BMSC from WM patients at different concentrations of TMP for 48 hours. Cell proliferation was assessed by [3H]thymidine uptake, and presented as percentage of growth of vehicle-treated cells cultured in the absence of BMSC (100% = control). (F) BCWM1 cells were injected s.c. in SCID mice. Treatment started following detection of tumor (approximately 3 weeks from cell injection). Mice were treated either with 50 mg/kg of TMP or placebo s.c. daily for 2 weeks. Tumors were measured in two perpendicular dimensions once every week.
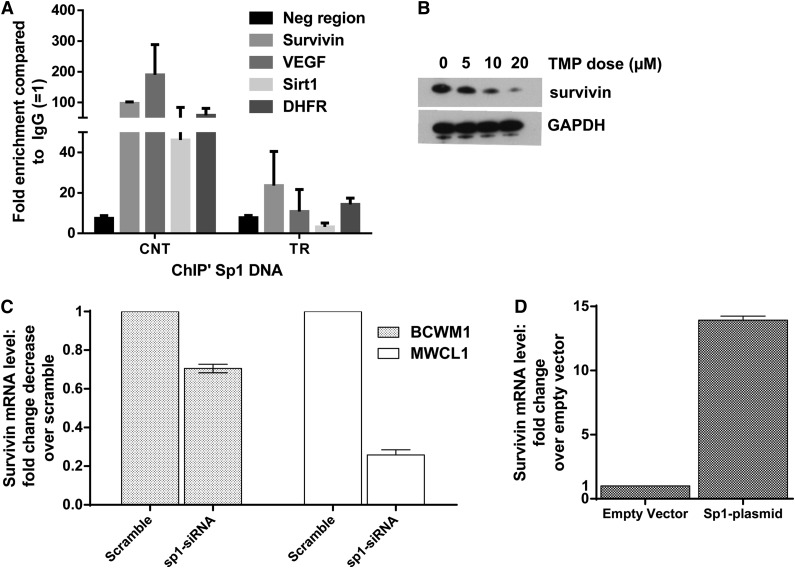
We further confirmed inhibition of Sp1 binding by TMP to known Sp1 responsive promoters by chromatin immunoprecipitation assay and reduction of survivin at protein levels by WB in cells treated with TMP (Figure 3A-B). We also evaluated and confirmed reduced messenger RNA levels of survivin in WM cell lines after Sp1 knockdown (Figure 3C). Conversely, overexpression of Sp1 increased the survivin level (Figure 3D), confirming that survivin is an Sp1-transcriptionally regulated gene in WM cells.
Figure 3.
Survivin is transcriptionally regulated by Sp1 in WM cells. (A) BCWM.1 cells were treated with 10 µM of TMP for 24 hours, and purified DNA from chromatin immunoprecipitated with anti-Sp1 and anti-IgG polyclonal antibody was subjected to PCR using primers specific for selected gene promoter regions. The y-axis represents average enrichment over IgG from at least 2 independent experiments normalized to input. (B) BCWM.1 cells with or without TMP treatment at stated concentrations for 24 hours. Cells were then subjected to WB analysis using anti-survivin and anti-GAPDH abs. (C) Survivin expression in BCWM1 and MWCL1 cells transfected with either Sp1-specific siRNA or Scr were analyzed by qRT-PCR. Data were first normalized to GAPDH and then to the signal from control cells. Data are presented as mean of fold change compared with control ± SEM of 3 biological replicates. (D) A plasmid encoding human Sp1, pCAGGS, was transfected in BCWM1 cells using nucleofection. BCWM.1 cells overexpressing Sp1 were evaluated for the expression of survivin by qRT-PCR 2 days after transfection.
Inhibition of Sp1 activity impairs NF-κB and STAT3 signaling pathways in WM cells
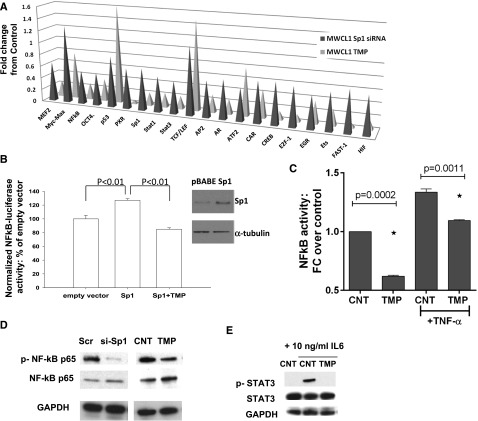
Since Sp1 interacts with other TFs influencing their activity, we analyzed activities of various TFs in nuclear extracts from MWCL1 cells following Sp1 knockdown or following TMP treatment using the TF activity array. We observed decreased activity of 17 TFs (out of 47 studied) following Sp1 knockdown and/or TMP treatment, including STAT1, STAT3, and NF-κB; whereas activity of other TFs such as p53, TCF/LEF, and Myc-Max, were not affected (Figure 4A). Enforced expression of Sp1 significantly induced NF-κB p65 (RelA) luciferase activity, and TMP was able to overcome this effect (Figure 4B). Moreover, we observed inhibition of basal and TNFα-induced NF-κB p65 DNA binding activity in nuclear extracts from TMP-treated cells (Figure 4C). Finally, immunoblotting analysis showed inhibition of NF-κB p65 phosphorylation after both siRNA-mediated and pharmacologic inhibition of Sp1 (Figure 4D). Taken together, these data demonstrate that both Sp1 siRNA and TMP significantly suppress basal and TNFα-stimulated NF-κB transcriptional activity in WM cells. Similarly, we observed inhibition of IL-6–induced STAT3 phosphorylation by TMP (Figure 4E). NF-κB and janus kinase-STAT3 signaling mediate the production of IL-6, which have a well-established role in the maintenance of many hematologic malignancies.11 We have confirmed reduced IL-6 expression levels by TMP- and siRNA-mediated Sp1 knockdown in BCWM1 and MWCL1 WM cell lines by qRT-PCR (data not shown).
Figure 4.
Inhibition of Sp1 activity impacts NF-κB and STAT3 pathways in WM cells. (A) Nuclear extracts from Sp1 knockdown or TMP-treated MWCL1 cells were analyzed for TF activation using a TF profiling array. Relative fold changes from corresponding control are plotted. (B) BCMW1 cells were electroporated with control or Sp1 expression vector, NF-κB luciferase reporter plasmid, and pRL-TK to normalize for different transfection efficiencies; following electroporation, cells were treated with vehicle or 10 µM TMP, and 24 hours later luminescence was measured using the Dual-Luciferase assay kit and the GloMax microplate luminometer. Results are expressed as a percentage of Firefly/Renilla ratio of control-transfected cells. Whole lysates from these transfected cells were immunoblotted using anti-Sp1 and anti-α-tubulin (Santa Cruz Biotechnology) antibodies. (C) BCWM.1 cells were cultured with TMP (10 µM) for 24 hours, and then TNF-α was added for the last 20 minutes. NF-κB p65 TF binding to its consensus sequence on the plate-bound oligonucleotide was analyzed in nuclear extracts. The results represent means ± SD of triplicate experiments. (D) Whole cell lysates from Scr, or Sp1-specific siRNA- or control, or TMP-treated BCWM.1 cells were subjected to WB using anti–p-NF-κB p65, -NF-κB p65, and -GAPDH antibodies. (E) BCWM1 cells were treated with IL-6 with and without TMP and assessed by WB analysis using anti–p-STAT3, -STAT3, and -GAPDH antibodies.
Sp1 inhibition decreases BTK and induces synergistic growth inhibitory effects with inhibitors of MYD88 pathways in WM
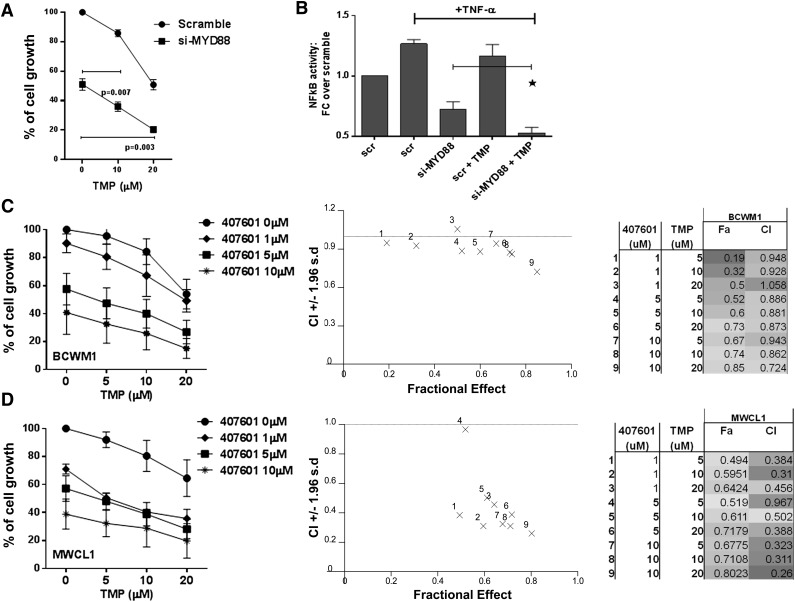
MYD88 L265P mutation has been reported in more than 90% of tumor samples from patients with WM or non-IgM lymphoplasmacytic lymphoma. MYD88 is an adaptor molecule in toll-like receptor and interleukin-1 receptor signaling. Inhibition of MYD88 signaling decreased NF-κB activity and survival of WM cell lines expressing MYD88 L265P, and activated B-cell–type diffuse large-cell lymphoma. We evaluated the interaction between Sp1 and MYD88 pathways in WM. For these studies, we have used BCWM1 and MWCL1 cell lines which expressed the MYD88 L265P mutation as a heterozygous variant, as reported in previous reports.1,2,12,13 We first investigated the impact of MYD88 on the sensitivity of WM cells to Sp1 inhibition, analyzing the effect of TMP on MYD88-silenced cells. MYD88 knockdown significantly inhibits BCWM1 cell growth compared with scrambled cells, and the antitumor effect was more pronounced upon treatment with TMP (Figure 5A). Increased tumor cell killing in response to dual Sp1 and MYD88 inhibition was associated with more robust inhibition of basal and induced NF-κB binding (Figure 5B). Both MYD88 L265P expressing BCWM1 and MWCL1 WM cells were then cultured in the absence or presence of the IRAK 1/4 kinase inhibitor 407601, for 24 hours. The combination treatment drugs markedly reduced WM cell growth (Figure 5C-D). We also observed a significant inhibition of cell survival after 48 hours of treatment with combination therapy (data not shown).
Figure 5.
Dual inhibition of MYD88 and Sp1 activity leads to synergistic killing in WM cells, and suppression of NF-κB activity. (A) BCWM.1 cells were transfected with either Scr or MYD88-specific siRNA; 24 hours after transfection, BCWM1 cells were treated with either vehicle or TMP and cultured for an additional 24 hours. Cell proliferation was assessed by thymidine uptake and presented as a percentage of control. (B) BCWM.1 cells were transfected with either Scr or MYD88-specific siRNA; 48 hours after transfection, WM cells were treated with either vehicle or TMP and cultured for an additional 4 hours. TNF-α was added for the last 20 minutes. NF-κB p65 TF binding to its consensus sequence on the plate-bound oligonucleotide was analyzed in nuclear extracts. The results represent means ± SD of triplicate experiments. (C-D) BCWM1 and MWCL1 cells were cultured in the absence or presence of IRAK 1/4 inhibitor peptide 407601 and TMP for 24 hours. Cell growth was assessed by [3H]thymidine uptake assay and presented as a percentage of control cells (untreated cells). Data are mean ± SD (n = 3). CIs and fractions affected were generated with CalcuSyn software for each set of combination. CI <1, CI values = 1, and CI values >1 indicate synergism, additive effect, and antagonism, respectively.
These results prompted us to investigate the nature of the interaction between Sp1 and MYD88 pathways. We have first observed that MYD88 knockdown failed to modulate Sp1 expression and/or activity (data not shown). In addition, no changes in the expression of MYD88 or its downstream molecular intermediates IRAK 1/4 after genetic and pharmacologic inhibition of Sp1 in WM cells were observed (data not shown).
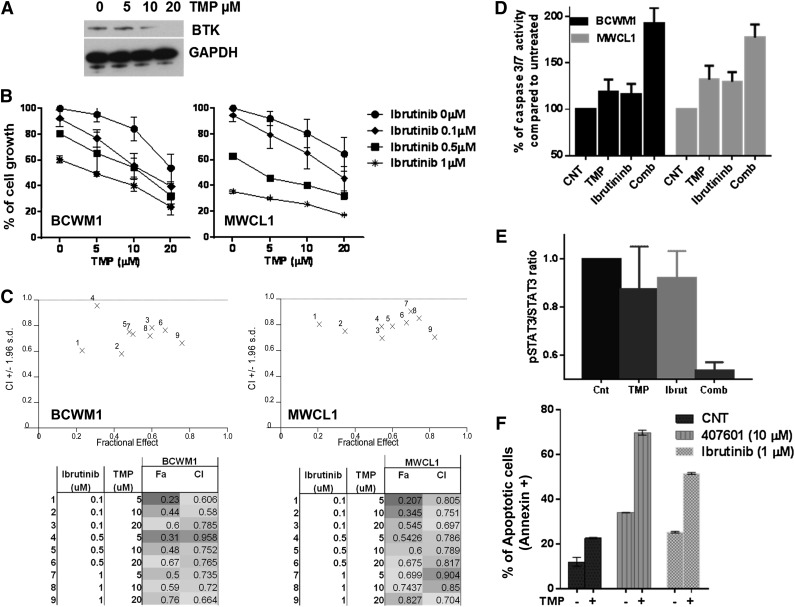
Recent studies have shown that BTK binds to, and is activated in response to MYD88 L265P mutation in WM cells.2 BTK is an important downstream adapter for B-cell–receptor signaling that is constitutively active in MWCL1 and BCWM1 cells. Recent clinical data suggest remarkable activity of ibrutinib, the first-in-class covalent inhibitor of BTK, in WM.14 Interestingly, Sp1 has been identified as one of the major TF regulators of BTK expression by binding and activating its promoter.15,16 We observed that treatment with TMP dramatically reduced BTK at messenger RNA (data not shown) and protein levels in WM cells in a time- and dose-dependent way (Figure 6A). Based on these results showing that Sp1 regulates BTK expression in WM cells, we next examined the impact of dual inhibition of Sp1 with TMP and BTK with the inhibitor ibrutinib on WM cell growth. BCWM1 and MWCL1 cells were simultaneously treated with different concentrations of both drugs for 24 hours. The combination treatment resulted in significant time- (data not shown) and dose-dependent inhibition of cell growth evaluated by thymidine uptake (Figure 6B), as well as inhibition of cell survival evaluated by CTG (data not shown). The Chou and Talalay analysis confirmed synergistic anti-WM activity of TMP plus ibrutinib, with a CI <1.0 with all tested doses (Figure 6C-D). Moreover, a significant activation of caspase 3/7 was observed after treatment with combined versus single-agent therapy (Figure 6E), as well as a more pronounced inhibition of p-STAT3 activity (Figure 6F).
Figure 6.
TMP decreases BTK expression and enhances growth inhibitory effect of ibrutinib against WM cells. (A) BCWM1 cells with or without TMP treatment at various concentrations for 24 hours were subjected to WB analysis using anti-BTK and GAPDH. (B) BCWM1 (left) and MWCL1 cells (right) were cultured in the presence of different doses of ibrutinib and TMP alone, or in combination for 24 hours. Cell growth was assessed by [3H]thymidine uptake and presented as a percentage of control cells (untreated cells). Data are means ± SD (n = 3). (C) CIs and fractions affected are shown in the graphs (upper panel) and in the tables (lower panel) for BCWM1 (left) and MWCL1 (right) cell lines. (D) Caspase 3/7 activity was evaluated in BCWM1 and MWCL1 after single and combination drug treatment using Caspase-Glo 3/7 assay. (E) Total and phosphorylated STAT3 levels in cell lysates from cells treated with or without TMP and ibrutinib alone or in combination were evaluated with STAT3 (Total/Phospho) InstantOne ELISA. (F) BCWM1 cells were treated with TMP (10 µM), 407601 peptide (10 µM), ibrutinib (1 µM), or combined therapy for 48 hours, followed by Annexin V/propidium iodide staining and flow cytometry analysis.
We next evaluated whether these combinations could lead to either additive or synergistic induction of apoptosis on WM cells. BCWM.1 cells were cultured with TMP (10 µM) for 24 hours, in the presence or absence of ibrutinib and/or IRAK 1/4 kinase inhibitor; 22% of BCWM.1 cells was in early apoptosis after treatment with TMP, which was increased to 52% and 69% in the presence of 1 µM ibrutininb, or 10 µM 407601, respectively, indicating synergistic effect (Figure 6G). Finally, we observed that neither the single agent nor the combination triggered death of healthy donor peripheral blood mononuclear cells, suggesting a favorable therapeutic index (data not shown).
Discussion
Recent studies have reported a high frequency of the MYD88 L265P somatic mutation in patients with WM. The mutation confers a survival advantage in WM cells, providing the rationale for the use of inhibitors targeting MYD88 and/or its downstream pathways for the treatment of WM. However, additional MYD88-independent pathways may drive the disease. Deregulation of Sp1 has been observed in many cancers and diseases, including myeloma.17 Our study demonstrates Sp1 as being one of the most activated TFs in WM cells when compared with normal B cells. By gain of and loss of Sp1 function studies, we demonstrate that Sp1 signaling was supportive of WM growth and survival. Moreover, targeting Sp1 activity with the small molecule TMP, significantly inhibits cell proliferation and induces cytotoxicity in a dose- and time-dependent manner in WM cells, suggesting that specific inhibition of Sp1 activity may be an interesting potential therapeutic. TMP is a semi-synthetic small molecule that disrupts the interaction between Sp1 and guanine-cytosine-rich motifs, thereby inhibiting Sp1 transcriptional activity.8,18-20 We have observed the inhibition of Sp1 binding to known Sp1-responsive promoters by TMP using chromatin immunoprecipitation assay, and decreased expression levels of Sp1-regulated proteins, confirming that the antitumor activity of TMP in WM occurs via selective targeting of Sp1 activity.
Moreover, the effect of Sp1 inhibition on WM cell growth and survival was partly achieved through interference with NF-κB and STAT3 signaling pathways. These functional data establish a new oncogenic pathway in WM: Sp1 activity promoting NF-κB and janus kinase-STAT3 signaling, which mediate cell growth and survival. Both NF-κB and STAT3 are downstream targets of MYD88 and key pathways involved in the pathophysiology of WM, raising the possibility that MYD88 and Sp1 pathways are interconnected in WM cells. MYD88 knockdown significantly increased the sensitivity of WM cells to Sp1 inhibition, without affecting its expression and/or activity. These results provided the rationale to investigate the activity of combination treatment with TMP and known inhibitors of the MYD88 pathway signaling.
Yang et al have recently established that BTK, a critical component in the signalosome that regulates B-cell proliferation and differentiation, as a downstream target of MYD88 L265P signaling in WM cells.2 Therefore, inhibition of BTK with a selective inhibitor may have indirect effects on MYD88 L265P-related activation in WM. Importantly, BTK is transcriptionally regulated by Sp1,21 and in this study, we confirm this interaction by demonstrating the dramatic reduction in BTK levels in WM cells with TMP treatment. Ibrutinib is a covalent, irreversible, and highly selective BTK inhibitor,22 which is already showing promising clinical activity in WM and other B-cell malignancies.14 The combined use of Sp1 and BTK inhibitors resulted in significant and synergistic dose-dependent decrease of growth and survival in WM cells. We also report that MYD88 L265P and Sp1 independently mediate the activation of NF-κB and STAT3. WM patients may therefore, benefit from therapies targeting Sp1 alone or in combination with agents targeting the MYD88 pathway or its downstream targets. Sp1 inhibition could also be a good combination therapy with the MYD88 inhibitor in activated B-cell–like, diffuse large B-cell lymphoma with L265P mutation23 and in multiple myeloma in combination with BTK inhibitors. However, further detailed analysis of single agent and combination in vitro and in vivo in animal models is required as preclinical studies in order to plan future clinical trials.
In conclusion, the results obtained in this study demonstrate Sp1 as an important TF in WM and provide a rationale to therapeutically target Sp1 in WM using small molecule inhibitors of Sp1 expression and activity. TMP provides an important possibility for therapeutic targeting of Sp1; its safety has been established in several clinical trials,24,25 indicating that TMP could be a useful therapeutic for treating WM. Importantly, our data indicate the need for further detailed investigations that may eventually lead to combination clinical trials in WM.
Acknowledgments
This work was supported in part by grants from the Veterans Administration (I01-BX001584) and National Institutes of Health (RO1-124929) (N.C.M.), and grants from the National Institutes of Health (P50-100007, PO1-78378, and PO1-155258) (N.C.M. and K.C.A.), and (RO1-50947) (K.C.A.). K.C.A. is an American Cancer Society Professor in Oncology.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.F. and N.C.M. designed research; M.F., N.A., R.L., and M.M. performed research; G.Y., L.X., Z.H., and P.T. contributed reagents/analytic tools; M.F., K.C.A., S.P.T., and N.C.M. analyzed data; and M.F. and N.C.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nikhil C. Munshi, Dana-Farber Cancer Institute, 440 Brookline Ave, M230, Boston, MA 02115; e-mail: nikhil_munshi@dfci.harvard.edu.
References
- 1.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367(9):826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 2.Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood. 2013;122(7):1222–1232. doi: 10.1182/blood-2012-12-475111. [DOI] [PubMed] [Google Scholar]
- 3.Vijay A, Gertz MA. Waldenström macroglobulinemia. Blood. 2007;109(12):5096–5103. doi: 10.1182/blood-2006-11-055012. [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Wang L, Wei D, et al. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004;10(12, pt 1):4109–4117. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 5.Shi Q, Le X, Abbruzzese JL, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61(10):4143–4154. [PubMed] [Google Scholar]
- 6.Kumar AP, Butler AP. Enhanced Sp1 DNA-binding activity in murine keratinocyte cell lines and epidermal tumors. Cancer Lett. 1999;137(2):159–165. doi: 10.1016/s0304-3835(98)00351-6. [DOI] [PubMed] [Google Scholar]
- 7.Park R, Chang CC, Liang YC, et al. Systemic treatment with tetra-O-methyl nordihydroguaiaretic acid suppresses the growth of human xenograft tumors. Clin Cancer Res. 2005;11(12):4601–4609. doi: 10.1158/1078-0432.CCR-04-2188. [DOI] [PubMed] [Google Scholar]
- 8.Castro-Gamero AM, Borges KS, Moreno DA, et al. Tetra-O-methyl nordihydroguaiaretic acid, an inhibitor of Sp1-mediated survivin transcription, induces apoptosis and acts synergistically with chemo-radiotherapy in glioblastoma cells. Invest New Drugs. 2013;31(4):858–870. doi: 10.1007/s10637-012-9917-4. [DOI] [PubMed] [Google Scholar]
- 9.Heller JD, Kuo J, Wu TC, Kast WM, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces G2 arrest in mammalian cells and exhibits tumoricidal activity in vivo. Cancer Res. 2001;61(14):5499–5504. [PubMed] [Google Scholar]
- 10.Fulciniti M, Amin S, Nanjappa P, et al. Significant biological role of sp1 transactivation in multiple myeloma. Clin Cancer Res. 2011;17(20):6500–6509. doi: 10.1158/1078-0432.CCR-11-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23(1):10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Hunter ZR, Yang G, et al. MYD88 L265P in Waldenström macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction [published correction appears in Blood. 2013;121(26):5259]. Blood. 2013;121(11):2051–2058. doi: 10.1182/blood-2012-09-454355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulain S, Roumier C, Decambron A, et al. MYD88 L265P mutation in Waldenstrom macroglobulinemia. Blood. 2013;121(22):4504–4511. doi: 10.1182/blood-2012-06-436329. [DOI] [PubMed] [Google Scholar]
- 14.Brown JR. Ibrutinib in chronic lymphocytic leukemia and B cell malignancies. Leuk Lymphoma. 2014;55(2):263–269. doi: 10.3109/10428194.2013.803226. [DOI] [PubMed] [Google Scholar]
- 15.Müller S, Sideras P, Smith CI, Xanthopoulos KG. Cell specific expression of human Bruton’s agammaglobulinemia tyrosine kinase gene (Btk) is regulated by Sp1- and Spi-1/PU.1-family members. Oncogene. 1996;13(9):1955–1964. [PubMed] [Google Scholar]
- 16.Müller S, Maas A, Islam TC, et al. Synergistic activation of the human Btk promoter by transcription factors Sp1/3 and PU.1. Biochem Biophys Res Commun. 1999;259(2):364–369. doi: 10.1006/bbrc.1999.0677. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wei D, Huang S, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9(17):6371–6380. [PubMed] [Google Scholar]
- 18.Chen H, Teng L, Li JN, et al. Antiviral activities of methylated nordihydroguaiaretic acids. 2. Targeting herpes simplex virus replication by the mutation insensitive transcription inhibitor tetra-O-methyl-NDGA. J Med Chem. 1998;41(16):3001–3007. doi: 10.1021/jm980182w. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Heller JD, Kuo J, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc Natl Acad Sci U S A. 2004;101(36):13239–13244. doi: 10.1073/pnas.0405407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Giacalone NJ, Lu B. Terameprocol (tetra-O-methyl nordihydroguaiaretic acid), an inhibitor of Sp1-mediated survivin transcription, induces radiosensitization in non-small cell lung carcinoma. J Thorac Oncol. 2011;6(1):8–14. doi: 10.1097/JTO.0b013e3181fa646a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himmelmann A, Thevenin C, Harrison K, Kehrl JH. Analysis of the Bruton’s tyrosine kinase gene promoter reveals critical PU.1 and SP1 sites. Blood. 1996;87(3):1036–1044. [PubMed] [Google Scholar]
- 22.Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2(1):58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 23.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez RA, Goodman AB, Rhodes M, Blomberg JA, Heller J. The anticancer activity of the transcription inhibitor terameprocol (meso-tetra-O-methyl nordihydroguaiaretic acid) formulated for systemic administration. Anticancer Drugs. 2007;18(8):933–939. doi: 10.1097/CAD.0b013e32813148e0. [DOI] [PubMed] [Google Scholar]
- 25.Tibes R, McDonagh KT, Lekakis L, et al. Phase I study of the novel survivin and cdc2/CDK1 inhibitor terameprocol in patients with advanced leukemias [abstract]. Blood. 2009;114 doi: 10.1007/s10637-014-0198-y. Abstract 1039. [DOI] [PubMed] [Google Scholar]