Key Points
Vimentin expressed on the platelet surface serves as adhesive receptor for VWF.
Abstract
The interaction between platelet receptor glycoprotein Ibα and the A1 domain of von Willebrand factor (VWF) mediates tethering/translocation of platelets to sites of vascular injury. Unexpectedly, we observed platelets translocating over A1A2A3 domains protein slower than on A1 domain at high shear stress. This observation suggests an additional interaction between A domains and an adhesive receptor. We investigated vimentin because we have data showing the interaction of vimentin with the A2 domain of VWF. Moreover, vimentin is expressed on the platelet surface. This novel interaction was analyzed by using purified VWF, recombinant proteins, anti-vimentin antibodies, parallel flow chamber adhesion assays, flow cytometry, and vimentin-deficient murine platelets. The active form of VWF bound to vimentin, and the purified A2 domain blocked that binding. The interaction of a gain-of-function A1A2A3 mutant with platelet was reduced using anti-vimentin antibody. Platelet adhesion to wild-type (WT) A1A2A3 protein, collagen, and fibrin(ogen) was inhibited (32-75%) by anti-vimentin antibody under high shear stress. Compared with WT mice, platelets from vimentin-deficient mice had a reduced flow-dependent adhesion to both collagen and purified murine VWF. Last, the vimentin knockout mice had a prolonged tail bleeding time. The results describe that platelet vimentin engages VWF during platelet adhesion under high shear stress.
Introduction
Atherothrombotic events, including acute coronary syndrome and stroke, are the result of platelet adhesion and activation on the ruptured atherosclerotic plaques. This platelet-mediated arterial thrombosis starts with the contact of the rapidly flowing platelets to components of the damaged blood vessel. von Willebrand factor (VWF), a multimeric plasma and subendothelial glycoprotein, is relevant in mediating platelet adhesion and activation at sites of lesions in the coronary arteries, where high shear conditions prevail.1,2 VWF captures the circulating platelets through its interaction with the platelet receptor glycoprotein (GP)Ib/IX/V complex. This interaction is responsible for the tethering, rolling, and activation of platelets that eventually become firmly adhered, leading to thrombus formation within a coronary artery.3,4
Mature VWF consists of a 2050-residue subunit formed by domains arranged in the order of D′-D3-A1-A2-A3-D4-B-C.5 The A1 domain contains the binding site for the platelet receptor GPIbα6; the cleavage site for the enzyme ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)-13 is localized in the A2 domain,7 and the A3 domain binds to collagen.8 Unlike the A3 domain, both the A1 and A2 domains do not have access to their ligands until their domain structure is altered.9 This structural modification can be induced by mutations,10 hydrodynamic forces,11 immobilization on a surface, or artificially with the modulator ristocetin.12
VWF mediates platelet adhesion through its interaction with 2 platelet receptors: GPIbα and GPIIb/IIIa.4,13,14 For this reason, 1 way to analyze the interaction of platelet GPIbα with VWF, independently of GPIIb/IIIa, has been by using the isolated A1 domain of VWF under hydrodynamic conditions.15-18 Importantly, the binding of GPIbα to the A1 domain decelerates the fast flowing platelets at high shear stress. We performed comparative analysis between proteins comprised of the single A1 domain and the A1A2A3 domains to examine the effect of the neighboring A2A3 domains on the binding of A1 domain to platelet GPIbα. The use of the triple A domain protein, which functions similar to full-length VWF and lacks the binding site for GPIIb/IIIa,9,19 has been beneficial in shedding new understanding on VWF-mediated flow dependent platelet adhesion.
Now, we are proposing that vimentin is a novel binding protein for VWF on platelets. Emerging studies indicate that this cytoskeletal protein can be found on the surface of different cell types, including platelets.20-25 This study reports that vimentin expressed on platelets may function as a receptor for VWF, and this interaction apparently works in concert with the classical GPIbα-VWF binding in mediating platelet adhesion at high shear stress.
Methods
Reagents and antibodies
The monoclonal antibody against human vimentin, V9, was obtained from Invitrogen. The polyclonal sheep anti-human vimentin and sheep IgG were obtained from Affinity Biologicals (Ancaster, ON, Canada). The polyclonal sheep anti-human vimentin-horseradish peroxidase (HRP) conjugate was purchased from Thermo Scientific. Mouse IgG was purchased from Pierce, whereas the human fibrinogen was obtained from Calbiochem (Gibbstown, NJ). Monoclonal antibody VP-1,26 against the human VWF-A2 domain, was a gift from Dr Z. M. Ruggeri (Scripps Research Institute, La Jolla, CA). The rabbit polyclonal anti-human VWF antibody was obtained from Dako (Carpinteria, CA), and the goat anti-rabbit FITC antibody was from Abcam. Purified recombinant human vimentin was obtained from R&D Systems. Collagen fibril (equine) was purchased from Helena Laboratories (Beaumont, TX), whereas human placenta collagen type III was purchased from Sigma-Aldrich. The recombinant A1A2A3 variants and the A1 domain (amino acids 1238-1480) were expressed in mammalian (HEK293) cells, purified from the conditioned medium, and subjected to gel electrophoresis and gel filtration chromatography to verify the purity and monomeric state as we previously described.9 Recombinant VWF A1, A2, and A3 were expressed in bacteria and purified as we described.8,27 Purified human and murine VWF was obtained from fresh plasma using a size exclusion column chromatography as we previously described.8 The fractions corresponding to the eluted VWF was further concentrated using Aquacide (Calbiochem) and dialyzed against Tris-Cl and NaCl, pH 7.4 buffer.
Binding assays
The analyses of the interaction of purified plasma VWF, A1A2A3 variants, and A2 protein with vimentin were performed as we described.27 Briefly, the microtiter wells were coated with vimentin (supplemental Figure 4, available on the Blood Web site) (96 nmol/L) in 50 mM carbonate buffer, pH 9.5, and incubated overnight at 4°C. After blocking with bovine serum albumin (BSA), the wells were incubated with increasing concentrations of either purified plasma VWF (without or with ristocetin, 0.24 mmol/L) or A2 protein for 1 hour. To analyze the binding of the A1A2A3 variants to vimentin, a fixed concentration of each protein (250 nmol/L) added to the wells was coated with vimentin and incubated for 1 hour. VWF and A1A2A3 proteins bound to vimentin were detected by enzyme-linked immunosorbent assay (ELISA) with rabbit polyclonal anti-VWF-HRP conjugate (Dako), whereas the antibody VP-1 was used for the A2 protein, followed by a secondary goat anti-mouse IgG-HRP conjugate (Pierce).
For competition assays, microtiter wells containing immobilized VWF (0.5-1.0 μg/mL) were incubated with vimentin (96 nmol/L) mixed with increasing concentrations of A2 protein (0-1.0 μmol/L) Bound vimentin was detected using anti-human vimentin-HRP antibody. Similarly, microtiter wells containing immobilized vimentin were incubated with A2 protein (50 nmol/L) mixed with either mouse IgG or antibody V9 (500 nmol/L). Bound A2 was detected using a monoclonal anti-his tag HRP antibody (Sigma-Aldrich). Then, the wells were again washed, and the substrate tetramethylbenzidine (Sigma-Aldrich) was added. Substrate conversion reactions were stopped with 0.025 mL of 2 N H2SO4, and the plates were read at 490 nm. Net specific binding was determined by subtracting optical density values from wells coated only with BSA from the total binding values obtained. The apparent binding affinity for the A2-vimentin interaction was determined as we previously described.19,27
Flow assays
Plates coated with purified VWF, fibrinogen, collagen, and mammalian cell-derived A1 or A1A2A3 protein (2.0 μmol/L) were prepared as previously described.9,16,28 To obtain blood, approval was attained from the Baylor College of Medicine institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Perfusion assays were carried out as described elsewhere.9,29 One milliliter of citrated whole blood containing either the sheep irrelevant IgG or sheep polyclonal anti-human vimentin (0.6 μmol/L) was perfused over the analyte surface-coated plate followed by PBS. Positive control was represented with whole blood incubated with the buffer only. Platelets were observed with phase contrast objectives, recorded by video-microscopy, and analyzed by using the MacBiophotonics ImageJ program. From these recorded videos, the translocation velocity of platelets was determined by measuring the time that 530 platelets travel a fixed distance using the imaging software Nis-Elements. The experiments were performed using 3 different blood donors.
Flow cytometry
Washed platelets were prepared as described elsewhere with some modifications.29 Briefly, blood was collected from normal donors into plastic syringes containing acid-citrate-dextrose (pH 4.5) at a ratio of 1:10 (volume/volume). The blood was supplemented with 75 nM of prostacyclin E1 to block platelet activation and was centrifuged at 200g for 10 minutes at room temperature. The platelet-rich plasma was collected and centrifuged again at 1000g for 10 minutes. The supernatant was removed, and the pellet was resuspended to the original volume with Tyrode’s buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 138 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 10 mM NaHCO3, and 5 mM dextrose), pH 6.5, containing 75 nM prostacyclin E1. The process was repeated 1 more time. After the last centrifugation, the cells were resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Tyrode’s buffer, pH 7.4, containing 0.3. mmol/L BSA.
To analyze the effect of the antibody V9 on the binding of A1(R1450E)A2A3 mutant to stimulated washed platelets, we followed a protocol previously described.25 Thrombin (0.5 U/mL) or ADP (20 μmol/L) was added to the washed platelets (1 × 108/mL) containing irrelevant mouse IgG as a negative control or antibody V9 (25 μg/mL) followed by an incubation of 5 to 10 minutes at room temperature. The platelets were briefly (3-4 minutes) fixed with 1% formaldehyde and washed by centrifugation. Then, the platelets were incubated with the A1A2A3 mutant (250 nmol/L) for 15 minutes, followed by the addition of rabbit polyclonal anti-human VWF and goat anti-rabbit IgG-FITC antibody. After fixation, platelets were analyzed as we previously described.29
Mice
The studies involving mice were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. The vimentin-deficient and wild-type (WT) mice (strain 129/SV) were obtained from Dr David M. Markovitz, University of Michigan Health Center.30 The mice expressing human (h)GPIbα subunit in platelets (strain C5BL/6J) were obtained from Dr Jerry Ware, University of Arkansas.31 For most studies, 10- to 18-week-old mice matched for age and gender were used. Blood was collected from the inferior vena cava of isoflurane-anesthetized mice into sodium citrate at a ratio of 1:10 (volume/volume). Platelet count was performed, and the blood was subjected to flow studies as described above. Some experiments involving the use of animals were performed by investigators who were blinded to the genotype of the mice.
Bleeding time assay
As previously described with some modifications,32 mice were anesthetized with ketamine (85 mg/kg), and 5 mm of the distal tail were cut using a scalpel. The amputated tail was immersed immediately in a beaker containing physiologic saline at 37°C. Bleeding time was measured from the moment of sever until bleeding stopped.
Statistical analysis
Data are presented as means ± standard deviation (SD) and are representative of ≥2 independent experiments. Data were evaluated by unpaired Student t test. P < .05 was considered statistically significant.
Results
Activated form of VWF binds to vimentin
The translocation velocity of platelets over a surface coated with A1A2A3 domains protein (2.8 ± 0.8 μm/s) was significantly lower than on single A1 protein (6.3 ± 2.2 μm/s, P < .05). The average platelet translocation velocity on A1 protein was comparable to previous reports at the same flow rate.18,33 This result indicated the possibility of another contact point, besides A1-GPIbα, between the A2A3 domains and platelets. We thought that this outcome might be connected with the results obtained from another ongoing research project in our laboratory (supplemental Figure 1A, inset), in which vimentin was identified as a ligand for the isolated A2 domain of VWF. The A2 domain bound to immobilized human vimentin in a saturable and concentration-dependent manner (supplemental Figure 1A). The bound A2 domain was detected with the monoclonal antibody against human A2 domain,VP-1.26 The half-maximal binding occurred at 65 ± 20 nM. This interaction was blocked by the monoclonal antibody V9 against human vimentin (supplemental Figure 1B). Because vimentin can also be found on the surface of platelets,25 we further tested the hypothesis that the interaction between the A2 domain of VWF and platelet vimentin plays a role in platelet adhesion to VWF at high shear stress.
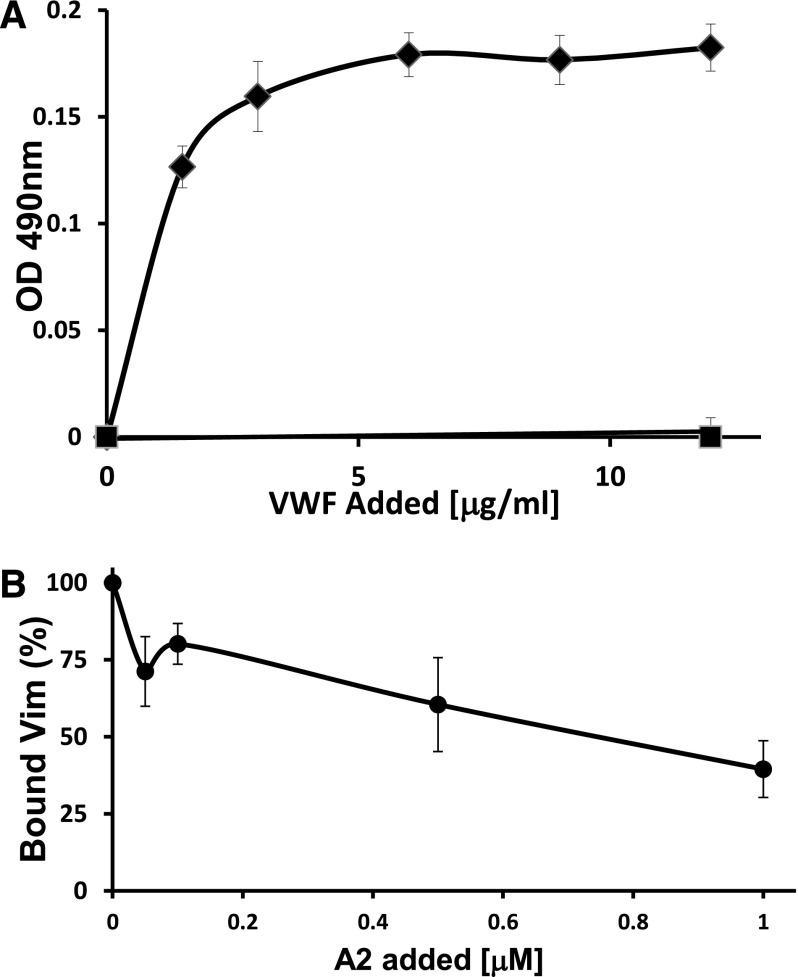
Because the A1A2A3 protein, like full-length VWF, is activated on its immobilization,9 we first examined whether the activation of VWF is a prerequisite to interact with vimentin. We analyzed the binding of purified plasma VWF to immobilized human vimentin in the presence or absence of the modulator ristocetin, which artificially induces conformational changes in the A1 and A2 domain structures. Figure 1A depicts that VWF bound effectively to vimentin in a ristocetin-dependent manner, demonstrating that the activated form of VWF exposes the contact site for vimentin.
Figure 1.
VWF binds to immobilized human vimentin. (A) Increasing concentrations of purified plasma VWF were mixed with ristocetin (0.2 mmol/L) (♦) or buffer (▪) and incubated with immobilized vimentin. Bound VWF was determined by ELISA using the polyclonal anti-VWF antibody. The VWF bound to vimentin in a ristocetin-dependent manner. Each point represents mean ± SD of 5 determinations, with a P < .01. (B) The binding of vimentin (96 nmol/L) to immobilized VWF was measured in the presence of the A2 domain at the indicated concentrations or the same volume of buffer in the control mixture. Bound vimentin was detected by using an HRP-conjugated anti-vimentin antibody. 100% is defined as the fraction of added vimentin bound in the absence of the A2 domain. Each point represents the mean ± SD of 4 determinations, P < .05 for each point.
We also assessed the capacity of the purified A2 domain in blocking the interaction between vimentin and immobilized VWF. In this experiment, both vimentin and A2 domain were in solution, excluding the possibility of a conformational change induced by the surface adsorption of the proteins. The ability of the A2 domain to block the binding of vimentin to the immobilized VWF is shown in Figure 1B.
Anti-vimentin antibodies blocked the interaction between platelets and VWF
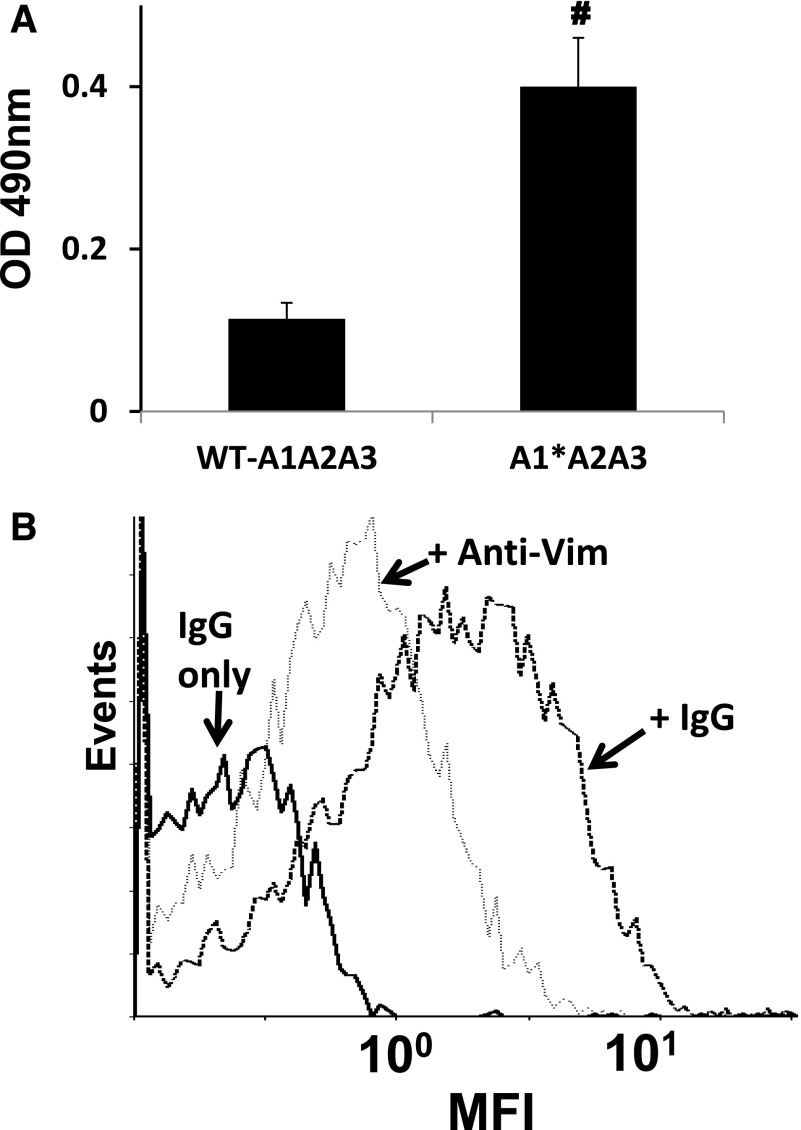
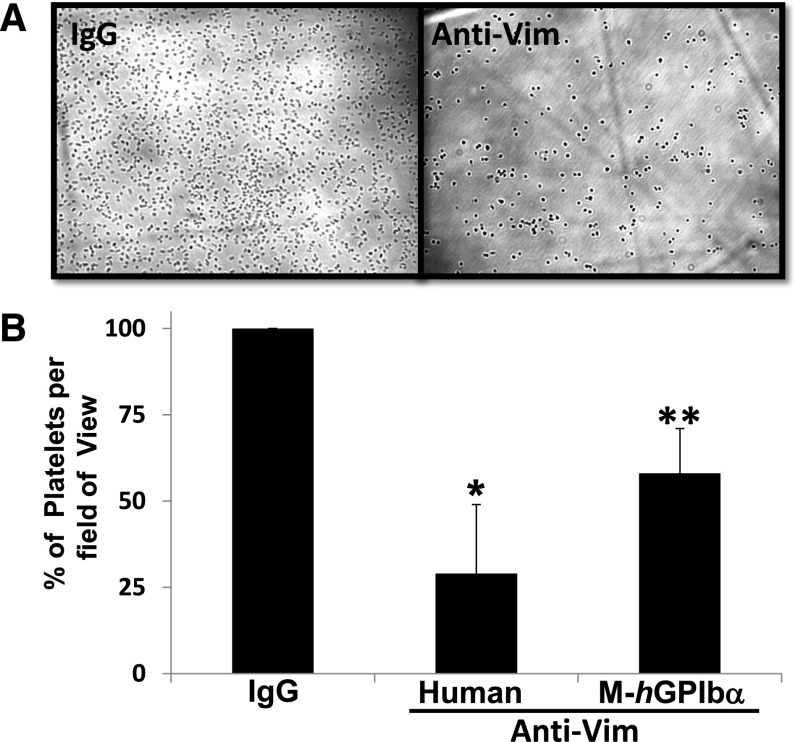
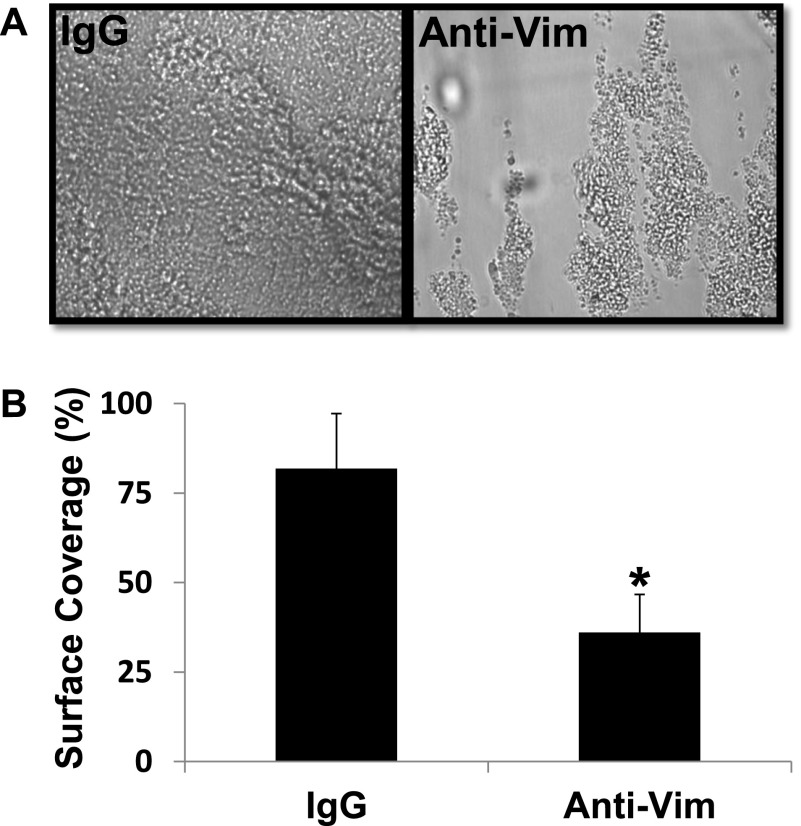
To further confirm that vimentin binds to the A2 domain in the activated form of VWF, we tested the vimentin-binding activity of a gain-of-function A1(R1450E)A2A3 mutant. This mutant, like the activated full-length VWF, has an increased binding activity for GPIbα compared with its counterpart WT protein.9,18 Figure 2A demonstrates that the mutant had a binding activity for the immobilized vimentin that was significantly higher (250% WT binding) than WT A1A2A3 protein in the absence of ristocetin. We then used whole blood and flow cytometry to examine the expression of vimentin on the platelet surface. Supplemental Figure 2 depicts the detection of vimentin on the platelet surface only on platelet activation, confirming a previous report25 and excluding the possibility that plasma vimentin could be bound to the membrane of the resting platelets. We next tested if VWF binds to vimentin on the activated platelets. Washed platelets containing irrelevant IgG as negative control or anti-vimentin antibody were stimulated with ADP and were subsequently incubated with the mutant A1(R1450E)A2A3 protein. Flow cytometric studies demonstrated that the binding of the mutant A1A2A3 to activated platelets was blocked (∼45%) by anti-vimentin antibody V9 but not by an irrelevant IgG (Figure 2B). This result implied that blocking vimentin on platelets may reduce the interaction between platelets and VWF. To test this hypothesis, human whole blood mixed with a polyclonal anti-human vimentin antibody (0.6 μmol/L) was perfused over a surface coated with WT A1A2A3 protein, at high shear rates equivalent to 1500 seconds−1. In comparison with whole blood mixed with irrelevant IgG molecule as negative control or buffer only as a positive control (data not shown), the anti-vimentin antibody markedly reduced (∼70%) the number of platelets adhered to the triple-A domain protein (Figure 3B). Similarly, the anti-vimentin antibody, which cross-reacts with murine vimentin, reduced murine platelet adhesion to the A1A2A3 protein when we used blood from transgenic mice that express the (h)GPIbα in platelets31 (Figure 3B). Next, we took advantage of a method that we recently designed to analyze the binding of soluble plasma VWF to the GPIbα of circulating platelets under high shear stress. In this assay, the combination of a subthreshold concentration of ristocetin and hydrodynamic force cause platelet activation, adhesion, and aggregation on a surface coated with fibrinogen at high shear stress.9,19 By using human whole blood, the anti-vimentin antibody reduced approximately 55% of the platelet adhesion to the immobilized fibrin(ogen), which exposes fibrin-specific sequences on surface adsorption (Figure 4). Moreover, the flow-dependent platelet adhesion to collagen fibrils, which is primarily mediated by the collagen-bound VWF under high shear stress, was also reduced (35%) by the anti-vimentin antibody (Figure 5). Similar results were obtained when we used human placenta collagen type III (supplemental Figure 3). These results indicate a physiological role of vimentin in platelet adhesion under high shear stress.
Figure 2.
Interaction of the A1A2A3 proteins with vimentin. (A) WT-A1A2A3 protein or gain-of-function A1A2A3 mutant (250 nmol/L) was incubated with immobilized vimentin in the absence of ristocetin. The bound protein was determined by ELISA, using the antibody against human VWF as described in “Methods.” Bars represents the mean ± SD of values obtained from 3 determinations, #P < .01. (B) ADP-stimulated (20 μM) washed platelets were incubated with anti-vimentin antibody, V9, or mouse IgG (25 μg/mL), followed by the addition of the mutant A1A2A3 (250 nmol/L). Mouse IgG without the A1A2A3 mutant was used as negative control and is represented by the dark solid line. The V9 decreases the interaction of the mutant A1A2A3 with platelets by an average of 45% (dotted line), based on mean fluorescence intensities (MFIs). The data represent 2 separate experiments.
Figure 3.
Effect of anti-vimentin antibody on flow-dependent platelet adhesion to A1A2A3 protein. Whole blood from human or mice expressing human (h)GPIbα in platelets was mixed with sheep IgG molecule (negative control) or sheep anti-vim antibody (100 μg/mL) and perfused over a surface coated with WT A1A2A3 protein at a shear rate of 1500 seconds−1. After a 2-minute perfusion, the plates were washed with buffer, and attached platelets were recorded and quantified as described in “Methods.” (A) A representative photomicrograph of the effect of the anti-vim antibody on platelet adhesion to the A1A2A3 surface using human blood. (B) The bar graph shows the percent of platelets (mean ± SD) on the surface of 15 different fields of view for human, *P < .03, or mice, **P < .05.
Figure 4.
Effect of anti-vimentin antibody on flow-dependent platelet adhesion to fibrin(ogen). Whole blood containing sheep IgG molecule or sheep anti-vim antibody was mixed with ristocetin (0.5 mg/mL) and perfused over surfaces coated with fibrin(ogen) at a shear rate of 1500 seconds−1. After a 2-minute perfusion, the plates were washed with buffer, and attached platelets were recorded and quantified as described in “Methods.” Photomicrographs represent 3 separate experiments. The bar graph shows the percent of surface covered by firmly adhered platelets in 15 fields of view (mean ± SD, *P < .01).
Figure 5.
Effect of anti-vimentin antibody on flow-dependent platelet adhesion to collagen. Whole blood containing sheep IgG molecule or sheep anti-human vimentin was perfused over surfaces coated with collagen fibrils and analyzed as described in Figure 4. The bar graph shows the percent of surface covered by the adhered platelets in 20 different fields of view (mean ± SD, *P < .05).
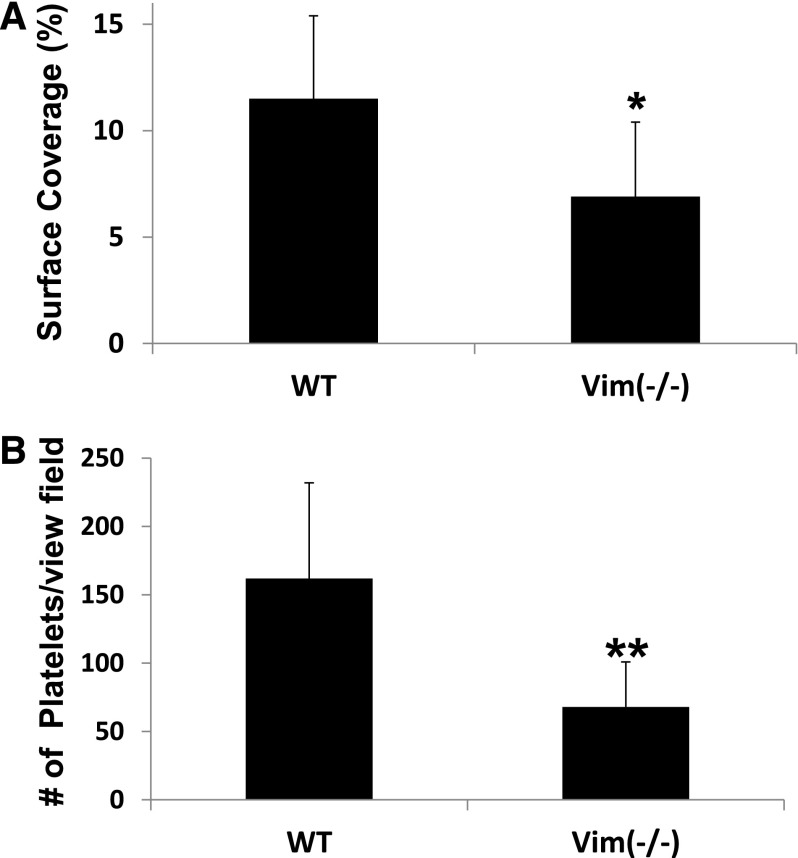
Vimentin-null murine platelets display decreased adhesion to VWF
To further corroborate the findings from anti-vimentin antibodies, we used vimentin-null murine platelets. In comparison with WT mice, platelets from the vimentin-deficient mice had a reduced flow-dependent adhesion to collagen fibrils and purified murine plasma VWF (supplemental Figure 4) at high shear stress (Figure 6A-B). Furthermore, the vimentin-deficient mice had a significant prolonged tail bleeding time compared with WT mice (supplemental Figure 5).
Figure 6.
Vimentin-deficient platelets have a reduced platelet adhesion to collagen and VWF. Whole blood from WT (n = 6) or vimentin knockout mice (n = 7) was perfused over surfaces coated with (A) collagen fibrils or (B) purified plasma murine VWF at a shear rate of 1500 seconds−1. After perfusion, the plates were washed with buffer, and the attached platelets were recorded and quantified as described in “Methods.” The bar graph in A shows the percent of surface covered by the adhered platelets in 20 different fields of view (mean ± SD, *P < .05,), whereas B shows the average number of adhered platelets per view field as described in Figure 3 (**P = .10).
Discussion
Historically, it has been accepted that the capacity of VWF in supporting platelet adhesion at sites of vascular injury under high shear stress is primarily due to its intrinsic property of interacting with 2 platelet receptors: GPIb/XI/V and GPIIb/IIIa. Our understanding of the molecular basis of platelet adhesion via the interaction of the GPIb/XI/V complex with VWF has advanced primarily through the use of purified fragments of the GPIbα and the A1 domain of VWF.16,17,34 For this reason, the observation that the translocation velocity of platelets over the A1A2A3 domains protein was lower than over the single A1 domain was intriguing and unexpected.
In the present study, we identified vimentin as a ligand for VWF, a surprising result, supported by several means. Purified plasma VWF bound to purified recombinant human vimentin in a concentration- and ristocetin-dependent manner. The requirement of the modulator to increase the binding of VWF to vimentin clearly argues that the putative contact site for vimentin is restricted in the A domains complex, particularly within the A2 domain. In fact, the purified A2 domain bound specifically to vimentin and blocked effectively the binding of vimentin to the immobilized (activated) VWF. Additionally, the interaction between platelet vimentin and the gain-of-function A1(R1450E)A2A3 mutant, which binds to vimentin (this study) and GPIbα in a ristocetin-independent manner,9,18 was efficiently reduced by the anti-vimentin antibody. These outcomes are consistent with the information that VWF exposes the cleavage site for ADAMTS-13 in the A2 domain on a conformational change induced by ristocetin,35 indicating that vimentin interacts specifically with the active form of VWF.
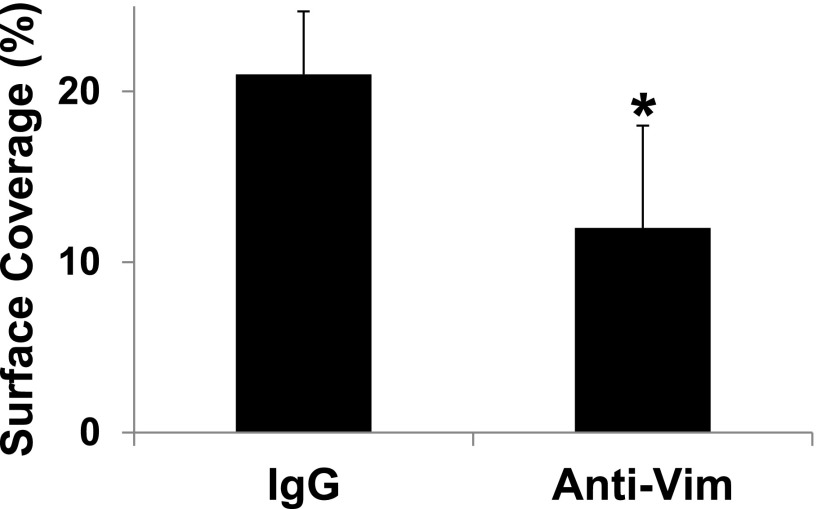
We are proposing vimentin as a novel binding protein for VWF in platelets. Vimentin is a cytoskeletal intermediate filament protein that plays an important role in intracellular dynamics and architecture.36 Several studies have indicated that this cytoskeletal protein can be found on the surface of different cell types, including platelets.20-22,24 Vimentin is not the only cytoskeletal protein found on the cell surface because ≥3 other intermediate filaments are also expressed on the surface of various cells.37 Because vimentin is a component of the platelet cytoskeleton,25 it is unclear the mechanism by which it is translocated to the cell surface. Some studies have suggested that the amino- and carboxyl-terminal domain and rod domain of vimentin contain amino acid sequences that other proteins used for translocation or are found in many membrane-associated proteins.38,39 Additionally, platelets could passively uptake vimentin from plasma23 as they are known to internalize plasma proteins,23 store them in the granules that are released on platelet activation.40,41 Another way to explain the expression of vimentin on platelets is via a direct mechanical signal transmitted to shift vimentin to the membrane surface when torque is applied to the VWF-engaged GPIb/IX/V complex. This notion is interesting when one considers that the GPIbα-VWF bond is subjected to a great torque as the platelets attach from flowing blood to the immobilized vessel wall.17 This speculation is supported by the fact that the translocation velocity of platelets over a surface coated with a mixture of A1 protein and anti-vimentin antibody was significantly lower than over A1 protein and isotype IgG (supplemental Figure 6). Thus, signals originating from GPIb after it binds A1 rapidly induce the surface expression of vimentin that can interact with the immobilized anti-vimentin antibody.
On the basis of the results obtained from using vimentin-deficient mice, it is reasonable to speculate that the A2-vimentin interaction cooperates with the A1-GPIbα binding. The fact that vimentin is ubiquitously expressed in cells of mesenchymal origin explains its involvement in many cell functions36 and does not allow us to conclude that the increased tail bleeding time observed in this study is primarily due to defective platelets. Nonetheless, all the outcomes from our studies strongly provide evidence that vimentin may participate with GPIbα in the initial contact of circulating platelets with VWF at high shear stress, including the observation that platelet adhesion to collagen and murine VWF was reduced for platelets from vimentin-deficient mice. This cooperation augments the intrinsic capacity of the VWF to quickly decelerate the rapidly moving platelets. This novel concept is supported by the fact that vimentin may act in synergy with GPIbα in platelets because vimentin (1) is a receptor for ≥1 pathogenic bacterium on the surface of brain microvascular endothelial cells,24 (2) is found in the membrane rafts of those cells, (3) functions as a signaling protein that modulates the invasion of the bacteria,42 and (4) probably binds to filamin.43
The antibody V9 recognizes a region in the tail domain of vimentin.22 However, in this study, we have not clearly elucidated whether the A2 domain binds specifically to the tail domain of vimentin. It is possible that V9 blocked the A2-vimentin binding by steric hindrance rather than competing for the same site in vimentin. In addition, several studies have demonstrated that different cells express a distinct region or domains of vimentin on their surfaces.22,44 Therefore, further investigations are required to identify what region of vimentin is expressed and recognized by VWF on the surface of stimulated platelets. On the other hand, the putative contact site for vimentin apparently does not overlap with the cleavage site in the A2 domain. For example, we reported that the A2 domain can be effectively cleaved by ADAMTS-13 in plasma,45-47 implying that the plasma vimentin, which binds to the A2 domain (supplemental Figure 1C), does not impair the cleavage activity. Moreover, the A2 domain bound to vimentin was detected with the monoclonal antibody VP-1, which recognizes a sequence in the N-terminal side next to the ADAMTS-13 cleavage site in the A2 domain, and it is a potent blocker of ADAMTS-13 activity.45
The use of anti-vimentin antibody to interfere with platelet adhesion to different matrixes under high flow conditions suggest that the inhibition of the vimentin-VWF interaction on platelets may decrease VWF-mediated platelet adhesion, activation, and consequently, the accumulation of platelets on ruptured atherosclerotic plaques. In fact, platelet adhesion has been considered as a target to treat thrombosis.48 Furthermore, this newly identified interaction of vimentin with VWF could have other several clinical implications. (1) Variations in the platelet vimentin levels in different disease states could serve as markers of altered platelet adhesiveness to VWF.25 (2) Conversely, high levels of vimentin in plasma in certain disorders could serve as an inhibitor of platelet adhesion.23 (3) VWF and vimentin have been independently identified as active players in tumor growth and metastasis.49,50 Because platelets are a key component in the development of metastasis,51 it is possible that the vimentin expressed on the surface of tumor cells, platelets, and the endothelium might serve as a receptor for VWF. Thus, vimentin-VWF binding could mediate the interaction between tumor cells and platelets and the vessel wall, facilitating the extravasation of the tumor cells from circulation.
In summary, vimentin expressed on the surface of the stimulated platelets can cooperate with GPIbα in mediating the initial contact of circulating platelets with VWF at arterial shear rates. This interaction, which is through the A2 domain, is effectively reduced by anti-vimentin antibodies. The results reported in this study add vimentin to the list of human ligands for VWF.52
Supplementary Material
Acknowledgments
This work was supported by the Mary R. Gibson Foundation, Alkek Foundation, Fondren Foundation, National Institutes of Health, National Heart, Lung and Blood Institute, grant HL 081613 (K.V.V.), and American Heart Association, grant GRNT18150006 (M.A.C.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Q.D. performed experiments and analyzed data; M.B. and J.I.C. performed experiments; K.V.V. designed experiments and wrote the paper; and M.A.C. designed and performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Miguel A. Cruz, Cardiovascular Research Section, Baylor College of Medicine, One Baylor Plaza, N1319, Houston, TX 77030; e-mail: miguelc@bcm.edu.
References
- 1.Alevriadou BR, Moake JL, Turner NA, et al. Real-time analysis of shear-dependent thrombus formation and its blockade by inhibitors of von Willebrand factor binding to platelets. Blood. 1993;81(5):1263–1276. [PubMed] [Google Scholar]
- 2.Bonnefoy A, Vermylen J, Hoylaerts MF. Inhibition of von Willebrand factor-GPIb/IX/V interactions as a strategy to prevent arterial thrombosis. Expert Rev Cardiovasc Ther. 2003;1(2):257–269. doi: 10.1586/14779072.1.2.257. [DOI] [PubMed] [Google Scholar]
- 3.Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28(3):403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 4.Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 5.Bonthron DT, Handin RI, Kaufman RJ, et al. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- 6.Sixma JJ, Schiphorst ME, Verweij CL, Pannekoek H. Effect of deletion of the A1 domain of von Willebrand factor on its binding to heparin, collagen and platelets in the presence of ristocetin. Eur J Biochem. 1991;196(2):369–375. doi: 10.1111/j.1432-1033.1991.tb15826.x. [DOI] [PubMed] [Google Scholar]
- 7.Lankhof H, Damas C, Schiphorst ME, et al. von Willebrand factor without the A2 domain is resistant to proteolysis. Thromb Haemost. 1997;77(5):1008–1013. [PubMed] [Google Scholar]
- 8.Cruz MA, Yuan H, Lee JR, Wise RJ, Handin RI. Interaction of the von Willebrand factor (vWF) with collagen. Localization of the primary collagen-binding site by analysis of recombinant vWF a domain polypeptides. J Biol Chem. 1995;270(18):10822–10827. doi: 10.1074/jbc.270.18.10822. [DOI] [PubMed] [Google Scholar]
- 9.Auton M, Sowa KE, Smith SM, Sedlák E, Vijayan KV, Cruz MA. Destabilization of the A1 domain in von Willebrand factor dissociates the A1A2A3 tri-domain and provokes spontaneous binding to glycoprotein Ibalpha and platelet activation under shear stress. J Biol Chem. 2010;285(30):22831–22839. doi: 10.1074/jbc.M110.103358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Federici AB, Mannucci PM, Castaman G, et al. Clinical and molecular predictors of thrombocytopenia and risk of bleeding in patients with von Willebrand disease type 2B: a cohort study of 67 patients. Blood. 2009;113(3):526–534. doi: 10.1182/blood-2008-04-152280. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101(7):2637–2645. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 12.Azuma H, Sugimoto M, Ruggeri ZM, Ware J. A role for von Willebrand factor proline residues 702-704 in ristocetin-mediated binding to platelet glycoprotein Ib. Thromb Haemost. 1993;69(2):192–196. [PubMed] [Google Scholar]
- 13.Ruggeri ZM. Von Willebrand’s disease and the mechanisms of platelet function. Ciba Found Symp. 1995;189:35–45. doi: 10.1002/9780470514719.ch4. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita T, Sadler JE. Identification of amino acid residues essential for von Willebrand factor binding to platelet glycoprotein Ib. Charged-to-alanine scanning mutagenesis of the A1 domain of human von Willebrand factor. J Biol Chem. 1995;270(22):13406–13414. doi: 10.1074/jbc.270.22.13406. [DOI] [PubMed] [Google Scholar]
- 15.Miyata S, Ruggeri ZM. Distinct structural attributes regulating von Willebrand factor A1 domain interaction with platelet glycoprotein Ibalpha under flow. J Biol Chem. 1999;274(10):6586–6593. doi: 10.1074/jbc.274.10.6586. [DOI] [PubMed] [Google Scholar]
- 16.Cruz MA, Diacovo TG, Emsley J, Liddington R, Handin RI. Mapping the glycoprotein Ib-binding site in the von willebrand factor A1 domain. J Biol Chem. 2000;275(25):19098–19105. doi: 10.1074/jbc.M002292200. [DOI] [PubMed] [Google Scholar]
- 17.Doggett TA, Girdhar G, Lawshé A, et al. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIb(α)-vWF tether bond. Biophys J. 2002;83(1):194–205. doi: 10.1016/S0006-3495(02)75161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yago T, Lou J, Wu T, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118(9):3195–3207. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auton M, Sowa KE, Behymer M, Cruz MA. N-terminal flanking region of A1 domain in von Willebrand factor stabilizes structure of A1A2A3 complex and modulates platelet activation under shear stress. J Biol Chem. 2012;287(18):14579–14585. doi: 10.1074/jbc.M112.348573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ise H, Kobayashi S, Goto M, et al. Vimentin and desmin possess GlcNAc-binding lectin-like properties on cell surfaces. Glycobiology. 2010;20(7):843–864. doi: 10.1093/glycob/cwq039. [DOI] [PubMed] [Google Scholar]
- 21.Moisan E, Girard D. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J Leukoc Biol. 2006;79(3):489–498. doi: 10.1189/jlb.0405190. [DOI] [PubMed] [Google Scholar]
- 22.Päll T, Pink A, Kasak L, et al. Soluble CD44 interacts with intermediate filament protein vimentin on endothelial cell surface. PLoS ONE. 2011;6(12):e29305. doi: 10.1371/journal.pone.0029305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, deWaal RM, Mor-Vaknin N, Hibbard C, Markovitz DM, Kahn ML. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol Cell Biol. 2004;24(20):9198–9206. doi: 10.1128/MCB.24.20.9198-9206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou Y, He L, Huang SH. Identification of a surface protein on human brain microvascular endothelial cells as vimentin interacting with Escherichia coli invasion protein IbeA. Biochem Biophys Res Commun. 2006;351(3):625–630. doi: 10.1016/j.bbrc.2006.10.091. [DOI] [PubMed] [Google Scholar]
- 25.Podor TJ, Singh D, Chindemi P, et al. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J Biol Chem. 2002;277(9):7529–7539. doi: 10.1074/jbc.M109675200. [DOI] [PubMed] [Google Scholar]
- 26.Dent JA, Berkowitz SD, Ware J, Kasper CK, Ruggeri ZM. Identification of a cleavage site directing the immunochemical detection of molecular abnormalities in type IIA von Willebrand factor. Proc Natl Acad Sci U S A. 1990;87(16):6306–6310. doi: 10.1073/pnas.87.16.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin C, Morales LD, Cruz MA. Purified A2 domain of von Willebrand factor binds to the active conformation of von Willebrand factor and blocks the interaction with platelet glycoprotein Ibalpha. J Thromb Haemost. 2007;5(7):1363–1370. doi: 10.1111/j.1538-7836.2007.02536.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith C, Estavillo D, Emsley J, Bankston LA, Liddington RC, Cruz MA. Mapping the collagen-binding site in the I domain of the glycoprotein Ia/IIa (integrin α(2)β(1)). J Biol Chem. 2000;275(6):4205–4209. doi: 10.1074/jbc.275.6.4205. [DOI] [PubMed] [Google Scholar]
- 29.Cruz MA, Chen J, Whitelock JL, Morales LD, López JA. The platelet glycoprotein Ib-von Willebrand factor interaction activates the collagen receptor alpha2beta1 to bind collagen: activation-dependent conformational change of the alpha2-I domain. Blood. 2005;105(5):1986–1991. doi: 10.1182/blood-2004-04-1365. [DOI] [PubMed] [Google Scholar]
- 30.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5(1):59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 31.Guerrero JA, Shafirstein G, Russell S, et al. In vivo relevance for platelet glycoprotein Ibalpha residue Tyr276 in thrombus formation. J Thromb Haemost. 2008;6(4):684–691. doi: 10.1111/j.1538-7836.2008.02916.x. [DOI] [PubMed] [Google Scholar]
- 32.Gushiken FC, Hyojeong H, Pradhan S, et al. The catalytic subunit of protein phosphatase 1 gamma regulates thrombin-induced murine platelet alpha(IIb)beta(3) function. PLoS ONE. 2009;4(12):e8304. doi: 10.1371/journal.pone.0008304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coburn LA, Damaraju VS, Dozic S, Eskin SG, Cruz MA, McIntire LV. GPIbα-vWF rolling under shear stress shows differences between type 2B and 2M von Willebrand disease. Biophys J. 2011;100(2):304–312. doi: 10.1016/j.bpj.2010.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasudevan S, Roberts JR, McClintock RA, et al. Modeling and functional analysis of the interaction between von Willebrand factor A1 domain and glycoprotein Ibalpha. J Biol Chem. 2000;275(17):12763–12768. doi: 10.1074/jbc.275.17.12763. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Ling M, Fu X, López JA, Chung DW. Simultaneous exposure of sites in von Willebrand factor for glycoprotein Ib binding and ADAMTS13 cleavage: studies with ristocetin. Arterioscler Thromb Vasc Biol. 2012;32(11):2625–2630. doi: 10.1161/ATVBAHA.112.254144. [DOI] [PubMed] [Google Scholar]
- 36.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313(10):2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Hasan AA, Zisman T, Schmaier AH. Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc Natl Acad Sci U S A. 1998;95(7):3615–3620. doi: 10.1073/pnas.95.7.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perides G, Harter C, Traub P. Electrostatic and hydrophobic interactions of the intermediate filament protein vimentin and its amino terminus with lipid bilayers. J Biol Chem. 1987;262(28):13742–13749. [PubMed] [Google Scholar]
- 39.Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277(5325):556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 40.Escolar G, Lopez-Vilchez I, Diaz-Ricart M, White JG, Galan AM. Internalization of tissue factor by platelets. Thromb Res. 2008;122(Suppl 1):S37–S41. doi: 10.1016/S0049-3848(08)70017-3. [DOI] [PubMed] [Google Scholar]
- 41.Verheul HM, Lolkema MP, Qian DZ, et al. Platelets take up the monoclonal antibody bevacizumab. Clin Cancer Res. 2007;13(18 Pt 1):5341–5347. doi: 10.1158/1078-0432.CCR-07-0847. [DOI] [PubMed] [Google Scholar]
- 42.Chi F, Jong TD, Wang L, et al. Vimentin-mediated signalling is required for IbeA+ E. coli K1 invasion of human brain microvascular endothelial cells. Biochem J. 2010;427(1):79–90. doi: 10.1042/BJ20091097. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Nakamura F, Lee W, Hong C, Pérez-Sala D, McCulloch CA. Regulation of cell adhesion to collagen via beta1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp Cell Res. 2010;316(11):1829–1844. doi: 10.1016/j.yexcr.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Lahat G, Zhu QS, Huang KL, et al. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS ONE. 2010;5(4):e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Cruz MA, Whitelock J, Dong JF. Evaluation of ADAMTS-13 activity in plasma using recombinant von Willebrand Factor A2 domain polypeptide as substrate. Thromb Haemost. 2003;90(6):1204–1209. doi: 10.1160/TH03-06-0398. [DOI] [PubMed] [Google Scholar]
- 46.Whitelock JL, Nolasco L, Bernardo A, Moake J, Dong JF, Cruz MA. ADAMTS-13 activity in plasma is rapidly measured by a new ELISA method that uses recombinant VWF-A2 domain as substrate. J Thromb Haemost. 2004;2(3):485–491. doi: 10.1111/j.1538-7836.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 47.Dong JF, Whitelock J, Bernardo A, Ball C, Cruz MA. Variations among normal individuals in the cleavage of endothelial-derived ultra-large von Willebrand factor under flow. J Thromb Haemost. 2004;2(8):1460–1466. doi: 10.1111/j.1538-7836.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- 48.Krishnan A, Lopes RD, Alexander JH, Becker RC, Goldstein LB. Antithrombotic therapy for ischemic stroke: guidelines translated for the clinician. J Thromb Thrombolysis. 2010;29(3):368–377. doi: 10.1007/s11239-010-0439-7. [DOI] [PubMed] [Google Scholar]
- 49.Terraube V, Marx I, Denis CV. Role of von Willebrand factor in tumor metastasis. Thromb Res. 2007;120(Suppl 2):S64–S70. doi: 10.1016/S0049-3848(07)70132-9. [DOI] [PubMed] [Google Scholar]
- 50.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3(7):425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 52.Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10(12):2428–2437. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.