Abstract
Multipotent mesenchymal stromal cells (MSC), have the potential to differentiate into cells of the mesenchymal lineage and have non-progenitor functions including immunomodulation. The demonstration that MSCs are perivascular cells found in almost all adult tissues raises fascinating perspectives on their role in tissue maintenance and repair. However, some controversies about the physiological role of the perivascular MSCs residing outside the bone marrow and on their therapeutic potential in regenerative medicine exist. In brain, perivascular MSCs like pericytes and adventitial cells, could constitute another stem cell population distinct to the neural stem cell pool. The demonstration of the neuronal potential of MSCs requires stringent criteria including morphological changes, the demonstration of neural biomarkers expression, electrophysiological recordings, and the absence of cell fusion. The recent finding that brain cancer stem cells can transdifferentiate into pericytes is another facet of the plasticity of these cells. It suggests that the perversion of the stem cell potential of pericytes might play an even unsuspected role in cancer formation and tumor progression.
Keywords: Stem cell, Mesenchymal stem cell, Pericyte, Brain, Cell plasticity, Cancer stem cell, Glioma, Neurodegenerative disease
Core tip: Mesenchymal stem cells (MSCs), in addition to their potential to differentiate into cells of the mesenchymal lineage, have immunomodulatory properties and can transdifferentiate to generate neural cells at least in vitro. These stem cells are found in almost any adult tissue, including brain. The existence of similarities between MSC and pericytes points to brain pericytes as the other stem cell population of the adult brain in addition to neural stem cells. This raises fascinating perspectives on the potential of brain pericytes in nervous system maintenance and repair. The recent finding that brain cancer stem cells transdifferentiate into pericytes is another facet of the plasticity of these cells. It suggests that the perversion of the stem cell potential of pericyte might play an even unsuspected role in cancer formation and tumor progression.
INTRODUCTION
The history of multipotent mesenchymal stromal cells started when colony-forming unit fibroblastic cells (CFU-F) with osteogenic potential were obtained from bone marrow cultured cells[1-3]. Accordingly, CFU-F cells were defined as self-renewing non-hematopoietic bone marrow stromal stem cells (BMSCs). They were isolated on the basis of their plastic adherence, and characterized both by their ability to form colony when plated at low-density and to differentiate into osteoblasts[3]. Thereafter, BMSCs were shown to differentiate in vitro and in vivo into other cells of mesenchymal lineage including chondrocytes and adipocytes[4]. Cells similar to BMSCs are also isolated from non-marrow fetal tissue such as placenta, cord blood, fetal liver and lung, as well as from adult tissues including muscle, adipose tissue, dental pulp, lung and brain[5-8]. These fetal and adult stem cells have the same ability as BMSCs for self-renewal and for differentiation into osteoblasts, chondrocytes and adipocytes in vitro. They also exhibit, at least in vitro, transdifferentiation capacity (see below). These cells are referred as mesenchymal stem cells or as multipotent mesenchymal stromal cells (MSCs). However, the question remains if these ubiquitous cells behave in vivo as genuine stem cells or if their stem cell potential is a cell culture artifact[9]. The existence of these MSCs in virtually all postnatal organs does not necessarily mean that these cells behave as stem cells during development. For example, their physiological function could be limited to postnatal regenerative processes. Hence, the concept of mesenchymal stem cell, initially well-defined and restricted to a multipotent progenitor for skeletal tissues and residing within the bone marrow has progressively evolved towards an all-encompassing concept including multipotent perivascular cells of almost any tissue[9]. Importantly, there is not an exclusive and universal marker for immunophenotyping MSCs. Therefore, their immuno-characterization relies on a combination of both positive and negative markers. Positive markers can include CD11b, CD13, CD19A, CD73, CD105, CD146, CD271, nestin, nerve/glial antigen 2 (NG2), platelet-derived growth factor receptor β (PDGFR-β), while negative markers usually are endothelial, and hematopoietic stem cell proteins (Table 1)[10-12]. An additional remarkable feature is that MSCs lack or have a low expression of MHC class II and of the co-stimulatory molecules CD40, CD80, CD86, CD134 and CD142[13]. In relation to this, MSCs have strong anti-inflammatory and immunomodulating potentials[14]. MSCs exert their inhibitory effects on T-cell proliferation by mechanisms involving both cell to cell contact between MSC and T lymphocytes, and secreted factors such as prostaglandin E2 (PGE2), inoleamine 2,3-dioxygenase and nitric oxide[14]. As in many biological processes, this immunosuppressive effect is dose dependent and depends on the ratio between MSCs and T cells. Indeed low ratios of MSCs can even enhance T cell proliferation[14]. In addition, MSCs prevent the differentiation of monocyte into dendritic cells, and modulate natural killer cell activity by the release of inhibitory factors such as PGE2 and transforming growth factor-β[14]. MSCs also have anti-inflammatory action by reducing the production of tumor necrosis factor (TNF)-α and interleukin (IL)-12 and by increasing the synthesis of IL-10 by macrophages[14]. These anti-inflammatory and immuno-modulatory capacities of MSCs are already exploited in vivo. MSC-based treatment is beneficial in several models of graft-vs-host disease and in auto-immune diseases such as collagen-induced arthritis, experimental autoimmune encephalomyelitis, type 1 diabetes mellitus disease and inflammatory bowel disease models[14-17]. Clinical trials are currently underway for these different pathologies[15,18]. The ability of MSCs to home in damaged tissues, associated with their capacity to secrete bioactive molecules such as growth factors, and their immunosuppressive and anti-inflammatory properties, suggest that these cells protect tissues from damage and facilitate tissue repair independently of their capacity to generate differentiated cells[18].
Table 1.
Major positive and negative markers used for identifying bone marrow mesenchymal stem cells and pericytes
| Markers | MSCs | Pericytes | EC | HSPCs | NSPCs |
| CD10 | +[12] | +[12] | |||
| CD13 | +[12] | +[12] | |||
| CD29 | +[12] | +[12] | +[91] | +[92] | |
| CD44 | +[12] | +[12] | +[93] | +[92] | |
| CD73 | +[12] | +[12] | +[94] | ||
| CD90 | +[12] | +[12] | +[95] | +[92,96] | |
| CD105 | +[12] | +[12] | +[97] | ||
| CD140B | +[12] | +[12] | |||
| CD146 | +/low[12,90] | +[12] | +[98] | ||
| CD166 | +[12] | +[12] | +[99] | ||
| NG2 | +[12] | +[12] | -[11] | -[100] | |
| Nestin | +[101,102] | +[72] | +[103] | ||
| CD14 | -[12] | -[12] | |||
| CD31 | -[12] | -[12] | +[104] | ||
| CD34 | -[12] | -[12] | +[105] | +[105] | |
| CD45 | -[12] | -[12] | |||
| CD133 | -[12] | -[12] | -[106] | +[107] | +[108] |
| CD117 | -[12] | -[12] | +[109] | +[110] | |
| CD144 | -[12] | -[12] | +[111] | ||
| vWF | -[112] | -[113] | +[114] |
In the absence of any universal and specific marker to define mesenchymal stem cells, their immunophenotyping relies on the use of combinations of both positive and negative markers. Note that MSCs profile may vary depending on the cell culture conditions[88], or with their in situ localization[89]. Expression of the cell surface antigens CD73, CD90, CD105 and non-expression of CD14, CD34, CD45 are useful criteria to define bone MSCs and pericytes. MSCs: Mesenchymal stem cells; EC: Endothelial cells; HSPCs: Hematopoietic stem and progenitor cells; NSPCs: Neural stem and progenitor cells.
For all these reasons, MSCs became the focus of intense researches in tissue engineering and regenerative medicine. These cells could provide an answer both to the ethical concerns raised by the therapeutic use of human embryonic stem cells and to their scarce availability. Furthermore, as MSCs are easily isolated from adult tissues, they offer the advantage to allow autologous transplantation. Importantly, experimental studies performed with MSCs revealed an additional property: MSCs have a greater differentiation plasticity potential than previously envisioned. For example, they can transdifferentiate into urothelial, myocardial, and epithelial cells[19-21]. Numerous studies also report the in vitro transdifferentiation of MSCs into neural and glial cells[22-30]. At the moment, the potential of MSCs to regenerate human tissues in vivo is not clearly defined. Current research is ongoing to resolve this critical issue by improving MSC culture engineering and cell transplantation technology. A better characterization of the therapeutic potential of MSCs according to their tissue of origin is also a critical issue.
WHEN MSCs TRANSDIFFERENTIATE INTO NEURAL CELLS: FACTS AND ARTIFACTS
The observation that MSCs transdifferentiate into neurons was first obtained with bone MSCs, and then extended to MSCs isolated from different adult tissues including adipose tissue, bone marrow, and brain[5,31-34]. Brain implanted marrow stromal cells also differentiate into glial cells[25]. Importantly, grafting MSCs in several brain lesion models reduces neuronal deficits[35-42]. However, current evidence suggests that in the experimental models used, the repair and functional improvements reported are primarily mediated by paracrine or cell-cell interactions rather than by the successful engraftment and the in situ transdifferentiation of implanted MSCs into neural cells[43-47]. Regarding MSC transdifferentiation into neural cells, a notable controversy arose when it was reported that, (1) the rapid in vitro morphological differentiation of MSC into neuron-like cells following administration of DMSO or cAMP elevating agents such as forskolin or IBMX can be linked to actin depolymerization resulting in cytoplasm retractation and not through neurite extension[48-50]; and (2) the transformation of MSCs into neurons in vivo can result from the fusion of MSCs with brain cells rather than to MSC transdifferentiation[51]. Therefore, additional criteria are now applied when studying MSC transdifferentiation. For example, reporting neuronal differentiation of MSCs now requires observation of morphological changes, the demonstration of neural biomarkers expression, neurotransmitter responsiveness or electrophysiological recording, and absence of cell fusion[28,33,49,52,53]. Note however, that all MSCs are not equal and that their differentiation potential can be related to their tissue of origin[6]. This suggests that brain-derived MSCs could have a greater potential for neural differentiation than bone MSCs. Hence, the difficulty to obtain functional mature neurons by differentiating bone MSC can be explained both by their origin and by cell culture conditions which are far to provide the cues found in the brain microenvironment. Accordingly, recent experiments using brain derived MSCs instead of bone marrow MSCs, provide additional evidence on the potential of brain MSCs to transdifferentiate into neuronal cells at the clonal level and on the basis of stringent criteria[54]. A notable point is that these observations are made in vitro. Therefore, it remains to establish whether the transdifferentiation of MSCs is a cell culture artifact with potential applications in cell replacement therapies for implanting pre-differentiated neurons, or is it also a physiological process contributing to brain development or repair. Part of the answer might be given by determining where MSCs reside in the organism and which cell behaves as MSC in vivo. Recent findings show that MSCs are perivascular cells such as pericytes[11,55,56].
MSCS ARE PERIVASCULAR CELLS
Pericytes are perivascular cells, or more strictly speaking peri-endothelial vascular mural cells (Figure 1). Pericytes form an incomplete layer on the abluminal surface of capillary endothelial cells. They wrap capillary endothelial cells and both cell types are surrounded by the basal lamina[57] (Figure 2). For many years, pericytes have been viewed as supportive vasculature cells involved in the regulation of capillaries blood flow and contributing to the blood-brain barrier[58]. Nowadays, known functions of pericytes also include a role in angiogenesis, in matrix proteins and bioactive molecules synthesis (vascular endothelial growth factor, placental growth factor, leukemia inhibitor factor, CXCL12, basic fibroblast growth factor, nerve growth factor, platelet-derived growth factor B…), in vessel stabilization and in the regulation of vascular tone[59]. Importantly, these cells are now considered as a potential reservoir of stem or progenitor cells for adult tissue repair. Regarding this stem cell potential, it has been known as early as 1995 that pericytes can differentiate into an osteogenic phenotype[60]. Ten years after, perivascular cells were also demonstrated to differentiate into adipocytes[61]. The definitive proof that MSCs are perivascular cells such as pericytes was done in 2008 in two landmark studies showing that a subset of perivascular cells from adult tissues, identified on CD146, NG2 and PDGF-Rβ expression, exhibit in culture the same osteogenic, chondrogenic, adipogenic and myogenic potentials than MSCs[11,55]. In addition, these perivascular cells express MSC markers including CD10, CD13, CD44, CD73, CD90 and CD105[11,12]. A consequence of the demonstration of a perivascular origin for MSCs was a burst of interest in pericyte research with the number of annual entries in PubMed for the keyword “pericyte” increasing from 83 in 1993 to 445 in 2013. With hindsight, the finding that some MSCs are pericytes is not incongruous[11,56]. Stem cells must reside in a specialized environment (the stem cell niche), and the presence of MSCs in almost all adult tissues suggests a ubiquitous distribution for MSC niches. This is consistent with the omnipresence of capillary blood vessel mural cells. In addition, this perivascular location allows the rapid recruitment of MSCs to the site of focal lesions where they could act as microenvironmental regulators for tissue regeneration[62]. Since tissue regeneration requires functional blood vessels, associating MSCs with endothelial cells in a same “regenerative/healing unit” makes sense. Note that in addition to capillaries, MSCs are also detected in the adventitia of large vessels[63-65].
Figure 1.
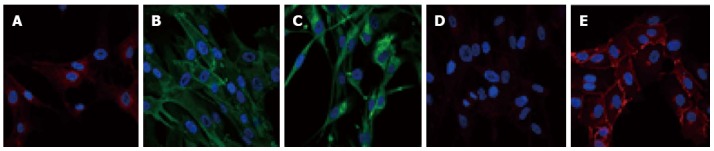
Pericyte immunophenotyping. Pericytes express antigens allowing their identification. However, there is currently no specific marker to identify them. Therefore, to distinguish pericytes from other cell types, both positive and negative markers are used. For example, pericytes are known to be positive for platelet-derived growth factor receptor β (PDGFR-β)/CD140b (A), Alanine aminopeptidase N/CD13 (B), and for the stem cell protein nestin (C). Pericytes are also negative for VE-Cad/CD144 (D) that is detected in human brain endothelial cells (E). Specific antigenic labeling is in green or red and nuclei are 4’,6-diamidino-2-phenylindole stained (blue).
Figure 2.
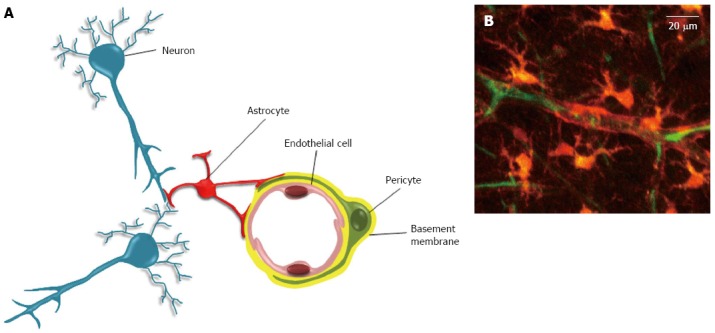
The neurovascular unit. A: The neurovascular unit. In the neurovascular unit, pericytes are located on the abluminal side of endothelial cells (EC). Both cells are ensheathed by the basement membrane (BM). The covering of EC by pericytes is incomplete, and interruptions in BM can allow direct contacts between pericyte and EC. These contacts occur through peg and socket structures, and adherent and gap junctions (not shown)[59]. The abluminal side of the basement membrane is also contacted by astrocytes endfeet. In addition to these cells, the neurovascular unit also includes neurons, and microglial cells (not shown); B: Two-photon microscopy of a neurovascular unit. Following injection in the rat tail vein, the sulforhodamine-B dye crosses the blood brain barrier and stains astrocytes and pericytes in orange (reproduced from[115]). The blood plasma is shown in green after iv injection of FITC-dextran (Mw 70 kDa). Neurons, endothelial and microglial cells are not shown here.
CNS PERICYTE AND THE NEUROVASCULAR UNIT
With a human brain capillary network estimated to 400 miles length[66], and a ratio of about one pericyte for three brain endothelial cells, the human brain pericyte population is far from negligible. Pericytes cover more than 30% of the cerebral capillary surface[67]. These cells are well-known to be involved in the regulation of angiogenesis, vascular tone and blood brain barrier function. They constitute with endothelial cells, astrocytes and neurons a critical brain structure named neurovascular unit (NVU). The NVU, in addition to selectively supplying nutrients and oxygen through the blood brain barrier structure, provides a permissive environment for neural stem cell homing and for their proliferation[68-70]. Note that if most pericytes are of mesoderm origin, forebrain pericytes originate from the neural crest[71]. The demonstration that MSCs originate at least in part from pericytes raises the question of the stem cell potential of brain pericytes. At a clonal level these cells have the potential to differentiate in vitro into adipocytes, chondroblasts and osteoblasts[54]. Moreover these cells are also able to differentiate in vitro toward a neuronal phenotype depending on cell culture conditions[33,54,72,73]. These observations revive the idea that CNS perivascular cells such as pericytes might contribute to brain repair either directly by generating new neurons or indirectly via their immunomodulatory properties or the secretion of neurotrophins[74]. Consistent with this idea is the observation that pericytes migrate away from the vascular wall and could generate neurons in response to injury[75,76].
THE CANCER PERICYTE MODEL: A PERPETUUM MOBILE
The NVU also plays a critical role in brain cancer since a contingent of brain cancer stem cells is found near the capillaries[77-79]. Importantly, glioblastoma stem cells are able to transdifferentiate into pericytes[80]. According to the function of pericytes in vessel formation, these cancer pericytes contribute to the glioblastoma microvas-culature[80,81]. The recent finding that MSCs are pericytes, and that glioblastoma cells generate cancer pericytes, suggests that the stemness potential of pericytes could play a yet unsuspected role in cancer formation and progression. In the synthetic hypothetical model depicted in Figure 3, a transformed dormant pericyte harboring oncogenic mutations and lying in its vascular niche is activated and released from its vascular location as a consequence of the up-regulation of matrix proteases (Figure 3, step 1). This activation can be triggered by inflammation or can occur following a local injury as observed in vivo with normal pericytes[76,82]. In the proposed model, and in accordance with the similarities between pericytes and MSCs, this cancer pericyte behaves as a cancer mesenchymal stem cell. In accordance to the described potential of MSCs to generate neural stem cell-like cells[30,72], cancer pericyte cells acquire a neural stem cell-like phenotype during their migration in brain parenchyma. This generates the cancer stem cell pool found in the tumor mass (Figure 3, step 2). Proliferation of these cancer stem cells generates hypoxia and triggers the angiogenic switch. Cancer stem cells are then recruited to develop vessels by endothelial cell-secreted cytokines such as CXCL12[83-85] (Figure 3, step 3). In this novel vascular microenvironment made of chaotic vessels, cancer stem cells reacquire a pericyte-like phenotype as described[80,81]. These pericyte-like cancer cells not only participate to tumor vascularization[80,81], but also re-express their mesenchymal potential by undergoing a mesenchymal transition reminiscent to the epithelial mesenchymal transition. This generates the perpetuum mobile described in Figure 3. Indeed, MSCs have already been characterized as cancer initiating cells in gastric cancer[86].
Figure 3.
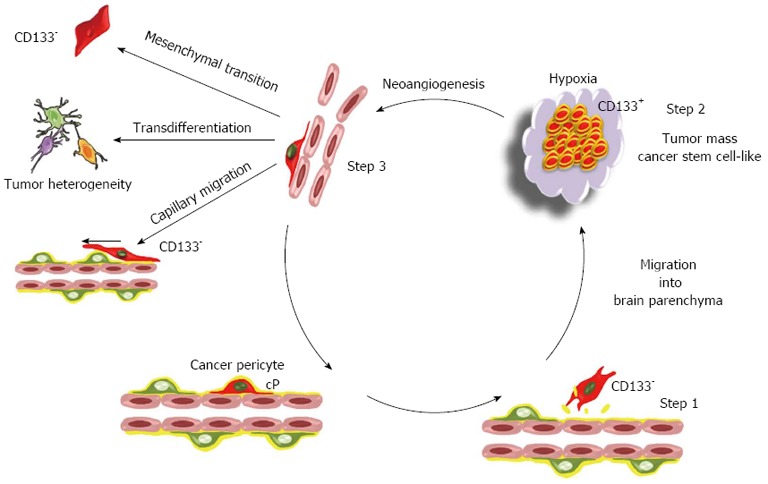
The cancer pericyte model: A perpetuum mobile. The proposed model of brain tumor is based on the mesenchymal stem cell potential of pericytes. In this model, the brain cancer initiating cell is a cancer pericyte (cP) harbouring oncogenic alterations and located on a brain capillary. After disruption of the basement membrane by proteases, it detaches from the vessel wall and migrates into brain parenchyma as normal pericytes do following injury[76] (step 1). During the passage from a vascular to a neural environment the pericyte acquires a CD133+ neural stem cell-like phenotype, as observed in vitro for non-transformed pericytes[72]. Such a transition towards a neural stem cell phenotype is already observed for non-transformed pericytes at least in vitro. This generates the CD133+ cancer stem cell pool (step 2). Amplification of the cancer stem cell pool generates hypoxia that triggers neoangiogenesis and the migration of endothelial cells towards the lesion as well as the migration of cancer stem cells towards endothelial capillaries[83] (step 3). Cancer stem cells within this new vascular microenvironment reacquire a CD133- pericyte-like phenotype. At this stage, they can either integrate into the tumor neovasculature and reinitiate a new cycle generating a perpetuum mobile, or migrate along capillaries and invade brain as previously described[116,117]. Alternatively, due to the mesenchymal stem cell potential of pericytes, these pericyte-like cancer cells can acquire mesenchymal traits and progress towards a more aggressive mesenchymal phenotype. The transdifferentiation potential of pericyte-like cancer cells could in turn participate to the cellular heterogeneity found in glioblastoma multiforme. Since CD133 is not detected in pericytes, the existence of CD133- pericyte-like cancer stem cells provides an issue to the controversy regarding the existence in glioma tumors of both CD133+ and CD133- cancer stem cells[118,119]. Note that this model is not exclusive. The transformation of a glial or neural stem cell might also generate cancer initiating cells.
CONCLUSION
Since the first observation of pericyte cells by Rouget[87], it has been a long road and winding road to get here. For many years, pericytes have been largely under-recognized and considered only as supportive cells of the vasculature. Their active role in angiogenesis and in cell-cell interactions with endothelial cells and astrocytes, as well as their in vitro stem cell functions, has only recently emerged. However, much remains to be done for a better understanding of the in vivo pericyte potential. For example, can pericytes/MSCs be considered as mobile “drugstores” migrating and delivering factors at the sites of injury[88]? Is the pericyte/MSC transdifferentiation potential an in vitro artifact or is it physiologically relevant? Is it an ancient feature of more primitive organisms which has been lost during the course of evolution and which is now reactivated in vitro? Alternatively, could it be an emerging evolutionary trait already engaged in vivo in some regenerative processes? Is the neural transdifferentiation potential of brain pericyte/MSC only efficient for repairing micro-lesions, which could explain why our current experimental paradigms which generate large infarcts might not be adequate to detect this potential? Do brain pericytes/MSCs behave like “sleeping beauties” awaiting the right physiological or pharmaceutical inducers for expressing their transdifferentiating and regenerative potentials? Conversely is the perversion of this potential involved in some brain tumors? The answers to these questions promise to be fascinating.
Footnotes
Supported by INSERM and the Ligue contre le Cancer Isère-Rhône Alpes
P- Reviewers: Cardinale V, Gassler N S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 3.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 8.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 9.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada N, Gronthos S, Bartold PM. Immunomodulatory effects of stem cells. Periodontol 2000. 2013;63:198–216. doi: 10.1111/prd.12024. [DOI] [PubMed] [Google Scholar]
- 11.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Péault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najar M, Raicevic G, Fayyad-Kazan H, De Bruyn C, Bron D, Toungouz M, Lagneaux L. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev. 2012;8:1188–1198. doi: 10.1007/s12015-012-9408-1. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan JM, Youd ME, Lodie TA. Immunomodulatory activity of mesenchymal stem cells. Curr Stem Cell Res Ther. 2011;6:297–316. doi: 10.2174/157488811797904353. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa FE, Carrión F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res. 2012;45:269–277. doi: 10.4067/S0716-97602012000300008. [DOI] [PubMed] [Google Scholar]
- 16.Greish S, Abogresha N, Abdel-Hady Z, Zakaria E, Ghaly M, Hefny M. Human umbilical cord mesenchymal stem cells as treatment of adjuvant rheumatoid arthritis in a rat model. World J Stem Cells. 2012;4:101–109. doi: 10.4252/wjsc.v4.i10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35:213–221. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 18.Rastegar F, Shenaq D, Huang J, Zhang W, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, et al. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J Stem Cells. 2010;2:67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi JG, Fu WJ, Wang XX, Xu YD, Li G, Hong BF, Hu K, Cui FZ, Wang Y, Zhang X. Transdifferentiation of human adipose-derived stem cells into urothelial cells: potential for urinary tract tissue engineering. Cell Tissue Res. 2012:Epub ahead of print. doi: 10.1007/s00441-011-1317-0. [DOI] [PubMed] [Google Scholar]
- 20.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 21.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaillie CM. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11854–11860. doi: 10.1073/pnas.1834196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Long Q, Jia C, Liu Y, Yi X, Yang H, Fei Z, Liu W. Over-expression of Mash1 improves the GABAergic differentiation of bone marrow mesenchymal stem cells in vitro. Brain Res Bull. 2013;99:84–94. doi: 10.1016/j.brainresbull.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Tu J, Yang F, Wan J, Liu Y, Zhang J, Wu B, Liu Y, Zeng S, Wang L. Light-controlled astrocytes promote human mesenchymal stem cells toward neuronal differentiation and improve the neurological deficit in stroke rats. Glia. 2014;62:106–121. doi: 10.1002/glia.22590. [DOI] [PubMed] [Google Scholar]
- 28.Tropel P, Platet N, Platel JC, Noël D, Albrieux M, Benabid AL, Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 29.Fu L, Zhu L, Huang Y, Lee TD, Forman SJ, Shih CC. Derivation of neural stem cells from mesenchymal stemcells: evidence for a bipotential stem cell population. Stem Cells Dev. 2008;17:1109–1121. doi: 10.1089/scd.2008.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexanian AR, Maiman DJ, Kurpad SN, Gennarelli TA. In vitro and in vivo characterization of neurally modified mesenchymal stem cells induced by epigenetic modifiers and neural stem cell environment. Stem Cells Dev. 2008;17:1123–1130. doi: 10.1089/scd.2007.0212. [DOI] [PubMed] [Google Scholar]
- 31.Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 32.Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–328. doi: 10.1016/j.expneurol.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 34.Zhang HT, Liu ZL, Yao XQ, Yang ZJ, Xu RX. Neural differentiation ability of mesenchymal stromal cells from bone marrow and adipose tissue: a comparative study. Cytotherapy. 2012;14:1203–1214. doi: 10.3109/14653249.2012.711470. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS One. 2011;6:e19808. doi: 10.1371/journal.pone.0019808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mora-Lee S, Sirerol-Piquer MS, Gutiérrez-Pérez M, Gomez-Pinedo U, Roobrouck VD, López T, Casado-Nieto M, Abizanda G, Rabena MT, Verfaille C, et al. Therapeutic effects of hMAPC and hMSC transplantation after stroke in mice. PLoS One. 2012;7:e43683. doi: 10.1371/journal.pone.0043683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang MJ, Sun JJ, Qian L, Liu Z, Zhang Z, Cao W, Li W, Xu Y. Human umbilical mesenchymal stem cells enhance the expression of neurotrophic factors and protect ataxic mice. Brain Res. 2011;1402:122–131. doi: 10.1016/j.brainres.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu Z, Wang P, Zhao C, Bi J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res Ther. 2013;4:76. doi: 10.1186/scrt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutiérrez-Fernández M, Rodríguez-Frutos B, Alvarez-Grech J, Vallejo-Cremades MT, Expósito-Alcaide M, Merino J, Roda JM, Díez-Tejedor E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. doi: 10.1016/j.neuroscience.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 41.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JR, Cheng GY, Sheu CC, Tseng GF, Wang TJ, Huang YS. Transplanted bone marrow stromal cells migrate, differentiate and improve motor function in rats with experimentally induced cerebral stroke. J Anat. 2008;213:249–258. doi: 10.1111/j.1469-7580.2008.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Eckert MA, Vu Q, Xie K, Yu J, Liao W, Cramer SC, Zhao W. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1322–1334. doi: 10.1038/jcbfm.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, Chen FF, Jiang XD. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456:120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004;77:192–204. doi: 10.1002/jnr.20147. [DOI] [PubMed] [Google Scholar]
- 49.Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004;77:174–191. doi: 10.1002/jnr.20148. [DOI] [PubMed] [Google Scholar]
- 50.Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 51.Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci USA. 2003;100:2088–2093. doi: 10.1073/pnas.0337659100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 53.Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, Rameshwar P. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1 alpha. Stem Cells. 2005;23:383–391. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- 54.Paul G, Özen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7:e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Covas DT, Panepucci RA, Fontes AM, Silva WA, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 58.Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 59.Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 60.Schor AM, Canfield AE, Sutton AB, Arciniegas E, Allen TD. Pericyte differentiation. Clin Orthop Relat Res. 1995;(313):81–91. [PubMed] [Google Scholar]
- 61.Brachvogel B, Moch H, Pausch F, Schlötzer-Schrehardt U, Hofmann C, Hallmann R, von der Mark K, Winkler T, Pöschl E. Perivascular cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development. 2005;132:2657–2668. doi: 10.1242/dev.01846. [DOI] [PubMed] [Google Scholar]
- 62.Paquet-Fifield S, Schlüter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–2806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM. The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Péault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ergün S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal. 2011;15:981–995. doi: 10.1089/ars.2010.3507. [DOI] [PubMed] [Google Scholar]
- 66.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–S15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 69.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 72.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 73.Montiel-Eulefi E, Nery AA, Rodrigues LC, Sánchez R, Romero F, Ulrich H. Neural differentiation of rat aorta pericyte cells. Cytometry A. 2012;81:65–71. doi: 10.1002/cyto.a.21152. [DOI] [PubMed] [Google Scholar]
- 74.Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, Kuroda J, Kamouchi M, Kitazono T. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res. 2012;83:352–359. doi: 10.1016/j.mvr.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Nakagomi T, Molnár Z, Nakano-Doi A, Taguchi A, Saino O, Kubo S, Clausen M, Yoshikawa H, Nakagomi N, Matsuyama T. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev. 2011;20:2037–2051. doi: 10.1089/scd.2011.0279. [DOI] [PubMed] [Google Scholar]
- 76.Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 77.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 79.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 80.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scully S, Francescone R, Faibish M, Bentley B, Taylor SL, Oh D, Schapiro R, Moral L, Yan W, Shao R. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J Neurosci. 2012;32:12950–12960. doi: 10.1523/JNEUROSCI.2017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 83.von Bülow C, Hayen W, Hartmann A, Mueller-Klieser W, Allolio B, Nehls V. Endothelial capillaries chemotactically attract tumour cells. J Pathol. 2001;193:367–376. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH810>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 84.Komatani H, Sugita Y, Arakawa F, Ohshima K, Shigemori M. Expression of CXCL12 on pseudopalisading cells and proliferating microvessels in glioblastomas: an accelerated growth factor in glioblastomas. Int J Oncol. 2009;34:665–672. doi: 10.3892/ijo_00000192. [DOI] [PubMed] [Google Scholar]
- 85.Salmaggi A, Gelati M, Pollo B, Frigerio S, Eoli M, Silvani A, Broggi G, Ciusani E, Croci D, Boiardi A, et al. CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. J Neurooncol. 2004;67:305–317. doi: 10.1023/b:neon.0000024241.05346.24. [DOI] [PubMed] [Google Scholar]
- 86.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 87.Rouget C. Note sur le developpement de la tunique contractile des vaisseaux. Compt Rend Acad Sci. 1874;59:559–562. [Google Scholar]
- 88.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hagmann S, Moradi B, Frank S, Dreher T, Kämmerer PW, Richter W, Gotterbarm T. Different culture media affect growth characteristics, surface marker distribution and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. BMC Musculoskelet Disord. 2013;14:223. doi: 10.1186/1471-2474-14-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M, Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liesveld JL, Winslow JM, Frediani KE, Ryan DH, Abboud CN. Expression of integrins and examination of their adhesive function in normal and leukemic hematopoietic cells. Blood. 1993;81:112–121. [PubMed] [Google Scholar]
- 92.Schwartz PH, Bryant PJ, Fuja TJ, Su H, O’Dowd DK, Klassen H. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J Neurosci Res. 2003;74:838–851. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- 93.Gunji Y, Nakamura M, Hagiwara T, Hayakawa K, Matsushita H, Osawa H, Nagayoshi K, Nakauchi H, Yanagisawa M, Miura Y. Expression and function of adhesion molecules on human hematopoietic stem cells: CD34+ LFA-1- cells are more primitive than CD34+ LFA-1+ cells. Blood. 1992;80:429–436. [PubMed] [Google Scholar]
- 94.Airas L, Hellman J, Salmi M, Bono P, Puurunen T, Smith DJ, Jalkanen S. CD73 is involved in lymphocyte binding to the endothelium: characterization of lymphocyte-vascular adhesion protein 2 identifies it as CD73. J Exp Med. 1995;182:1603–1608. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vogel W, Grünebach F, Messam CA, Kanz L, Brugger W, Bühring HJ. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88:126–133. [PubMed] [Google Scholar]
- 97.Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol. 1988;141:1925–1933. [PubMed] [Google Scholar]
- 98.George F, Poncelet P, Laurent JC, Massot O, Arnoux D, Lequeux N, Ambrosi P, Chicheportiche C, Sampol J. Cytofluorometric detection of human endothelial cells in whole blood using S-Endo 1 monoclonal antibody. J Immunol Methods. 1991;139:65–75. doi: 10.1016/0022-1759(91)90352-g. [DOI] [PubMed] [Google Scholar]
- 99.Chitteti BR, Bethel M, Kacena MA, Srour EF. CD166 and regulation of hematopoiesis. Curr Opin Hematol. 2013;20:273–280. doi: 10.1097/MOH.0b013e32836060a9. [DOI] [PubMed] [Google Scholar]
- 100.Smith FO, Rauch C, Williams DE, March CJ, Arthur D, Hilden J, Lampkin BC, Buckley JD, Buckley CV, Woods WG, et al. The human homologue of rat NG2, a chondroitin sulfate proteoglycan, is not expressed on the cell surface of normal hematopoietic cells but is expressed by acute myeloid leukemia blasts from poor-prognosis patients with abnormalities of chromosome band 11q23. Blood. 1996;87:1123–1133. [PubMed] [Google Scholar]
- 101.Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, Bron D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 102.Corselli M, Chin CJ, Parekh C, Sahaghian A, Wang W, Ge S, Evseenko D, Wang X, Montelatici E, Lazzari L, et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121:2891–2901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 104.Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 105.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 106.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 107.Kobari L, Giarratana MC, Pflumio F, Izac B, Coulombel L, Douay L. CD133+ cell selection is an alternative to CD34+ cell selection for ex vivo expansion of hematopoietic stem cells. J Hematother Stem Cell Res. 2001;10:273–281. doi: 10.1089/15258160151134980. [DOI] [PubMed] [Google Scholar]
- 108.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bühring HJ, Ullrich A, Schaudt K, Müller CA, Busch FW. The product of the proto-oncogene c-kit (P145c-kit) is a human bone marrow surface antigen of hemopoietic precursor cells which is expressed on a subset of acute non-lymphoblastic leukemic cells. Leukemia. 1991;5:854–860. [PubMed] [Google Scholar]
- 110.Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. 1995;15:1229–1239. doi: 10.1161/01.atv.15.8.1229. [DOI] [PubMed] [Google Scholar]
- 112.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 113.Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Péault B. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 114.Jones TR, Kao KJ, Pizzo SV, Bigner DD. Endothelial cell surface expression and binding of factor VIII/von Willebrand factor. Am J Pathol. 1981;103:304–308. [PMC free article] [PubMed] [Google Scholar]
- 115.Appaix F, Girod S, Boisseau S, Römer J, Vial JC, Albrieux M, Maurin M, Depaulis A, Guillemain I, van der Sanden B. Specific in vivo staining of astrocytes in the whole brain after intravenous injection of sulforhodamine dyes. PLoS One. 2012;7:e35169. doi: 10.1371/journal.pone.0035169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lugassy C, Haroun RI, Brem H, Tyler BM, Jones RV, Fernandez PM, Patierno SR, Kleinman HK, Barnhill RL. Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol. 2002;24:473–478. doi: 10.1097/00000372-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 117.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 118.Wang J, Sakariassen PØ, Tsinkalovsky O, Immervoll H, Bøe SO, Svendsen A, Prestegarden L, Røsland G, Thorsen F, Stuhr L, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 119.Beier CP, Beier D. CD133 negative cancer stem cells in glioblastoma. Front Biosci (Elite Ed) 2011;3:701–710. doi: 10.2741/e280. [DOI] [PubMed] [Google Scholar]


