Abstract
Acute myeloid leukemia (AML) represents a heterogeneous group of high-grade myeloid neoplasms of the elderly with variable outcomes. Though remission-induction is an important first step in the management of AML, additional treatment strategies are essential to ensure long-term disease-free survival. Recent pivotal advances in understanding the genetics and molecular biology of AML have allowed for a risk-adapted approach in its management based on relapse-risk. Allogeneic hematopoietic cell transplantation (allo-HCT) represents an effective therapeutic strategy in AML providing the possibility of cure with potent graft-versus-leukemia reactions, with a demonstrable survival advantage in younger patients with intermediate- or poor-risk cytogenetics. Herein we review the published data regarding the role of allo-HCT in adults with AML. We searched MEDLINE/PubMed and EMBASE/Ovid. In addition, we searched reference lists of relevant articles, conference proceedings and ongoing trial databases. We discuss the role of allo-HCT in AML patients stratified by cytogenetic- and molecular-risk in first complete remission, as well as allo-HCT as an option in relapsed/refractory AML. Besides the conventional sibling and unrelated donor allografts, we review the available data and recent advances for alternative donor sources such as haploidentical grafts and umbilical cord blood. We also discuss conditioning regimens, including reduced intensity conditioning which has broadened the applicability of allo-HCT. Finally we explore recent advances and future possibilities and directions of allo-HCT in AML. Practical therapeutic recommendations have been made where possible based on available data and expert opinion.
Keywords: Acute myeloid leukemia, Allogeneic hematopoietic cell transplantation, Reduced intensity conditioning, Myeloablative conditioning, Haploidentical, Umbilical cord blood
Core tip: Acute myeloid leukemia (AML) represents a heterogeneous group of high-grade myeloid neoplasms of the elderly with variable outcomes. We discuss the role of allo-hematopoietic cell transplantation (HCT) in AML patients stratified by cytogenetic- and molecular-risk in first complete remission, as well as allo-HCT as an option in relapsed/refractory AML.
INTRODUCTION
Acute myeloid leukemia (AML) comprises a group of high-grade clonal neoplasms of the myeloid progenitor cells. With a median age of 66 years, AML is a disease of the older age group with an annual incidence of 4.4 per 100000. It is estimated that approximately 15000 new cases of AML will be diagnosed in the United States in 2013[1]. While the goal of initial therapy in AML is attaining complete remission (CR), without additional post-remission therapy disease relapse is inevitable in vast majority of the cases[2]. In the past two decades little has changed in AML induction chemotherapy regimens, but our improved understanding of the disease biology in identifying high-risk groups with modern cytogenetics and molecular testing have led to better risk-stratification that facilitates customization of post-remission therapy based on the relapse-risk[3-5]. While allogeneic hematopoietic cell transplantation (allo-HCT) has been long considered a potentially curative therapy for AML[6], advances in human leukocyte antigen (HLA)-matching, supportive care, optimal pre-transplant conditioning and advent of alternative donor allografting have broadened the availability and improved transplant outcomes[7]. Herein we review the role of allo-HCT in adults with AML in first complete remission (CR1), discuss the allograft options in advanced AML (beyond CR1), and review the current state of reduced-intensity and alternative donor allo-HCT in the management of AML.
PROGNOSTIC FACTORS IN AML
Traditionally used prognostic factors in AML include age, leukocyte count at diagnosis, performance status, extra-medullary involvement, antecedent hematologic disorders and initial response to therapy. Cytogenetics by metaphase and interphase analysis are one of the most powerful prognostic factors in AML, providing us the ability to risk-stratify patients at diagnosis. Acute promyelocytic leukemeia t(15;17) and core binding factor (CBF) leukemia t(8;21) and inv(16)/t(16;16) are favorable-risk AML, largely retaining their good prognosis even with additional cytogenetic abnormalities[8-10]. Chromosomal abnormalities conferring poor outcomes include abnormalities of chromosome 3q (abnl 3q), deletions of 5q (-5q), monosomies of chromosome 5 or 7 (-5/-7), and complex karyotype. Large cooperative group studies have confirmed the impact of cytogenetics on survival rates, reporting 55%-65% and 5%-14% 5-year overall survival (OS) for patients with favorable- and poor-risk cytogenetics, respectively[8,11,12]. Grimawade et al[10] reported outcomes in 5876 patients treated on Medical Research Council (MRC) trials and identified abnl 3q (excluding t(3;5)(q25;q34)), inv(3)(q21q26)/t(3;3)(q21;q26), add5q/-5q, -5, -7, add(7q)/-7q, t(6;11)(q27;q23), t(10;11)(p11;13;q23), other t(11q23) (excluding t(9;11)(p21;22;q23) and t(11;19)(q23;p13)), t(9;22)(q34;q11), -17, abnl(17p) and complex karyotype as poor risk cytogenetic aberrations. Presence of monosomal karyotype (defined as 2 or more autosomal monosomies or combination of 1 monosomy with structural abnormalities) is associated with very poor prognosis with 4-year OS < 5%[13,14]. Similarly, the presence of subclones within the poor risk cytogenetic category (i.e., clonal heterogeneity) may confer poorer outcomes[15]. Recently, Middeke et al[16] found the presence of abnl(17p) and -5/-5q, within complex and monosomal karyotype AML characterized ultra high-risk disease.
Work done in the last decade has further enhanced our ability to stratify cytogenetically normal AML (CN-AML) based on presence of molecular aberrations into poor-risk [e.g., FMS-like tyrosine kinase 3 gene-internal tandem duplication (FLT3-ITD), mixed-lineage leukemia gene-partial tandem duplication (MLL-PTD), overexpression of Wilms’ tumor gene 1 (WT1), brain and acute leukemia, cytoplasmic gene (BAALC), ETS-related gene (ERG), KIT-gene and ecotropic viral integration site 1 gene (EVI1)] and good-risk [nucleophosmin (NPM1), isocitrate dehydrogenase (IDH 1/2) and CCAAT enhancer binding protein alpha (CEPBA)] categories[5,17-23]. Integrating conventional cytogenetics and the commonly utilized molecular testing markers (FLT3-ITD, CEBPA and NPM1), the European LeukemiaNet validated the effect of prognostic factors on remission rates, disease-free survival (DFS) and OS (Table 1)[24,25]. The improved understanding of the molecular basis of AML and its ramifications on patient outcomes has important relevance in clinical decision making, heralding the era of “individualized” post-remission therapy (Figure 1).
Table 1.
The European LeukemiaNet Standardized Reporting System for risk stratification of acute myeloid leukemia based on cytogenetics and molecular testing1
| Risk category | Cytogenetic abnormalities | Molecular abnormalities |
| Favorable risk | t(15;17) inv(16)/t(16;16)2t(8;21)2 | CN-AML with biallelic CEBPA mutationCN-AML with NPM1 mutated but FLT3-ITD negative |
| Intermediate risk | CN-AMLt(9;11)All others abnormalities not classified as favorable or adverse risk | CN-AML with:NPM1 mutated/FLT3-ITD positiveNPM1 wild type/FLT3-ITD negativet(8;21)/inv (16) with c-KIT mutation |
| Adverse risk | inv (3)/t(3;3) | CN-AML with FLT3-ITD positive |
| t(6;9) | ||
| t(v;11)/MLL rearranged | ||
| - 5/-5q | ||
| -7 | ||
| Monosomal karyotype | ||
| Abnormal 17p | ||
| Complex cytogenetics |
1Table modified from Mrózek et al[24];
The good prognosis of inv(16) and t(8;21) is maintained even with additional cytogenetic abnormalities. The presence of concomitant c-KIT mutation may increase relapse risk in t(8;21) and to lesser extend inv(16). CN-AML: Cytogenetically normal acute myeloid leukemia; CEBPA: CCAAT enhancer binding protein alpha; FLT3-ITD: FMS-like tyrosine kinase 3 gene-internal tandem duplication; MLL: Mixed lineage leukemia; NPM: Nucleophosmin.
Figure 1.
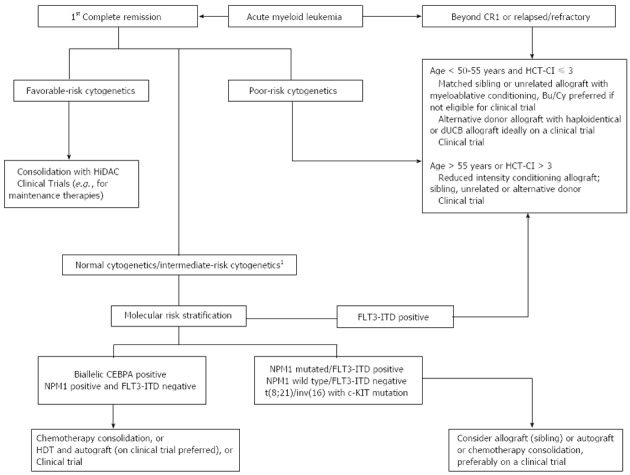
Clinically useful algorithm for optimal consolidation for acute myeloid leukemia patients based on cytogenetic and molecular genetic aberrations, based on available data and practice preference. 1Allogeneic HCT may be considered in medically fit AML patients with intermediate risk/normal cytogenetics in CR1. Bu/Cy: Busulfan/cyclophosphamide; CEBPA: CCAAT enhancer binding protein alpha; CR: Complete remission; dUCB: Double umbilical cord blood; FLT3-ITD: FMS-like tyrosine kinase 3 gene-internal tandem duplication; HCT-CI: Hematopoietic cell transplantation-comorbidity index; HDT: High dose therapy; HiDAC: High dose cytarabine; NPM: Nucleophosmin.
CONSOLIDATION WITH ALLOGENEIC HCT IN CR1
Remission induction reduces the leukemic burden roughly from 1 × 1012 cells to approximately 1 × 109 cells, if the patient achieves a morphologic CR. Hence additional consolidative therapy is necessary to eradicate a sizeable leukemic clone in patients in morphologic CR to achieve long-term DFS. Generally using chemotherapy-based consolidation approaches alone, the relapse rates in intermediate- and poor-risk cytogenetic groups remain unacceptably high[26] and represent an area where alternative consolidation approaches are warranted. Allogeneic HCT for patients in CR, not only provides a “tumor-free” graft, but more importantly the donor effector T-cells recognize and mount an effective immune response against the leukemia cells [i.e., the graft-versus leukemia (GVL) effect], to provide patients with durable disease control. While the potent GVL effects of allogeneic HCT provide the most effective post-remission therapy for AML patients in CR1, the associated morbidity and mortality warrants careful selection of high-risk patients, likely to benefit the most from this approach, and sparing the toxicity in lower-risk cohorts.
SIBLING DONOR ALLOGENEIC HCT IN CR1
Prospective single institution studies comparing allo-HCT with consolidation chemotherapy (CC) in the 1980s and early 1990s showed lower relapse rates and improved DFS with allo-HCT for AML patients in CR1, but none conclusively demonstrated an OS advantage[27,28]. Subsequently, six cooperative group trials (Table 2) have examined the role of allo-HCT in AML in CR1[28-33]. Those with HLA-matched siblings were offered allo-HCT (“genetic randomization”) while the others were randomized to autologous transplantation or CC on an intention-to-treat analysis. In the European Organization for Research and Treatment of Cancer (EORTC)-Gruppo Italiano Malattie Ematologiche Maligne Ddell’Adulto (GIMEMA) trial[29], superior 4 year DFS was noted with allo-HCT (55%) and autologous HCT (48%) compared to CC (30%). However, no OS improvement was seen with either transplant modality[34]. In the Groupe Ouest-Est Leucémies Aigues Myeloblastiques study, the relapse rates following allo-HCT were unusually high (37% at 4 years) and likely explain the lack of therapeutic benefit with allografting in this study[30]. The MRC reported improved DFS but not OS in the MRC AML-10 patients randomized to allo-HCT[31]. Similarly the US intergroup trial showed that the higher treatment related mortality (TRM) in patients randomized to allo-HCT arm negated the benefits of lower relapse rates in this group, resulting in no net OS advantage with transplantation in CR1 over chemotherapy alone[28]. Although provocative, the data from these cooperative group trials failed to provide any concrete guideline for selecting the optimal post-remission strategy for individual patients with a matched sibling donor available in CR1.
Table 2.
Cooperative group trial of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first complete remission
| Cooperative group |
Relapse rate |
Disease free survival |
Overall survival |
||||||
| Allo | Auto | CC | Allo | Auto | CC | Allo | Auto | CC | |
| EORTC/GIMEMA AML-8 | 24%1 | 41% | 57% | 55%1 | 48%1 | 30% | 59% | 56% | 46% |
| GOELAM | 37% | 45% | 55% | 49% | 48% | 43% | 55% | 52% | 58% |
| ECOG/CALGB/SWOG | 29%1 | 48% | 61% | 43% | 34% | 34% | 46% | 43% | 52%1 |
| EORTC/GIMEMA AML-10 | 30%1 | 52% | - | 52%1 | 42% | - | 58% | 50% | - |
| UK MRC AML-1023 | 36%1 | 52% | 50%1 | 42% | 55% | 42% | |||
| HOVON-SAKK3 | 32%1 | 59% | 48%1 | 37% | 54% | 46% | |||
Represents statistically significant and favorable outcome with the treatment modality;
The 4-year relapse rate, disease free survival and overall survival shown in all studies, except the UK-MRC AML-10 which reported 7-year outcomes;
All studies designed to compare outcomes between allograft vs autograft vs consolidation chemotherapy except the UK MRC AML-10 and HOVON-SAKK trial which did not differentiate between autograft and chemotherapy. Allo: Allogeneic transplantation; auto: Autologous transplantation; CC: Consolidation chemotherapy.
Impact of cytogenetic and molecular markers on allo-HCT in CR1
Integrating information regarding cytogenetic-risk categories in the outcome analysis of aforementioned cooperative group trials was the next logical step. Reanalysis of the EORTC/GIMEMA AML-10 trial by cytogenetic-risk stratification showed superior DFS (43% vs 18%) and OS (50% vs 29%) with allo-HCT compared to autografting in patients with poor-risk cytogenetics[32]. However allo-HCT did not benefit patients with good-risk [t(8;21), inv(16)] or intermediate-risk (normal or -Y) cytogenetics. Similar cytogenetic-risk stratification of the US intergroup trial showed a 5-year OS of 44%, 13% and 15% with allo-HCT, autologous-HCT and CC respectively, in patients with poor-risk cytogenetics[12]. No improvement in OS was observed in patients with good or intermediate-risk disease. Unlike the prior studies, the Dutch-Belgian Haemato-Oncology Co-operative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) trial demonstrated superior DFS with allo-HCT for both intermediate and poor cytogenetic-risk patients[33]. It may be noted that risk stratification in the HOVON-SAKK trial included additional variables. Patients with intermediate-risk cytogenetics requiring two induction cycles to achieve CR1 were classified as poor-risk, only t(8;21) AML patients with a white blood cell count of < 20 × 109/L were considered favorable and patients with unknown cytogenetics (n = 89) were considered intermediate-risk group. Two separate meta-analyses conducted by the HOVON-SAKK group and Koreth et al[35] have confirmed survival benefit with allo-HCT in patients with intermediate- and poor-risk cytogenetics in CR1. Allogeneic HCT in CR1 also appears to improved DFS and OS in AML with monosomal karyotype, compared to other consolidation strategies.
Recognition of the prognostic value of additional molecular markers is facilitating further risk stratification of the heterogeneous group of patients with CN-AML. The German-Austrian Acute Myeloid Leukemia Study Group showed that transplantation might have an important role in a molecular subset of patients with CN-AML. Patients with normal cytogenetics were randomized based on availability of an HLA-identical sibling donor for allo-HCT in CR1 vs chemotherapy alone. No benefit of allogeneic transplantation was seen in patients whose leukemia was NPM1 mutated without FLT3-ITD. Conversely, patients with the FLT3-ITD mutation or the genotype consisting of wild-type NPM1 and CEBPA without FLT3-ITD, benefited from an allogeneic transplant performed during CR1[36]. In double mutant CEBPA allo-HCT or autografting in CR1 improved DFS without impacting OS compared to CC[37].
Matched sibling allo-HCT in medically fit AML patients, with poor- and intermediate-risk (at least in the FLT3-ITD+ or NPM1-/CEBPA-/FLT3-ITD- subgroups) cytogenetics, who are able to achieve CR1 should be considered a standard option.
UNRELATED DONOR ALLOGENEIC HCT IN CR1
The strength of evidence presented above supports allo-HCT from a sibling donor in intermediate-/poor-risk AML in CR1. Unfortunately only approximately 25%-30% of AML patients have an HLA-identical sibling. No randomized trials have looked at unrelated donor (URD) allo-HCT for AML in CR1. Yakoub-Agha et al[38] reported similar outcomes with respect to acute graft-versus-host disease (GVHD), TRM, and OS in patients with standard-risk hematologic malignancies who received HLA-A, -B, -C, -DRB1, and -DQ (10/10) allele-matched allografts from either sibling or unrelated donors. Although randomized, prospective trials of URD transplantation for AML in CR1 are lacking, a number of retrospective studies provide evidence in support of the approach. Sierra et al[39] reported outcomes of URD transplantation in 161 AML patients at various stages of disease including 16 patients with poor-cytogenetic risk AML in CR1 with a 5-year DFS of 50%. The corresponding DFS for those undergoing allo-HCT in CR2, relapse, or primary induction failure were 28%, 7%, and 19%, respectively. Bashir et al[40] reported a 3-year OS and TRM of 78% and 15% respectively in a cohort of 44 patients (59% poor risk cytogenetics) who underwent URD allo-HCT in CR1. In a Center for International Blood and Marrow Transplant Registry (CIBMTR) analysis of 476 patients undergoing URD allo-HCT; adjusted 3-year OS, and DFS, in CR1 were 44%, and 43% respectively[41]. Interestingly, Tallman et al[42] found no difference in survival by cytogenetic-risk stratification for AML patients undergoing URD allo-HCT in CR1. However, the reported 5-year DFS of 30% in cytogenetically poor-risk AML likely represents a better outcome than with other non-HCT treatment strategies[43]. The presence of complex cytogenetics (> 3), however likely represent a high- risk group with poorer outcomes even with allo-HCT in CR[44].
European Group for Blood and Marrow Transplantation (EBMT) recently reported outcomes of 206 CN-AML patients in CR1 undergoing HLA-identical sibling or matched URD allo-HCT with reference to their FLT3-ITD status (present: n = 120, 58%; absent: n = 86, 42%)[45]. FLT3/ITD-positive patients, compared with FLT3/ITD-negative patients had higher 2-year relapse incidence (30% vs 16%, P = 0.006) and lower DFS (58% vs 71%, P = 0.04). More importantly, more than half of the patients harboring this mutation who received matched sibling or URD allo-HCT were alive and leukemia free at 2 years. URD allo-HCT in CR1 however may be associated with a higher TRM as noted in a registry study that reported trends of outcomes over the last two decades, underlining the need to carefully select patients for URD allo-HCT. For poor-risk cytogenetics and FLT3-ITD+ CN AML patients in CR1 lacking an HLA-matched sibling donor, it is certainly reasonable to consider matched URD allo-HCT.
OPTIMAL CONDITIONING REGIMENS
Myeloablative conditioning regimens (MAC) utilizing chemotherapy and/or total body irradiation (TBI) have been the basis of most of the studies discussed thus far. The two most commonly utilized MAC regimens are busulfan/cyclophosphamide (Bu/Cy) and cyclophosphamide/TBI (CY/TBI). Although prior studies showed inferior DFS and OS with Bu/Cy conditioning[46,47], a large meta-analysis did not show any difference between the two regimens with regards to survival and relapse[48]. It has been widely noted that the erratic bioavailability of oral busulfan was the likely cause inferior outcomes. Recent EBMT data comparing intravenous Bu/Cy to CY/TBI in AML found increased incidence of GVHD with TBI conditioning, and a trend towards improved TRM with Bu/Cy but no difference in DFS at 2-year[49]. A larger CIBMTR analysis clearly showed better DFS (RR = 0.70, 95%CI: 0.55-0.88, P = 0.003) and OS (RR = 0.68, 95%CI: 0.52-0.88, P = 0.003) in AML patients receiving IV, but not oral busulfan compared to TBI[50]. Similar observations (lower TRM with Bu/Cy and better OS compared to TBI-based regimens) were made in a prospective cohort study of CIBMTR[50,51]. Collectively these data suggest that in the era of pharmacokinetically driven adjustment of intravenous busulfan dosing, in younger (< 50-55 year) AML patients Bu/Cy should be considered the preferred MAC regimen for allo-HCT.
The use MAC is limited to medically fit, younger AML patients. The observed lower TRM rates using the so-called non-myeloablative (NMA) or reduced-intensity conditioning (RIC) regimens have broadened the applicability of allo-HCT to elderly patients or younger patients with comorbidities. Unlike MAC regimens; the NMA/RIC allo-HCT relies more heavily on the GVL effects to eradicate disease in the recipient. The decision to use NMA or RIC regimens for AML patients undergoing allo-HCT is not always clearly delineated, and significant variations exist in the selection criteria used by transplant centers across the globe. Sorror et al[52] evaluated the impact of a priori medical comorbidities on transplant outcomes by using the HCT-Comorbidity Index (HCT-CI), and reported significantly higher TRM rates and inferior OS in patients with an HCT-CI score of ≥ 3. While not validated in prospective clinical trials, it is increasingly becoming common practice to offer RIC allo-HCT to AML patients of advanced age (generally > 50-55 years), and/or HCT-CI > 3 (regardless of age), or with a prior history of autologous transplantation or less optimal performance status[53,54].
The acute leukemia working party of the EBMT compared transplantation outcomes for 315 RIC and 407 MAC recipients[55]. While the incidence of grade II-IV acute GVHD (22% vs 31%) and 2-year TRM (18% vs 36%) significantly favored the RIC group, more patients with RIC allograft experienced disease relapse compared to MAC regimens (41% vs 24%). The DFS and OS did not differ between the two groups. Another report noted grade II-IV acute GVHD rates and 2-year relapse rates of 40% and 39% respectively in 122 AML patients who received a RIC regimen with 2-year DFS of 44%[56]. A Spanish prospective, multicenter trial of patients with poor-risk AML/myelodysplastic syndrome reported 4-year DFS and OS rates of 43% and 45% with RIC and showed that development of chronic GVHD was strongly associated with reduced risk of relapse and improved OS and DFS, providing proof of concept for clinically relevant GVL effects with RIC allotransplantation[57].
RIC in AML has generally shown lower TRM with comparable OS and DFS to MAC regimens, but follow up is relatively short thus limiting conclusions. The ongoing prospective randomized BMT-CTN 0901 clinical trial (NCT01339910) comparing RIC regimens against MAC in AML/myelodysplastic syndrome will hopefully clarify the optimal conditioning intensity in AML. The advent of RIC allo-HCT has indeed extended the feasibility and applicability of allogeneic transplantation to include those with advanced age and multiple co-morbidities, thus offering them possibly a better chance for long term DFS.
ALTERNATIVE DONOR TRANSPLANTATION
Umbilical cord blood transplantation
For those high-risk patients who do not have an HLA-identical sibling or unrelated donor available, alternative donor sources may be necessary. Umbilical cord blood transplantation (UCBT) is an attractive alternative donor option due to its rapid and easy availability[58-62]. UCBT is associated with lower GVHD rates for the degree of HLA-disparity. In a direct comparison of outcomes in adults with hematological malignancies, Laughlin et al[61] reported no difference in TRM or relapse rates between UCBT and mismatched URD bone marrow transplantation, although outcomes were inferior to matched bone marrow allografts. Similarly, Rocha et al[59] in a study that included patients with acute leukemia who received UCB or matched URD marrow (n = 582) grafts showed no difference in TRM, relapse rate, DFS, and OS between the two groups.
The low cell dose available from individual cord blood units has been the major limitation against the widespread use of UCBT in adults with AML or other hematologic malignancies. However work done by the group in University of Minnesota has firmly established the feasibility of combining two cord blood units, in the so-called double UCBT (dUCBT), to overcome dose limitation of a single cord unit for adult patients[63]. A large multicenter collaborative effort comparing dUCBT, matched-sibling allo-HCT, matched URD allo-HCT and mismatched URD allo-HCT showed similar 5-year DFS with all 4 modalities. dUCBT was associated with lower relapse rates but higher TRM[64]. The preliminary results of Societe Française De Greffe De MoelleOsseuse Et Therapie Cellulaire and Eurocord’s multicenter phase II trial for UCBT in patients with AML were presented in abstract form[65]. At 1 year the rates of OS, DFS, relapse and TRM for the 65 AML patients on the study were 60%, 52%, 30% and 18%, respectively. The wider acceptance of UCBT has markedly extended the application of allogeneic transplantation, particularly to minority populations who are underrepresented in current volunteer donor databases.
Haploidentical transplantation
Almost all AML patients without an HLA-identical donor will find a haploidentical related (parents, sibling or children) donor. Enthusiasm for this modality was subdued early on due to the increased risks of GVHD, TRM, graft rejection and opportunistic infections. However, renewed interest in haploidentical transplants has been noted with T-cell depleted as well as unmanipulated allografts with novel strategies for GVHD prevention[66,67]. The Perrugia group reported DFS of 30%-45% in AML with rigorous ex-vivo T-cell depletion and intense myeloablative conditioning[68-70]. Although such transplantation has been demonstrated as feasible, it is associated with slow immune reconstitution and high rates of TRM, in smaller centers.
Recently, an alternative approach to haploidentical allo-HCT was developed with the addition of post-transplant cyclophosphamide to prevent GVHD and graft rejection in the setting of a marrow allograft after reduced intensity conditioning[71,72]. This approach has demonstrated promising results, including acceptable rates of TRM and severe GVHD in single- and multi-institution studies. Variations including myeloablative conditioning and use of peripheral blood grafts with post transplant cyclophosphamide treatment are being studied in prospective trials[73]. Limited retrospective data suggest comparable outcomes of matched sibling HCT, URD all-HCT and haploidentical transplantation utilizing post-transplant cyclophosphamide administration, in patients with hematological malignancies[74]. Bone Marrow Transplantation-Clinical Trials Network’s (BMT-CTN) two parallel multicenter phase II trials (BMT-CTN 0603 and BMT-CTN 0604) showed comparable 1-year OS and progression-free survival with RIC dUCBT (54% and 46%, respectively) and haploidentical bone marrow transplantation (62% and 48%, respectively) in hematological malignancies[75]. These trials have paved the way for the ongoing BMT-CTN 1101 trial (NCT01745913) randomizing patients with hematological malignancies to either haploidentical transplantation or dUCBT. This study will hopefully guide us further in choosing the optimal alternative donor source.
Continued research is needed to better define preferred conditioning regimens, methods and degree of T-cell depletion, reduce high relapse rates with haploidentical transplantation and improved delayed immune-reconstitution inherent to all alternative donor HCT. Recently, allelic polymorphism in donor natural killer-cell immunoglobulin like receptor (KIR) gene has been shown to impact allograft outcome and may play important role in donor selection, including alternative sources[76]. In centers with available expertise, alternative donor allo-HCT for carefully selected high- or intermediate-risk AML patients in CR, or those beyond CR1 is reasonable, however enrollment of such patients on any available protocols is preferred.
ALLOGENEIC-HCT FOR AML BEYOND CR1
Second complete remission (CR2)
Relapsed AML patients, who are able to achieve a second CR (CR2), typically do not enjoyed sustained responses with chemotherapy alone. A retrospective matched-pair analysis that compared the outcomes of autologous HCT versus HLA-identical sibling allo-HCT in AML CR2 (n = 288) showed that while allograft recipients had higher TRM it was offset by a much lower relapse rate leading to better OS (39% vs 30%) at 4-years[77]. Burnett et al[78] reported outcomes of 1271 patients aged 16-49 years who entered the MRC AML10, AML12, and AML15 trials and did not receive a transplant in CR1 and then subsequently relapsed. Fifty-five percent of patients who relapsed entered CR2. Sixty-seven percent of remitters received an allotransplant that delivered superior OS compared with patients who did not receive a HCT (42% vs 16%). A more-stringent assessment of a transplant by using delayed-entry (Mantel-Byar) analysis confirmed the benefit of transplant overall and within intermediate- and poor-risk groups but not the favorable-risk subgroup. Allo-HCT is the preferred option for most medically fit patients with AML in CR2, including carefully planned alternative donor allografts. For those unable to undergo an allograft (due to comorbidities, personal preference, etc.) are best treated in the context of a clinical trial when available.
Beyond CR2
Allogeneic HCT offers the best prospect of long term DFS for patients with relapsed/refractory AML beyond CR2[79,80]. Sierra et al[39] reported 5-year DFS of 50%, 28%, 27% and 7% with allo-HCT in CR1, CR2, beyond CR2 and in untreated relapse respectively. The corresponding relapse rates were 19%, 23%, 25% and 44%, respectively. A history of prior autologous transplantation adversely affects the success of a subsequent allo-HCT[79].
The first relapse of AML poses a management dilemma regarding whether to proceed directly with allo-HCT or to administer salvage chemotherapy to attain remission. Retrospective data indicate 3-year DFS rates of approximately 30% for patients transplanted in untreated first relapse[81,82]. Salvage chemotherapy generally induces subsequent CR in approximately 30% of relapsed AML patients[83]. Considering that only 35%-45% of these patients may achieve long-term DFS with allo-HCT (approximately 15% of all relapsing patients), theoretically allografting in untreated relapse may cure more patients than additional chemotherapy. However, in clinical practice the logistics of HLA-typing, identifying and evaluating potential donors, and stem cell collection generally necessitate administration of chemotherapy for disease control before transplantation. Moreover, relapse/refractory patients may not be prime candidates for myeloablative conditioning regimes that are likely required for optimal disease control to facilitate graft-versus-leukemia effect. This fact also highlights the importance of initiating the donor search in AML patients at the time of diagnosis[84,85].
Primary refractory AML
Allo-HCT likely represents the only curative option for patients with primary refractory AML[83]. Retrospective analyses have shown long-term survival in a subset of patients receiving allo-HCT for primary refractory AML[86-89]. Despite the relatively high TRM (30%-50%), the reported 3-year OS and DFS of approximately 20%-30% are encouraging for this otherwise poor prognosis group. CIBMTR reported outcomes of 1673 AML patients undergoing allo-HCT with refractory/active disease[90]. Five adverse pre-transplantation variables significantly influenced survival: first CR duration < 6 mo, circulating blasts, non-HLA-identical sibling donor, Karnofsky score < 90, and poor-risk cytogenetics. Patients who had 0 adverse factors had 42% OS at 3 years, whereas OS was 6% for a score ≥ 3. These important results highlight that allo-HCT can salvage a highly select subgroup of AML patients, who are not able to achieve a CR before transplantation. Based on promising phase I/II data, the use of novel clofarabine and busulfan conditioning is being explored in this population (NCT01457885)[91].
FUTURE DIRECTIONS
Great strides have been made in the field of AML and allo-HCT resulting in a steady increase in the number of allogeneic transplantation done for AML. Risk stratification of AML based on conventional cytogenetics and now molecular profiling has been instrumental in identifying higher-risk groups who may benefit from early allo-HCT. Studies looking at whole-genome and whole-exome sequencing have been reported[92] and this information will be vital not only in prognostication but is likely to lead to discovery of novel therapeutic targets. The cytogenetic and molecular signature of AML has become expansive and its clinical application ought to be carefully interpreted. The identification of higher-risk cytogenetic groups, novel molecular stratifications incorporating coinciding aberrations and the presence of clonal heterogeneity in poor-risk AML may allow us to better predict relapse risk, recommend allo-HCT and other strategies to improve disease control and survival in an individualized fashion. The presence of minimal residual disease (MRD) is another area of active interest that may help identify those subsets of AML with the highest risk of early relapse and thus may benefit from early interventions such as allo-HCT. This may be especially important in good-risk and intermediate-risk group AML[93]. Similarly evidence of persistent MRD post allo-HCT is a marker of poor outcomes. Such AML patients with evidence of MRD post allografting could be enrolled in trials designed to eradicate persistent low level disease (e.g., by rapid taper of immune suppression, planned/escalated donor-lymphocyte infusions, low-dose chemotherapies, or novel targeted agents, etc.).
Allogeneic HCT itself has indeed undergone tremendous advancement in the last 2 decades. High-resolution allele level HLA-typing, improvements in supportive care, use of alternative donor allograft and RIC has widely broadened the use of allo-HCT in AML. The newest concept of adoptive cellular therapy is the so-called “microtransplantation” where HLA-mismatched peripheral blood stem cells are infused into the recipient after consolidative chemotherapy with cytarabine, the hypothesis being that the alloreactive HLA-mismatched cells would not engraft, but during their transitory period will destroy AML clone without causing GVHD[94]. Concerted efforts are needed to devise strategies to prevent relapse post allo-HCT using novel maintenance or consolidation strategies (e.g., FLT3 inhibitors post allo-HCT in FLT3+ patients, hypomethylating agent administration to eradicate minimal residual disease). Rigorous research efforts in the development of novel preparative regimens able to provide better early disease control and limiting TRM are need. In this regard total marrow irradiation programs and/or immune-radioisotope-based conditioning appear promising. Additional avenues include using propylene glycol free melphalan (to limited renal toxicity), and pharmacokinetically dose busulfan (to limited organ damage, and prevent underdosing) as safer conditioning drugs. Immunological strategies to modulate patient or donor’s immune system, so that they mount response against tumor specific antigens are ongoing. Various antigens (Wilms Tumor gene, NOTCH, PR1, etc.) are being tested to develop vaccine to achieve a lasting immune response in the setting of relapsed leukemia or MRD after transplant. Newer mobilization regimens (e.g., plerixafor for sibling donor mobilization) and more effective methods to prevent GVHD[95-101] as well as increased availability of alternative-donor approaches, are ongoing and will add to our ability to cure patients with AML in the coming years.
Footnotes
P- Reviewers: Chen SS, Sharma P, Thomas X S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.American cancer society: Cancer facts and figures 2013. Atlanta, GA: American cancer society, 2013. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. Accessed Oct 24, 2013.
- 2.Cassileth PA, Harrington DP, Hines JD, Oken MM, Mazza JJ, McGlave P, Bennett JM, O’Connell MJ. Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia. J Clin Oncol. 1988;6:583–587. doi: 10.1200/JCO.1988.6.4.583. [DOI] [PubMed] [Google Scholar]
- 3.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 4.Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clift RA, Thomas ED. Follow-up 26 years after treatment for acute myelogenous leukemia. N Engl J Med. 2004;351:2456–2457. doi: 10.1056/NEJM200412023512326. [DOI] [PubMed] [Google Scholar]
- 7.Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK, Bredeson CN, Carabasi MH, Gupta V, Hale GA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 9.Byrd JC, Dodge RK, Carroll A, Baer MR, Edwards C, Stamberg J, Qumsiyeh M, Moore JO, Mayer RJ, Davey F, et al. Patients with t(8; 21)(q22; q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J Clin Oncol. 1999;17:3767–3775. doi: 10.1200/JCO.1999.17.12.3767. [DOI] [PubMed] [Google Scholar]
- 10.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 12.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 13.Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, Nieuwint A, Jotterand M, Hagemeijer A, Beverloo HB, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–4797. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood. 2010;116:2224–2228. doi: 10.1182/blood-2010-02-270330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochtler T, Stölzel F, Heilig CE, Kunz C, Mohr B, Jauch A, Janssen JW, Kramer M, Benner A, Bornhäuser M, et al. Clonal heterogeneity as detected by metaphase karyotyping is an indicator of poor prognosis in acute myeloid leukemia. J Clin Oncol. 2013;31:3898–3905. doi: 10.1200/JCO.2013.50.7921. [DOI] [PubMed] [Google Scholar]
- 16.Middeke JM, Beelen D, Stadler M, Göhring G, Schlegelberger B, Baurmann H, Bug G, Bellos F, Mohr B, Buchholz S, et al. Outcome of high-risk acute myeloid leukemia after allogeneic hematopoietic cell transplantation: negative impact of abnl(17p) and -5/5q- Blood. 2012;120:2521–2528. doi: 10.1182/blood-2012-03-417972. [DOI] [PubMed] [Google Scholar]
- 17.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, Walker H, Wheatley K, Bowen DT, Burnett AK, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 18.Döhner K, Tobis K, Ulrich R, Fröhling S, Benner A, Schlenk RF, Döhner H. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–3261. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 19.Baldus CD, Tanner SM, Kusewitt DF, Liyanarachchi S, Choi C, Caligiuri MA, Bloomfield CD, de la Chapelle A. BAALC, a novel marker of human hematopoietic progenitor cells. Exp Hematol. 2003;31:1051–1056. [PubMed] [Google Scholar]
- 20.Bergmann L, Miething C, Maurer U, Brieger J, Karakas T, Weidmann E, Hoelzer D. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90:1217–1225. [PubMed] [Google Scholar]
- 21.Marcucci G, Maharry K, Whitman SP, Vukosavljevic T, Paschka P, Langer C, Mrózek K, Baldus CD, Carroll AJ, Powell BL, et al. High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol. 2007;25:3337–3343. doi: 10.1200/JCO.2007.10.8720. [DOI] [PubMed] [Google Scholar]
- 22.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, La Starza R, Diverio D, Colombo E, Santucci A, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 23.Preudhomme C, Sagot C, Boissel N, Cayuela JM, Tigaud I, de Botton S, Thomas X, Raffoux E, Lamandin C, Castaigne S, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA) Blood. 2002;100:2717–2723. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]
- 24.Mrózek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, Metzeler KH, Schwind S, Wu YZ, Kohlschmidt J, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arcese W, Rocha V, Labopin M, Sanz G, Iori AP, de Lima M, Sirvent A, Busca A, Asano S, Ionescu I, et al. Unrelated cord blood transplants in adults with hematologic malignancies. Haematologica. 2006;91:223–230. [PubMed] [Google Scholar]
- 26.Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, Patil SR, Davey FR, Berg DT, Schiffer CA, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179. [PubMed] [Google Scholar]
- 27.Appelbaum FR, Dahlberg S, Thomas ED, Buckner CD, Cheever MA, Clift RA, Crowley J, Deeg HJ, Fefer A, Greenberg PD. Bone marrow transplantation or chemotherapy after remission induction for adults with acute nonlymphoblastic leukemia. A prospective comparison. Ann Intern Med. 1984;101:581–588. doi: 10.7326/0003-4819-101-5-581. [DOI] [PubMed] [Google Scholar]
- 28.Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, Willman C, Hurd DD, Bennett JM, Blume KG, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339:1649–1656. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 29.Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, Leoni F, Damasio E, Visani G, Papa G. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332:217–223. doi: 10.1056/NEJM199501263320403. [DOI] [PubMed] [Google Scholar]
- 30.Harousseau JL, Cahn JY, Pignon B, Witz F, Milpied N, Delain M, Lioure B, Lamy T, Desablens B, Guilhot F, et al. Comparison of autologous bone marrow transplantation and intensive chemotherapy as postremission therapy in adult acute myeloid leukemia. The Groupe Ouest Est Leucémies Aiguës Myéloblastiques (GOELAM) Blood. 1997;90:2978–2986. [PubMed] [Google Scholar]
- 31.Burnett AK, Wheatley K, Goldstone AH, Stevens RF, Hann IM, Rees JH, Harrison G. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118:385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 32.Suciu S, Mandelli F, de Witte T, Zittoun R, Gallo E, Labar B, De Rosa G, Belhabri A, Giustolisi R, Delarue R, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. 2003;102:1232–1240. doi: 10.1182/blood-2002-12-3714. [DOI] [PubMed] [Google Scholar]
- 33.Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, van Marwijk Kooy M, Wijermans P, Schouten H, Huijgens PC, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109:3658–3666. doi: 10.1182/blood-2006-06-025627. [DOI] [PubMed] [Google Scholar]
- 34.Keating S, de Witte T, Suciu S, Willemze R, Hayat M, Labar B, Resegotti L, Ferrini PR, Caronia F, Dardenne M, et al. The influence of HLA-matched sibling donor availability on treatment outcome for patients with AML: an analysis of the AML 8A study of the EORTC Leukaemia Cooperative Group and GIMEMA. European Organization for Research and Treatment of Cancer. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. Br J Haematol. 1998;102:1344–1353. doi: 10.1111/j.1365-2141.1998.896hm3674.x. [DOI] [PubMed] [Google Scholar]
- 35.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, Habdank M, Späth D, Morgan M, Benner A, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 37.Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L, Gaidzik VI, Paschka P, Corbacioglu A, Göhring G, et al. The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood. 2013;122:1576–1582. doi: 10.1182/blood-2013-05-503847. [DOI] [PubMed] [Google Scholar]
- 38.Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, Chehata S, Esperou H, Vernant JP, Michallet M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 39.Sierra J, Storer B, Hansen JA, Martin PJ, Petersdorf EW, Woolfrey A, Matthews D, Sanders JE, Storb R, Appelbaum FR, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant. 2000;26:397–404. doi: 10.1038/sj.bmt.1702519. [DOI] [PubMed] [Google Scholar]
- 40.Bashir Q, Andersson BS, Fernandez-Vina M, de Padua Silva L, Giralt S, Chiattone A, Wei W, Sharma M, Anderlini P, Shpall EJ, et al. Unrelated donor transplantation for acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17:1067–1071. doi: 10.1016/j.bbmt.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarus HM, Pérez WS, Klein JP, Kollman C, Bate-Boyle B, Bredeson CN, Gale RP, Geller RB, Keating A, Litzow MR, et al. Autotransplantation versus HLA-matched unrelated donor transplantation for acute myeloid leukaemia: a retrospective analysis from the Center for International Blood and Marrow Transplant Research. Br J Haematol. 2006;132:755–769. doi: 10.1111/j.1365-2141.2005.05947.x. [DOI] [PubMed] [Google Scholar]
- 42.Tallman MS, Dewald GW, Gandham S, Logan BR, Keating A, Lazarus HM, Litzow MR, Mehta J, Pedersen T, Pérez WS, et al. Impact of cytogenetics on outcome of matched unrelated donor hematopoietic stem cell transplantation for acute myeloid leukemia in first or second complete remission. Blood. 2007;110:409–417. doi: 10.1182/blood-2006-10-043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 44.Armand P, Kim HT, Zhang MJ, Perez WS, Dal Cin PS, Klumpp TR, Waller EK, Litzow MR, Liesveld JL, Lazarus HM, et al. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2012;18:280–288. doi: 10.1016/j.bbmt.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunet S, Labopin M, Esteve J, Cornelissen J, Socié G, Iori AP, Verdonck LF, Volin L, Gratwohl A, Sierra J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30:735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 46.Ringdén O, Remberger M, Ruutu T, Nikoskelainen J, Volin L, Vindeløv L, Parkkali T, Lenhoff S, Sallerfors B, Mellander L, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 47.Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP, Milpied N, Attal M, Michallet M, Ifrah N. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79:2578–2582. [PubMed] [Google Scholar]
- 48.Hartman AR, Williams SF, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant. 1998;22:439–443 ]. doi: 10.1038/sj.bmt.1701334. [DOI] [PubMed] [Google Scholar]
- 49.Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A, Volin L, Poire X, Aljurf M, Masszi T, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen--a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 50.Copelan EA, Hamilton BK, Avalos B, Ahn KW, Bolwell BJ, Zhu X, Aljurf M, van Besien K, Bredeson C, Cahn JY, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bredeson C, LeRademacher J, Kato K, Dipersio JF, Agura E, Devine SM, Appelbaum FR, Tomblyn MR, Laport GG, Zhu X, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122:3871–3878. doi: 10.1182/blood-2013-08-519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, Deeg HJ, Appelbaum FR, Storer B, Storb R. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1259–1268. doi: 10.1038/bmt.2010.94. [DOI] [PubMed] [Google Scholar]
- 54.Hamadani M, Mohty M, Kharfan-Dabaja MA. Reduced-intensity conditioning allogeneic hematopoietic cell transplantation in adults with acute myeloid leukemia. Cancer Control. 2011;18:237–245. doi: 10.1177/107327481101800404. [DOI] [PubMed] [Google Scholar]
- 55.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, Frassoni F, Boiron JM, Yin JL, Finke J, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 56.Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H, Cordonnier C, Rio B, Gratwohl A, Lange T, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24:444–453. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 57.Valcárcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, Sampol A, Bernal MT, Piñana JL, Vazquez L, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26:577–584. doi: 10.1200/JCO.2007.11.1641. [DOI] [PubMed] [Google Scholar]
- 58.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 59.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M, Finke J, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 60.Hwang WY, Samuel M, Tan D, Koh LP, Lim W, Linn YC. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444–453. doi: 10.1016/j.bbmt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, Fukuno K, Uchiyama M, Takasugi K, Iseki T, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 63.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, McGlave PB, Wagner JE. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, Verneris MR, Appelbaum FR, Wagner JE, Delaney C. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rio B, Chevret S, Vigouroux S, Chevallier P, Furst S, Sirvent A, Bay J, Socie G, Ceballos P, Huynh A, et al. Reduced intensity conditioning regimen prior to unrelated cord blood transplantation in patients with acute myeloid leukemia: Preliminary analysis of a prospective phase II multicentric trial on behalf of societe franaise de greffe de moelle osseuse et therapie cellulaire (SFGM-TC) and eurocord. Blood. 2010;116:911 (ASH Annual Meeting Abstracts). [Google Scholar]
- 66.Anasetti C, Perkins J, Nieder ML, Field T. Are matched unrelated donor transplants justified for AML in CR1? Best Pract Res Clin Haematol. 2006;19:321–328. doi: 10.1016/j.beha.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Anasetti C, Aversa F, Brunstein CG. Back to the future: mismatched unrelated donor, haploidentical related donor, or unrelated umbilical cord blood transplantation? Biol Blood Marrow Transplant. 2012;18:S161–S165. doi: 10.1016/j.bbmt.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 69.Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, Iacucci R, Zei T, Martelli MP, Gambelunghe C. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84:3948–3955. [PubMed] [Google Scholar]
- 70.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 71.O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, Rhubart P, Cowan K, Piantados S, Fuchs EJ. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 72.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, Morris LE, Bashey A. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18:1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, Morris LE, Solomon SR. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 75.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, Devine SM, Wingard JR, Aljitawi OS, Cutler CS, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bari R, Rujkijyanont P, Sullivan E, Kang G, Turner V, Gan K, Leung W. Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2013;31:3782–3790. doi: 10.1200/JCO.2012.47.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorin NC, Labopin M, Fouillard L, Meloni G, Frassoni F, Iriondo A, Brunet Mauri S, Goldstone AH, Harousseau JL, Reiffers J, et al. Retrospective evaluation of autologous bone marrow transplantation vs allogeneic bone marrow transplantation from an HLA identical related donor in acute myelocytic leukemia. A study of the European Cooperative Group for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 1996;18:111–117. [PubMed] [Google Scholar]
- 78.Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J, Wheatley K, Hunter A, Russell N. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013;31:1293–1301. doi: 10.1200/JCO.2011.40.5977. [DOI] [PubMed] [Google Scholar]
- 79.Breems DA, Löwenberg B. Autologous stem cell transplantation in the treatment of adults with acute myeloid leukaemia. Br J Haematol. 2005;130:825–833. doi: 10.1111/j.1365-2141.2005.05628.x. [DOI] [PubMed] [Google Scholar]
- 80.Gale RP, Horowitz MM, Rees JK, Gray RG, Oken MM, Estey EH, Kim KM, Zhang MJ, Ash RC, Atkinson K, et al. Chemotherapy versus transplants for acute myelogenous leukemia in second remission. Leukemia. 1996;10:13–19. [PubMed] [Google Scholar]
- 81.Brown RA, Wolff SN, Fay JW, Pineiro L, Collins RH, Lynch JP, Stevens D, Greer J, Herzig RH, Herzig GP. High-dose etoposide, cyclophosphamide, and total body irradiation with allogeneic bone marrow transplantation for patients with acute myeloid leukemia in untreated first relapse: a study by the North American Marrow Transplant Group. Blood. 1995;85:1391–1395. [PubMed] [Google Scholar]
- 82.Clift RA, Buckner CD, Appelbaum FR, Schoch G, Petersen FB, Bensinger WI, Sanders J, Sullivan KM, Storb R, Singer J. Allogeneic marrow transplantation during untreated first relapse of acute myeloid leukemia. J Clin Oncol. 1992;10:1723–1729. doi: 10.1200/JCO.1992.10.11.1723. [DOI] [PubMed] [Google Scholar]
- 83.Song KW, Lipton J. Is it appropriate to offer allogeneic hematopoietic stem cell transplantation to patients with primary refractory acute myeloid leukemia? Bone Marrow Transplant. 2005;36:183–191. doi: 10.1038/sj.bmt.1705038. [DOI] [PubMed] [Google Scholar]
- 84.Appelbaum FR. Hematopoietic cell transplantation beyond first remission. Leukemia. 2002;16:157–159. doi: 10.1038/sj.leu.2402345. [DOI] [PubMed] [Google Scholar]
- 85.Appelbaum FR, Pearce SF. Hematopoietic cell transplantation in first complete remission versus early relapse. Best Pract Res Clin Haematol. 2006;19:333–339. doi: 10.1016/j.beha.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Michallet M, Thomas X, Vernant JP, Kuentz M, Socié G, Espérou-Bourdeau H, Milpied N, Blaise D, Rio B, Reiffers J, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Société Française de Greffe de Moelle (SFGM) Bone Marrow Transplant. 2000;26:1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- 87.Biggs JC, Horowitz MM, Gale RP, Ash RC, Atkinson K, Helbig W, Jacobsen N, Phillips GL, Rimm AA, Ringdén O. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–1093. [PubMed] [Google Scholar]
- 88.Fung HC, Stein A, Slovak Ml, O’donnell MR, Snyder DS, Cohen S, Smith D, Krishnan A, Spielberger R, Bhatia R, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant. 2003;9:766–771. doi: 10.1016/j.bbmt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Wong R, Shahjahan M, Wang X, Thall PF, De Lima M, Khouri I, Gajewski J, Alamo J, Couriel D, Andersson BS, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–114. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 90.Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, Kamble R, Copelan E, de Lima M, Gupta V, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farag SS, Wood LL, Schwartz JE, Srivastava S, Nelson RP, Robertson MJ, Abonour R, Secrest A, Cox E, Baute J, et al. Phase I trial and pharmacokinetic study of high-dose clofarabine and busulfan and allogeneic stem cell transplantation in adults with high-risk and refractory acute leukemia. Leukemia. 2011;25:599–605. doi: 10.1038/leu.2010.319. [DOI] [PubMed] [Google Scholar]
- 92.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ossenkoppele G, Schuurhuis GJ. MRD in AML: time for redefinition of CR? Blood. 2013;121:2166–2168. doi: 10.1182/blood-2013-01-480590. [DOI] [PubMed] [Google Scholar]
- 94.Guo M, Hu KX, Liu GX, Yu CL, Qiao JH, Sun QY, Qiao JX, Dong Z, Sun WJ, Sun XD, et al. HLA-mismatched stem-cell microtransplantation as postremission therapy for acute myeloid leukemia: long-term follow-up. J Clin Oncol. 2012;30:4084–4090. doi: 10.1200/JCO.2012.42.0281. [DOI] [PubMed] [Google Scholar]
- 95.Subbiah K, Hamlin DK, Pagel JM, Wilbur DS, Meyer DL, Axworthy DB, Mallett RW, Theodore LJ, Stayton PS, Press OW. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med. 2003;44:437–445. [PubMed] [Google Scholar]
- 96.Matthews DC, Martin PJ, Nourigat C, Appelbaum FR, Fisher DR, Bernstein ID. Marrow ablative and immunosuppressive effects of 131I-anti-CD45 antibody in congenic and H2-mismatched murine transplant models. Blood. 1999;93:737–745. [PubMed] [Google Scholar]
- 97.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, Hurley C, Kollman C, Anasetti C, Noreen H, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 98.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 99.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 100.Hamadani M, Gibson LF, Remick SC, Wen S, Petros W, Tse W, Brundage KM, Vos JA, Cumpston A, Bunner P, et al. Sibling donor and recipient immune modulation with atorvastatin for the prophylaxis of acute graft-versus-host disease. J Clin Oncol. 2013;31:4416–4423. doi: 10.1200/JCO.2013.50.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, Ho VT, Cutler C, Blazar BR, Antin JH, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]


