Patients with tumors that are ER-positive 1%–9% have clinical and pathologic characteristics different from those with tumors that are ER-positive 10%. Similar to patients with ER-negative tumors, those with ER-positive 1%–9% do not appear to benefit from endocrine therapy. Further study of the clinical benefit from endocrine therapy in patients whose tumors are ER-positive 1%–9% is warranted.
Keywords: estrogen receptor, breast cancer, survival, low positive
Abstract
Background
Guidelines for the use of chemotherapy and endocrine therapy recently recommended that estrogen receptor (ER) status be considered positive if ≥1% of tumor cells demonstrate positive nuclear staining by immunohistochemistry. In clinical practice, a range of thresholds are used; a common one is 10% positivity. Data addressing the optimal threshold with regard to the efficacy of endocrine therapy are lacking. In this study, we compared patient, tumor, treatment and survival differences among breast cancer patients using ER-positivity thresholds of 1% and 10%.
Methods
The study population consisted of patients with primary breast carcinoma treated at our center from January 1990 to December 2011 and whose records included complete data on ER status. Patients were separated into three groups: ≥10% positive staining for ER (ER-positive ≥10%), 1%–9% positive staining for ER (ER-positive 1%–9%) and <1% positive staining (ER-negative).
Results
Of 9639 patients included, 80.5% had tumors that were ER-positive ≥10%, 2.6% had tumors that were ER-positive 1%–9% and 16.9% had tumors that were ER-negative. Patients with ER-positive 1%–9% tumors were younger with more advanced disease compared with patients with ER-positive ≥10% tumors. At a median follow-up of 5.1 years, patients with ER-positive 1%–9% tumors had worse survival rates than did patients with ER-positive ≥10% tumors, with and without adjustment for clinical stage and grade. Survival rates did not differ significantly between patients with ER-positive 1%–9% and ER-negative tumors.
Conclusions
Patients with tumors that are ER-positive 1%–9% have clinical and pathologic characteristics different from those with tumors that are ER-positive ≥10%. Similar to patients with ER-negative tumors, those with ER-positive 1%–9% disease do not appear to benefit from endocrine therapy; further study of its clinical benefit in this group is warranted. Also, there is a need to better define which patients of this group belong to basal or luminal subtypes.
introduction
Estrogen receptor (ER) is a prognostic factor in breast cancer and a predictor of response to endocrine therapy [1]. Recently, guidelines from the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) recommended that ER status be considered positive if 1% or more of tumor cells demonstrate positive nuclear staining on immunohistochemistry [2]. However, in routine practice, a wide range of arbitrary cutoffs in percentage of stained cells are being used (i.e. >0% [3, 4], 1% [5], 5%–10% and 20% [3]). Many clinicians consider patient eligibility for endocrine therapy with 10% or greater nuclear staining [6–10]. Results of a meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group revealed tamoxifen was ineffective with low ER expression (<10 fmol/mg by ligand-binding assay, LBA) [11]. Prospective data addressing the optimal cutoff for defining positivity based on the efficacy of endocrine therapy are lacking.
A recent study demonstrated that half of tumors staining ER-positive 1%–9% on immunohistochemistry have molecular characteristics more similar to the ER-negative, basal-like phenotype [12]. Unfortunately, limited clinical information is available for these subtypes regarding prediction of treatment effect. In principle, those retrospective results need to be validated, particularly with regard to clinical endocrine responsiveness. In this study, we examined patient, tumor and treatment differences among patients with different ER status: at least 10% of cells staining positive (ER-positive ≥10%), between 1% and 9% of cells positive (ER-positive 1%–9%) and <1% positive (ER-negative). We compared survival outcomes among patients with different ER-positivity thresholds, for the whole cohort and for subgroups based on treatment with endocrine therapy.
patients and methods
We used the Surgical Breast Oncology Database at The University of Texas MD Anderson Cancer Center to identify patients with primary invasive breast carcinoma treated at our center from January 1990 to December 2011 with known ER status. Patients presenting with recurrent or metastatic disease were excluded. This study was approved by our center's Institutional Review Board.
Hormone receptor evaluation was carried out on core biopsy or surgical specimens. When ER and PR immunohistochemistry were carried out at a referring institution, the slides were evaluated at our institution. Less than 40% of cases were referred from other centers. Largely due to a lack of availability of tumor blocks, routine re-staining for ER/PR was not done on these outside cases. When immunohistochemistry slides were not available for review, ER and PR were repeated at our institution. Multiple pathologists were involved in signing the markers. The slides from 6% of tumors were available for re-review by a single pathologist (LH) for confirmation. From 2007 to present, the polymeric biotin-free horseradish peroxidase method was used for ER staining on a Leica Bond-Max stainer (Leica Microsystems, Buffalo Grove, IL). One whole-slide 4-μm-thick unstained tissue section from a representative paraffin block of the invasive carcinoma was incubated at 60°C for 20 min. Following heat-induced epitope retrieval with citrate buffer for 30 min at 100°C, slides were incubated with mouse monoclonal antibody to ER (clone 6F11, 1:35, Novocastra Laboratories, Leica Microsystems). The Refine Polymer Detection kit was used to detect bound antibody, with 3,3-diaminobenzidine as the chromogen (Leica Microsystems). Slides were counterstained with Mayer's hematoxylin and results evaluated with positive and negative tissue controls. ER staining was carried out using antibody clone 6F11 on a DAKO autostainer (Dako North America, Inc., Carpinteria, CA) from 2002 to 2007, and using antibody clone 1D5 (Dako North America, Inc.) before 2002. Any invasive tumor cell with strong, moderate or weak nuclear staining is considered positive (supplementary Figure S1, available at Annals of Oncology online). Assessment of percentage of stained tumor cells is an estimate of the entire invasive tumor on a given slide, regardless of whether there are heterogeneously stained tumor areas.
For analysis, patients were separated into three groups: ER-positive ≥10%, ER-positive 1%–9% and ER-negative. Patient, tumor and treatment characteristics were evaluated and compared between groups. Kaplan–Meier survival curves were calculated, and log-rank test used to compare overall survival (OS) (time from surgery to death from any cause), recurrence-free survival (RFS) (time from surgery to first recurrence), and distant recurrence-free survival (DRFS) (time from surgery to death due to breast cancer or first distant recurrence) between groups. A multivariate stratified Cox proportional hazards model was used to identify significant predictors of DFS stratifying by clinical TNM stage and tumor grade. STATA statistical software (SE 9, StataCorp LP, College Station, TX) was used for statistical analyses. All P values were two tailed, and P ≤ 0.05 was considered significant.
results
patient and tumor characteristics
Of 9639 patients included in this study, 7764 (80.5%) had tumors ER-positive ≥10%, 1625 (16.9) were ER-negative and 250 (2.6%) were ER-positive 1%–9%. Median percentage of ER positivity in the 1%–9% group was 4 (mean: 3.5, range: 1–9). Of the 250 ER-positive 1%–9%, 230 (92%) were ER-positive ≤5%. For the entire cohort, median age at diagnosis was 55 years (mean 56, range: 21–99). The majority had stage I (50.5%) or II (36.5%) disease and tumors were grade II in 48.2% and grade III in 38.4%.
Patient and tumor characteristics of the three groups are summarized in Table 1. Compared with patients whose tumors were ER-positive ≥10%, those with ER-positive 1%–9% were younger (median age 53 versus 56 years, P < 0.0001), less likely to be white (74.2% versus 66.4%, P = 0.008), more likely to have ductal histology (83.6% versus 73.0%, P < 0.0001) with more advanced disease (clinical stage II/III 61.6% versus 43.7%, P < 0.0001) and more likely to receive neoadjuvant chemotherapy (47.6% versus 29.2%, P < 0.0001). They were also more likely to have HER-2-positive (27.6% versus 13.1%, P < 0.0001) and grade III (81.6% versus 27.9%, P < 0.0001) disease. Of 250 patients with ER-positive 1%–9% status, 66 (26.4%) were progesterone (PR) positive 1%–9%, while only 609 (7.9%) patients with ER-positive ≥10% status and 110 (6.8%) of patients with ER-negative tumors were PR-positive 1%–9%. Overall, 63.7% of patients had tumors that were PR-positive ≥10% and 8.1% of patients had tumors that were PR-positive 1%–9%. Compared with patients with ER-negative tumors, patients with ER-positive 1%–9% tumors had earlier stage disease and were less likely to have ductal histology.
Table 1.
Comparison of patient, tumor and pathologic factors by level of ER staining in the primary tumor
| Factors | ER staining |
||||
|---|---|---|---|---|---|
| ≥10% (n = 7764) | n = 250 | P valuea | Negative (n = 1625) | P valueb | |
| Age at diagnosis, years | |||||
| Mean | 56.6 | 51.9 | <0.0001 | 52.3 | 0.7 |
| Median (range) | 56 (21–93) | 53 (22–84) | 52 (23–99) | ||
| Race | |||||
| White | 5762 (74.2) | 166 (66.4) | 0.007 | 1058 (65.1) | 0.2 |
| Black | 1061 (13.7) | 47 (18.8) | 331 (20.4) | ||
| Hispanic | 660 (8.5) | 22 (8.8) | 173 (10.6) | ||
| Asian | 232 (3.0) | 15 (6.0) | 54 (3.3) | ||
| Others | 49 (0.6) | 0 (0) | 9 (0.6) | ||
| Clinical TNM stage | |||||
| I | 4292 (55.3) | 93 (37.2) | <0.0001c | 486 (29.9) | 0.04c |
| II | 2654 (34.2) | 114 (45.6) | 749 (46.1) | ||
| III | 741 (9.5) | 40 (16.0) | 363 (22.3) | ||
| IV | 77 (1.0) | 3 (1.2) | 27 (1.7) | ||
| Clinical tumor size, cm | |||||
| Mean | 2.3 | 2.9 | <0.0001d | 3.1 | 0.2d |
| Median (range) | 1.8 (0.03–20) | 2.5 (0.05–18) | 2.5 (0.01–38) | ||
| Histology | |||||
| IDC/DCIS | 5671 (73.0) | 209 (83.6) | <0.0001c | 1432 (88.1) | 0.001c |
| ILC/DCIS | 768 (9.9) | 11 (4.4) | 20 (1.2) | ||
| Mixed | 718 (9.3) | 12 (4.8) | 36 (2.2) | ||
| Others | 607 (7.8) | 18 (7.2) | 137 (8.4) | ||
| Tumor grade | |||||
| I | 1148 (15.0) | 7 (2.9) | <0.0001 | 16 (1.0) | 0.049c |
| II | 4380 (57.1) | 38 (15.5) | 228 (14.2) | ||
| III | 2141 (27.9) | 200 (81.6) | 1362 (84.8) | ||
| Unknown | 95 | 5 | 19 | ||
| Pathologic nodal stage | |||||
| N0 | 4828 (62.2) | 182 (72.8) | 0.003c | 1080 (66.5) | 0.3c |
| N1 | 1979 (25.5) | 40 (16.0) | 332 (20.4) | ||
| N2 | 471 (6.1) | 14 (5.6) | 96 (5.9) | ||
| N3 | 486 (6.2) | 14 (5.6) | 117 (7.2) | ||
| HER-2 status | |||||
| Positive | 890 (13.1) | 64 (27.6) | <0.0001 | 436 (28.6) | 0.8c |
| Negative | 5903 (86.9) | 168 (72.4) | 1088 (71.4) | ||
| Unknown | 971 | 18 | 101 | ||
| PR status | |||||
| Positive ≥10% | 5956 (77.0) | 38 (15.2) | <0.0001 | 149 (9.2) | <0.0001 |
| Positive 1%–9% | 609 (7.9) | 66 (26.4) | 110 (6.8) | ||
| Negative | 1169 (15.1) | 146 (58.4) | 1360 (84.0) | ||
| Unknown | 30 | 0 | 6 | ||
| Preoperative chemotherapy | |||||
| No | 5496 (70.8) | 131 (52.4) | <0.0001 | 732 (45.1) | 0.03 |
| Yes | 2268 (29.2) | 119 (47.6) | 893 (54.9) | ||
IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma; PR, progesterone receptor.
aComparisons between ≥10% and 1%–9%.
bComparisons between 1%–9% and negative.
cFisher's exact test.
dWilcoxon scores rank sum test.
Adjuvant treatments, follow-up and recurrence in the three different groups based on ER status are shown in Table 2. Patients with ER-positive 1%–9% disease were more likely to receive adjuvant chemotherapy (49.2% versus 35.5%, P < 0.0001) and less likely to receive adjuvant endocrine therapy (20.4% versus 83.6%, P < 0.0001) than patients with ER-positive ≥10% tumors. Compared with patients with ER-negative tumors, patients with ER-positive 1%–9% were more likely to receive adjuvant endocrine therapy (20.4% versus 12.9%, P = 0.002). Follow-up time was longer in patients with ER-positive tumors at ≥10%. Patients with ER-positive 1%–9% tumors were more likely to experience recurrence (17.2%) than patients with ER-positive ≥10% tumors (9.1%) (P < 0.0001), including more local, regional and distant recurrences. There was no significant difference in recurrences between patients with ER-positive 1%–9% and ER-negative tumors (19.4%) (P = 0.5). For patients receiving endocrine therapy, recurrence rates were higher in patients whose tumors were ER-positive1%–9% compared with those that were ER-positive ≥10% (17.7% versus 7.7%, P = 0.02). There was no significant difference in total recurrences between these groups for patients who did not receive endocrine therapy.
Table 2.
Adjuvant therapy, follow-up and recurrence status among patients with three different levels of ER staining
| Factors | ER staining |
||||
|---|---|---|---|---|---|
| ≥10% (n = 7764) | n = 250 | P valuea | Negative (n = 1625) | P valueb | |
| Adjuvant chemotherapy | |||||
| Yes | 2742 (35.5) | 123 (49.2) | <0.0001 | 805 (49.7) | 0.9c |
| No | 4981 (64.5) | 127 (50.8) | 815 (50.3) | ||
| Unknown | 41 | 0 | 5 | ||
| Adjuvant endocrine therapy | |||||
| Yes | 6454 (83.6) | 51 (20.4) | <0.0001 | 208 (12.9) | 0.002c |
| No | 1265 (16.4) | 199 (79.6) | 1409 (87.1) | ||
| Unknown | 45 | 0 | 8 | ||
| Adjuvant radiation therapy | |||||
| Yes | 5174 (67.1) | 160 (64.3) | 0.4c | 1134 (70.0) | 0.08c |
| No | 2536 (32.9) | 89 (35.7) | 485 (30.0) | ||
| Unknown | 54 | 1 | 6 | ||
| Follow-up time, years | |||||
| Mean | 6.2 | 4.6 | <0.0001d | 5.8 | <0.0001d |
| Median (range) | 5.4 (1–19.8) | 3.8 (1–19.3) | 5.3 (1.1–19.5) | ||
| Recurrence | |||||
| Yes | 685 (9.1) | 42 (17.2) | <0.0001 | 307 (19.4) | 0.5c |
| No | 6841 (90.9) | 202 (82.8) | 1276 (80.6) | ||
| Unknown | 238 | 6 | 42 | ||
| Local recurrence | |||||
| Yes | 181 (2.4) | 12 (5.0) | 0.01c | 91 (5.8) | 0.8c |
| No | 7313 (97.6) | 230 (95.0) | 1478 (94.2) | ||
| Unknown | 270 | 8 | 56 | ||
| Regional recurrence | |||||
| Yes | 124 (1.7) | 9 (3.7) | 0.02c | 99 (6.3) | 0.1c |
| No | 7372 (98.3) | 232 (96.3) | 1469 (93.7) | ||
| Unknown | 268 | 9 | 57 | ||
| Distant recurrence | |||||
| Yes | 561 (7.5) | 35 (14.4) | <0.0001 | 264 (16.6) | 0.4c |
| No | 6963 (92.5) | 208 (85.6) | 1323 (83.4) | ||
| Unknown | 240 | 7 | 38 | ||
| Patients who received endocrine therapy | |||||
| Recurrence | |||||
| Yes | 500 (7.7) | 9 (17.7) | 0.02c | 48 (23.1) | 0.5c |
| No | 5954 (92.3) | 42 (82.3) | 160 (76.9) | ||
| Patients who did not receive endocrine therapy | |||||
| Recurrence | |||||
| Yes | 183 (14.5) | 33 (16.6) | 0.5c | 258 (18.3) | 0.6c |
| No | 1082 (85.5) | 166 (83.4) | 1151 (81.7) | ||
aComparisons between ≥10% and 1%–10%.
bComparisons between 1%–10% and negative.
cFisher's exact test.
dWilcoxon scores rank sum test.
survival outcomes
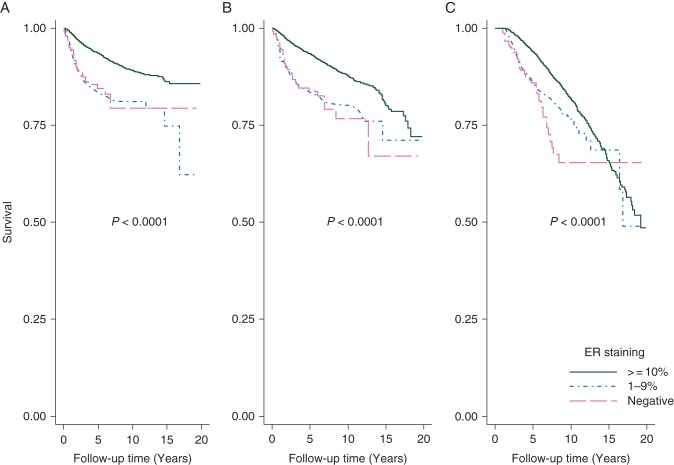
At a median follow-up of 5.1 years, patients with ER-positive tumors at 1%–9% or ER-negative tumors had worse DRFS (P < 0.0001), RFS (P < 0.0001) and OS (P < 0.0001) rates compared with ER-positive tumors at ≥10%. Patients with ER-positive tumors at 1%–9% had similar DRFS (P = 0.8), RFS (P = 0.96) and OS (P = 0.1) rates as ER-negative tumors (Figure 1).
Figure 1.
Comparison of survival outcomes among patients with three different levels of ER expression in the primary tumor: (A) distant recurrence-free survival, (B) recurrence-free survival, (C) overall survival.
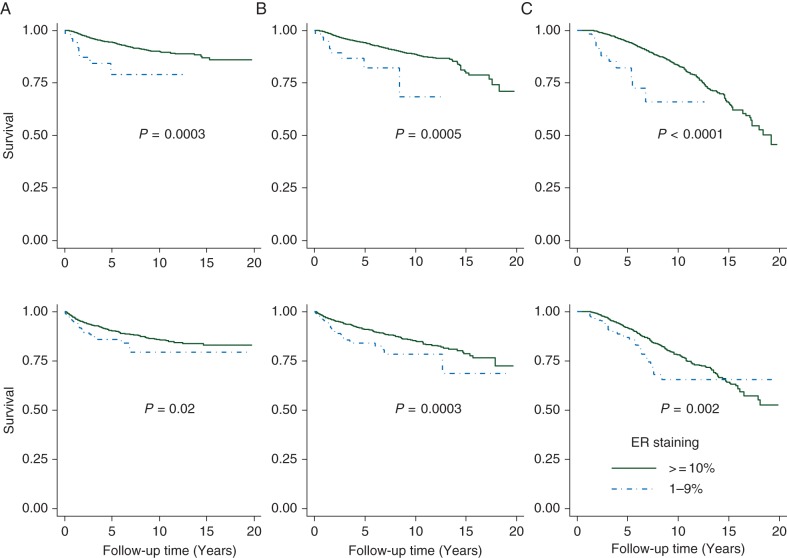
Figure 2 shows survival outcomes between patients with ER-positive tumors at 1%–9% and ER-positive tumors at ≥10% with/without endocrine therapy. Patients with ER-positive tumors at 1%–9% had worse DRFS (P = 0.0003), RFS (P = 0.0005) and OS (P < 0.0001) rates than did patients with ER-positive ≥10% tumors even in patients who received endocrine therapy (Figure 2, upper). Among patients who did not receive endocrine therapy (Figure 2, lower), those with ER-positive 1%–9% tumors had worse DRFS (P = 0.02), RFS (P = 0.0003) and OS (P = 0.0002) rates than those with ER-positive ≥10% tumors. There were no DRFS and RFS survival differences between patients with ER-positive 1%–9% tumors who received endocrine therapy and patients with ER-negative tumors who did not receive endocrine therapy (supplementary Figure S2, available at Annals of Oncology online).
Figure 2.
Comparison of survival outcomes between patients with ER-positive tumors at 1%–9% and patients with ER-positive tumors ≥10% among patients: (A) distant recurrence-free survival, (B) recurrence-free survival, (C) overall survival; upper received endocrine therapy; lower: not received endocrine therapy.
Table 3 shows the stratified Cox proportion regression models for different groups by ER positivity associated with survival outcomes. Patients with ER-positive ≥10% tumors had better DRFS, RFS and OS rates than patients with ER-positive 1%–9% tumors even when stratified by clinical stage and tumor grade. Patients with ER-negative tumors had similar DRFS and RFS rates as patients with ER-positive 1%–9% tumors when stratified by clinical stage and tumor grade.
Table 3.
Cox regression stratified model for survival outcomes among patients with different levels of ER staining in the primary tumor
| HRa | SE | P value | 95% CI |
||
|---|---|---|---|---|---|
| Distant recurrence-free survival | |||||
| ER staining | |||||
| 1%–9% | Reference | ||||
| ≥10% | 0.7 | 0.06 | <0.001 | 0.6 | 0.8 |
| Negative | 1.2 | 0.2 | 0.3 | 0.8 | 1.7 |
| Recurrence-free survival | |||||
| ER staining | |||||
| 1%–9% | Reference | ||||
| ≥10% | 0.7 | 0.06 | <0.001 | 0.6 | 0.8 |
| Negative | 1.2 | 0.2 | 0.2 | 0.9 | 1.7 |
| Overall survival | |||||
| ER staining | |||||
| 1%–9% | Reference | ||||
| ≥10% | 0.8 | 0.06 | 0.002 | 0.7 | 0.9 |
| Negative | 1.5 | 0.2 | 0.02 | 1.1 | 2.0 |
aStratified by tumor grade and clinical stage.
HR, hazard ratio; SE, standard error; CI, confidence interval.
discussion
Recent ASCO/CAP guidelines have decreased the threshold for ER positivity by immunohistochemistry to 1%. A finding of 1%–9% ER positivity is rare; our study indicates that only about 3% of breast cancers fit this category, a lower rate than other studies have reported [12, 13]. Our study shows that patients with ER-positive 1%–9% tumors have clinical and pathologic characteristics different from those with ER-positive ≥10% tumors. Similar to patients with ER-negative tumors, those with ER-positive 1%–9% disease do not appear to benefit from endocrine therapy. Our findings are consistent with reports from the Oxford Overview [11]; all 20 trials included in that meta-analysis defined values of 10 fmol/mg or greater on biochemical assays as ER-positive. The study showed little apparent benefit from adjuvant tamoxifen if ER levels were just below 10 fmol/mg, but a significant and increasing benefit with higher levels, beginning at the cutoff [11].
A number of methods have been developed to determine ER status; however, retrospective studies showed that semiquantitative immunohistochemistry analysis of ER expression was superior for prognostic and predictive purposes compared with standardized LBAs [7, 14].The heterogeneity of hormone receptor expression in breast cancer can be visualized with immunohistochemistry [15]. Tumors have variable expression ER and PR, with some cells staining positively, whereas the others do not [16]. St Gallen 2005 guidelines suggested three categories for scoring ER status: endocrine responsive, with strong ER expression; endocrine response uncertain, with low ER expression and endocrine nonresponsive, with no ER expression [17]. The boundary between ‘endocrine responsive’ and ‘endocrine response uncertain’ was not provided, although the authors suggest that tumors with 1%–10% positive cells are ‘usually considered’ to have low ER expression. This endocrine-response-uncertain group may have potential resistance to particular endocrine therapies due to lack of PR [17]. The panel suggested this group should receive endocrine therapy and adjuvant chemotherapy. In an effort to improve accuracy of hormone receptor testing by immunohistochemistry, ASCO/CAP guidelines were revised in 2010 to recommend a cutoff of 1% positive cells be used to define ER-positive status [2]. The panel recommended considering endocrine therapy when tumors show at least 1% ER-positive cells and withholding endocrine therapy if tumors had <1% ER-positive cells [2]. This would increase the proportion of patients receiving endocrine therapy; based on our study, 3% more patients could receive endocrine therapy than if a 10% threshold is used. Although updated guidelines recommend that patients whose tumors show at least 1% ER-positive cells be considered for endocrine therapy, clinicians must consider benefits of endocrine therapy versus cost and side-effects. Tamoxifen is the least expensive of all the endocrine therapies; a generic version costs about $100/month in the USA, according to Susan G. Komen for the Cure (http://ww5.komen.org/uploadedfiles/content_binaries/806–326a.pdf 3 January 2013, date last accessed). Aromatase inhibitors usually cost significantly more than tamoxifen [18]. The side-effects of tamoxifen include vasomotor symptoms, gynecologic symptoms, sexual dysfunction, and increased rates of endometrial cancer, stroke, pulmonary embolism and deep vein thrombosis [19, 20]. Aromatase inhibitors are better tolerated with fewer side-effects but are associated with increased risk of osteopenia, osteoporosis and fractures [21].
Studies have shown that the benefit of tamoxifen increases with increasing ER expression, and that there is a potential benefit for therapy in patients with as little as 1% ER expression [11, 22]. Other clinically relevant genes aside from ER are likely to affect therapeutic benefit. Oncotype DX® (Genomic Health, Redwood City, CA) is a commercial assay designed to assess recurrence probability in node-negative ER-positive breast cancers. Some have recommended Oncotype DX® replace immunohistochemistry for ER and PR. However, studies that investigated Oncotype DX and the predictive validity of the recurrence score in ER-positive breast cancer found no correlation between expression of the hormonal receptor-related genes to clinical outcome [23–25]. A recent study that compared immunohistochemistry with Oncotype DX® qRT-PCR assay for ER and PR found that immunohistochemistry is preferable to qRT-PCR for determining ER and PR expression [26].
The current study has limitations. First, we retrospectively collected data, and treatment was not assigned in a randomized fashion. Second, because of the limited sample size of patients with ER-positive 1%–9% tumors, we cannot perform subset analyses based on adjuvant chemotherapy and endocrine therapy. Also, we cannot assess predictive ability of ER at different cutoffs by examining the interaction at various cutoff points between patients who received endocrine therapy versus those who did not. Third, some patients had ER determined outside our center, and those ER slides were only reviewed and not re-stained. We cannot account for differences in ER evaluation method and heterogeneity in methodology may affect results.
In conclusion, patients with ER positive 1%–9% tumors have clinical and pathologic characteristics more similar to tumors that are ER-negative than those with ER positive ≥10% tumors. Similar to patients with ER-negative tumors, those with ER-positive 1%–9% tumors do not appear to benefit from endocrine therapy. Although the substantial benefit of endocrine therapy in ER-positive cases is indisputable, its application to patients with a low level of ER expression, specifically those with ER at 1%–9%, requires further study.
funding
This work was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant [CA016672].
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Hua Guo for her excellent technical assistance.
references
- 1.Bardou VJ, Arpino G, Elledge RM, et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 2.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Layfield LJ, Gupta D, Mooney EE. Assessment of tissue estrogen and progesterone receptor levels: a survey of current practice, techniques, and quantitation methods. Breast J. 2000;6:189–196. doi: 10.1046/j.1524-4741.2000.99097.x. [DOI] [PubMed] [Google Scholar]
- 4.Putti TC, Pinder SE, Elston CW, et al. Breast pathology practice: most common problems in a consultation service. Histopathology. 2005;47:445–457. doi: 10.1111/j.1365-2559.2005.02246.x. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Cuzick J, Wale C, et al. Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: an hypothesis-generating study. J Clin Oncol. 2005;23:7512–7517. doi: 10.1200/JCO.2005.01.4829. [DOI] [PubMed] [Google Scholar]
- 6.Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, alone or in combination trial. J Clin Oncol. 2008;26:1059–1065. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 7.Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89:111–117. [PubMed] [Google Scholar]
- 8.Regan MM, Viale G, Mastropasqua MG, et al. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98:1571–1581. doi: 10.1093/jnci/djj415. [DOI] [PubMed] [Google Scholar]
- 9.Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol. 2007;25:3846–3852. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 10.Viale G, Regan MM, Maiorano E, et al. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors—International Breast Cancer Study Group. J Clin Oncol. 2008;26:1404–1410. doi: 10.1200/JCO.2007.10.6393. [DOI] [PubMed] [Google Scholar]
- 11.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto T, Booser D, Valero V, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30:729–734. doi: 10.1200/JCO.2011.36.2574. [DOI] [PubMed] [Google Scholar]
- 13.Allred DC, Carlson RW, Berry DA, et al. NCCN Task Force Report: estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Canc Netw. 2009;7(Suppl 6):S1–S21. doi: 10.6004/jnccn.2009.0079. ; quiz S22–S23. [DOI] [PubMed] [Google Scholar]
- 14.Harvey JM, Clark GM, Osborne CK, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 15.Di Cosimo S, Baselga J. Management of breast cancer with targeted agents: importance of heterogeneity. [corrected] Nat Rev Clin Oncol. 2010;7:139–147. doi: 10.1038/nrclinonc.2009.234. [DOI] [PubMed] [Google Scholar]
- 16.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- 17.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 18.Stephan P. Compare the cost and effectiveness of tamoxifen and aromatase inhibitors. 2012 [Google Scholar]
- 19.Day R, Ganz PA, Costantino JP, et al. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 21.Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633–643. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 22.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 23.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 24.Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 25.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 26.Kraus JA, Dabbs DJ, Beriwal S, et al. Semi-quantitative immunohistochemical assay versus oncotype DX® qRT-PCR assay for estrogen and progesterone receptors: an independent quality assurance study. Mod Pathol. 2012;25:869–876. doi: 10.1038/modpathol.2011.219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.