Abstract
Hydrolysates of Trianthema portulacastrum in acidified methanol were evaluated for their total phenolic (TP) constituents and respective antioxidant activities using in vitro assays (i.e., 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, percent inhibition of linoleic acid peroxidation, and ferric reducing power). The observed results indicate that root, shoot, and leaf fractions of T. portulacastrum contain 50.75~98.09 mg gallic acid equivalents/g dry weight of TP. In addition, these fractions have substantial reducing potentials (0.10~0.59), abilities to inhibit peroxidation (43.26~89.98%), and DPPH radical scavenging capabilities (6.98~311.61 μg/mL IC50). The experimental data not only reveal T. portulacastrum as potential source of valuable antioxidants, but also indicate that acidified methanol may be an ideal choice for the enhanced recovery of phenolic compounds with retained biological potential for the food and pharmaceutical industry.
Keywords: Trianthema portulacastrum, effective extraction, antioxidant phenolics, reducing power
INTRODUCTION
Trianthema portulacastrum L. (Family: Azioaceae), commonly known as desert horse-purslane, carpet weed, pig, and it-sit (in Pakistan), is an annual or perennial weed plant. T. portulacastrum is recognized for its high potential for infestation of agricultural and vegetable crops and is primarily controlled by spraying herbicides (1,2). Epidemiological studies have revealed a therapeutic value of T. portulacastrum for bronchitis; heart disease; anemia; inflammation; piles; ascites; eye disorders; allergies; alcohol poisoning; and paracetamol-, thioacetamide-, and toxin-induced hepatocarcinogenesis (3–5). However, there is limited information available about the phytochemical attributes of T. portulacastrum. Awareness of the functional food and health benefits of phenolic anti-oxidants has enhanced the value of these biologically active compounds.
The advent of modern versatile chromatographic and spectroscopic techniques has revolutionized the era of analytical characterization. However, current methods used to extract phenolics for further characterization and other end uses lack fitness and are mostly selected because of their yield, economic concerns, and environmental concerns. A number of attempts have been made to develop green extraction technologies (6,7). Enzyme-, ultrasound-, microwave- and heat pulse-assisted extraction strategies have shown promising yields for lipophilic compounds (8–10). In all approaches, the soaking or distribution potential of the extraction solvent can be modified to enhance the liberation of target compounds. Unfortunately, in most of these cases, the antioxidant potential of most of the phenolic compounds obtained is decreased.
The present work was undertaken to improve the soaking and distribution power of the most frequently used extraction solvent (i.e., methanol) to enhance the recovery of phenolics with maximized retained antioxidant capacity. For this purpose, methanol that had been acidified to variable pH levels was used to extract phenolic antioxidants from different parts of a potentially important medicinal weed (T. portulacastrum). The acidified methanol hydrolysates obtained from the different parts of T. portulacastrum were evaluated for their anti-oxidant activities using standard in vitro assays.
MATERIALS AND METHODS
Materials and chemicals
T. portulacastrum weed samples were collected from the botanical garden of the University of Agriculture, Faisalabad (Pakistan). The specimens were identified by the Taxonomist of the Department of Botany, University of Agriculture, Faisalabad. Reagents, including Folin-Ciocalteu reagent (FCR), gallic acid (98.0%), 2,2-diphenyl- 1-picrylhydrazyl (DPPH) (90.0%), and linoleic acid (99.0%) were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). All other chemicals (anhydrous sodium carbonate, sodium hydroxide, sodium nitrite, ferrous chloride, ammonium thiocyanate, aluminum chloride, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, isooctane, chloroform, acetic acid, potassium iodide, and sodium thiosulphate) were of analytical grade and procured from Merck (Darmstadt, Germany).
Sample preparation
The leaf, root, and shoot portions of healthy and mature T. portulacastrum weeds were separated with sharp scissors, dried under ambient conditions (25°C), pulverized to pass through an 80-mesh sieve and stored in airtight polythene bags until further analysis.
Extraction
Powdered material (5 g) from the leaf, root, and shoot portions of T. portulacastrum were soaked overnight with 50 mL of 0.01, 0.5, 0.1, 1, 2, and 5 N acidified methanol in an orbital shaker (Gallenkamp, Lougborough, Leicestershire, UK) at 120 rpm and 30°C. The hydrolysates were separated from the residue by filtering through Whatman No. 1 filter paper (Sigma). The residues were re-extracted twice with the same solvents, and the final hydrolysates obtained were concentrated to dryness under reduced pressure at 45°C using a rotary evaporator (EYELA, N-N Series, Rikakikai Co., Ltd., Tokyo, Japan), weighed to calculate the yield, and stored in a freezer (4°C) until further analysis.
Total phenolics (TP)
The concentrations of TP in the hydrolysates of different parts of T. portulacastrum were estimated using FCR as described by Aaby et al. (11), with slight modifications. Briefly, 50 mg of crude hydrolysate was diluted with 7.5 mL of deionized water and mixed with 0.5 mL of FCR. The mixture was incubated at 30°C for 10 min and then 1.5 ml of 20% w/v sodium carbonate was added followed by consistent heating at 40°C for 20 min. The resultant mixture was chilled in an ice bath and the absorbance was recorded at 755 nm (U-2001, Hitachi Instrument Inc., Tokyo, Japan) to calculate TP (milligram gallic acid equivalents [GAE]/g dry weight [DW]) according to a gallic acid calibration curve (2~200 ppm; R2=0.9952).
DPPH antiradical capacity
The antiradical potential of T. portulacastrum weed hydrolysates was determined spectrophotometrically as described by Ilahi et al. (12). Six different concentrations of hydrolysates (1, 10, 100, 1,000, 2,000, and 5,000 μg/mL) were mixed with 100 μL of DPPH radical solution in a 96-well microplate and incubated for 20 min at room temperature. The resultant mixture was read spectrophotometrically at 517 nm against a methanol blank and the following equation was used to calculate the % inhibition of each hydrolysate:
where AControl and Asample indicate the absorbance of the DPPH solution and the reaction mixture, respectively. The effective dose of hydrolysate needed to neutralize 50% of the DPPH radical solution (IC50) was obtained from a plot comparing percent inhibition to hydrolysate concentration.
Antioxidant activity against linoleic acid peroxidation
The antioxidant activities of acidified methanol extracts were determined by measuring the ability of the extracts to inhibit linoleic acid peroxidation (13). Briefly, 5 mg of each hydrolysate was diluted with 10 mL of 0.2 M sodium phosphate buffer (pH 7) and mixed with a solution of linoleic acid (0.13 mL) and ethanol (10 mL). The mixture was diluted with distilled water to 25 mL and incubated at 40°C for 96 h. The thiocyanate method was used to measure the degree of oxidation (14). Briefly, 10 mL of ethanol (75% v/v), 0.2 mL of an aqueous solution of ammonium thiocyanate (30% w/v), and 0.2 mL of ferrous chloride (FeCl2) solution (20 mM in 3.5% HCl) were added sequentially to 0.2 mL of sample. A mixture containing all of the reagents except the sample was used as a blank, and synthetic antioxidants (i.e., butylated hydroxytoluene (BHT) and ascorbic acid [200 ppm]) were used as a positive control. The % inhibition of linoleic acid peroxidation was calculated as follows:
Ferric reducing power
The antioxidant power of acidified methanol hydrolysates of T. portulacastrum was determined by measuring ferric reducing ability (15). Different amounts of each hydrolysate were suspended in 5 mL of phosphate buffer to produce 2.5, 5, 7.5, and 10 mg/mL solutions. These solutions were then mixed with 5 mL of potassium ferri-cyanide [K3Fe(CN)6] (1% w/v) and incubated at 50°C for 20 min. The resulting solution was mixed with 5 ml of trichloroacetic acid (10% w/v) and centrifuged at 3,000 rpm for 10 min. The upper layer (5 mL) was collected, diluted with an equal amount of distilled water and 1 mL FeCl3 (0.1% w/v), and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Vitamin C was used as positive control.
Statistical analysis
All in vitro assays measuring the antioxidant potential of hydrolysates were performed in triplicate. All results are reported as mean±standard deviation (SD) of triplicate experiments. Minitab 16 Statistical Software (Minitab Inc., State College, PA, USA) was used for all statistical analyses. Two-way analysis of variance was used to determine differences among plant parts and solvent acidification levels.
RESULTS AND DISCUSSION
Yield of T. portulacastrum hydrolysates
Plant materials are organized polymeric cellulosic structures of sugars (alcohols) and acids (organic and phenolic acids), which are further fortified by lignin and, in certain cases, shielded by pectin (16). The recovery of phenolic compounds from plant material depends upon the soaking and distribution potentials of the extraction media and the technique applied. Acid hydrolysis can depolymerize the constitutional monomers of cell walls, which in turn can be used as an energy source for the industrial production of valuable compounds and enzymes (17,18). Phenolic compounds are primarily bound in the form of their glycosidic esters. Therefore, acidic media will not only hydrolyze and swell plant cell walls, but also convert bound phenolic esters into phenolic acids, which will ultimately enhance the distribution power of the extraction media. Therefore, in this study an organic solvent (i.e., methanol) that had been variably acidified with HCl was utilized to liberate phenolic antioxidants. Released phenolic compounds with a higher miscibility towards the polar solvent were simultaneously accumulated in the organic solvent and extracted (19).
The hydrolysate yield of T. portulacastrum root, shoot, and leaf portions was 10.43~54.23 g/100 g DW, 10.30~ 26.29 g/100 g DW, and 5.53~38.40 g/100 g DW, respectively. The extent of methanol acidification (0.01~5 N) produced significantly variable amounts of hydrolysate (P<0.05), as indicated by different subscripted letters (Table 1). The highest hydrolysate yield was produced with 0.1 N acidified methanol from root of T. portulacastrum, while the lowest yields were achieved with 80% aqueous methanol (i.e., control). Overall, the acidification of methanol enhanced the recovery of phytochemicals from T. portulacastrum up to four fold. The higher hydrolysate yield at a relatively lower acidification level (i.e., 0.1 N acidified methanol) might be attributed to the optimum maceration of the T. portulacastrum cell wall. Significant variations in the percent yield for root, shoot, and leaf portions were due to difference in the chemical composition of the plant parts.
Table 1.
Hydrolysate yields and total phenolics (TP) concentrations of different parts of T. portulacastrum
| Acidified methanol (N) | Extraction yield (g/100 g) | TP (mg GAE/100 g DW) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Root | Shoot | Leaf | Root | Shoot | Leaf | |
| 5 | 10.43±0.40cA | 10.30±0.28bA | 5.53±0.47aA | 63.60±2.73eA | 50.75±0.11aA | 54.21±2.86dA |
| 2 | 27.36±1.56cB | 12.63±0.15aB | 15.56±0.15bB | 66.46±0.34eA | 56.47±1.67aA | 61.18±0.08dA |
| 1 | 28.43±0.15cB | 15.43±0.45aB | 16.60±0.21bB | 75.23±5.06eB | 58.51±3.32aB | 67.18±0.61dB |
| 0.5 | 33.60±0.39cB | 16.30±0.36aB | 22.26±0.25bB | 76.20±2.12eC | 60.87±0.08aC | 83.37±0.33dC |
| 0.1 | 54.23±0.25cD | 26.29±0.89aD | 38.40±0.29bD | 98.09±0.50eD | 68.63±5.99aD | 91.86±3.86dD |
| 0.01 | 44.30±0.38cC | 17.53±0.25aC | 23.33±0.41bC | 86.08±2.88eC | 62.16±4.57aC | 87.77±0.11dC |
| Aqueous methanol | 16.55±0.45cA | 9.99±0.72aA | 10.42±0.37bA | 61.56±0.67eA | 52.27±1.25aA | 58.13±0.63dA |
The results are mean±SD of experiments conducted in triplicate for each T. Portulacastrum part and solvent acidification level. Superscripted capital and small letters denote variation (P<0.05) among solvent acidification levels and plant parts, respectively.
A thorough review of previously published research indicates that this is the first report of the extraction of antioxidant phenolics from T. portulacastrum. The methanol extract used in the current study results in a similar yield to that of fruits, as reported by our group (20,21), and that of Lantana camara (12.68%), as reported by Ali-Emmanuel et al. (22). Together, these results indicate that acidified methanol is a suitable choice for the recovery of phenolic antioxidants.
Total phenolics
The concentrations of TP in the crude hydrolysates of root, shoot, and leaf portions of T. portulacastrum obtained using variably acidified methanol were 63.60~98.09 mg GAE/100 g DW, 50.75~68.63 mg GAE/100 g DW, and 54.21~91.86 mg GAE/100 g DW, respectively. Overall, the yield of TP, as determined using FCR and gallic acid as positive controls, varied significantly (P<0.05) with the level of acidification applied and the plant part investigated (Table 1). The results indicate that acidification of the extraction solvent (i.e., methanol) increases phenolic recovery 1~1.5 fold. The root, shoot, and leaf hydrolysate fractions obtained using 0.1 N acidified methanol contained the highest concentrations of TP, whereas further acidification lowered the amount of phenolic liberation. The reason for this may be a change in the structural features of the hydrolysate substituents or the deterioration of phenolic compounds under severe acidic conditions. No previous technical data were found regarding phenolic and antioxidant attributes for comparison. The concentration of TP found in T. portulacastrum was higher than that of methanol extracts from Vitex doniana (0.20~7.60 mg GAE/100 g) reported by Muanda et al. (23) and methanol extracts from ambient dried cauliflower (24); however, the concentrations of TP found in this study were comparable to those found in certain oriental herb medicines (25).
The markedly high concentrations of TP that were observed in different parts of T. portulacastrum during the present investigation indicate that the reported folk medicinal applications of T. portulacastrum may be due to the presence of biologically active phenolic metabolites. The observed levels of TP also suggest that acidified methanol is a good choice for the recovery of phenolic compounds. However, there is some concern in the literature that acid or alkaline hydrolysis causes a degradation of phenolic compounds that decreases the biological activity of the hydrolysate (26). Therefore, to confirm that appropriate antioxidant activities were retained, all extracts were subjected to in vitro experiments to determine radical scavenging potential, peroxidation inhibition capacity, and reducing power.
Ferric reducing power (FRP)
The antioxidant activities of T. portulacastrum hydrolysates were evaluated by determining FRP. FRP is thought to imitate some aspects of the antioxidant activity of extracts. According to a few reports, FRP is controlled by the hydrogen donating ability of the compounds under investigation (27). In this assay, ferric ions are reduced to ferrous ions, resulting in a change of color from yellow to bluish-green. The intensity of the bluish-green color reflects the reducing potential of the compounds: a more intense color is associated with a higher absorption and a greater antioxidant activity (28).
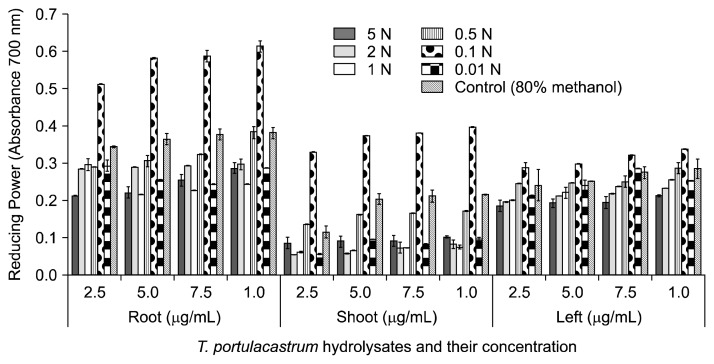
The reducing potentials of T. portulacastrum root, shoot, and leaf hydrolysates were assessed over a concentration range of 2.5 to 10 mg/mL. The graphical presentation of assimilated data (Fig. 1) reveals that the root of T. portulacastrum contains the highest amount of reducing species, followed by the leaf and the shoot portions of T. portulacastrum. In addition, the reducing potentials of the 0.1 N acidified methanol hydrolysates were higher for all T. portulacastrum concentrations tested (Fig. 2). Therefore, the ferric reducing power of T. portulacastrum phenolic metabolites is maintained when phenolic metabolites are liberated using acidified methanol. FRP varied significantly (P<0.05) in response to solvent system, plant part, and hydrolysate concentration. At the maximum hydrolysate concentration investigated (10 mg/mL), FRP was greatest in the root portion (0.268~0.595), followed by the shoot portion (0.103~0.398) and the leaf portion (0.213~0.339). Among methanol acidification levels (0.01~5 N), the 0.1 N acidified methanol condition produced hydrolysates with the highest concentrations of valuable reductants (phenolics). Further acidification lowered the reducing potential of extracts, possibly because of a change in structural features or the availability of interchangeable hydrogen atoms. Furthermore, a strong correlation was found between the observed reducing power and concentration of TP (Table 2). This finding supports the hypothesis that the anti-oxidant character of plant biodiversity may be due to the presence of phenolic compounds (28).
Fig. 1.
Comparison of the reducing power of acidified methanol hydrolysates of T. portulacastrum.
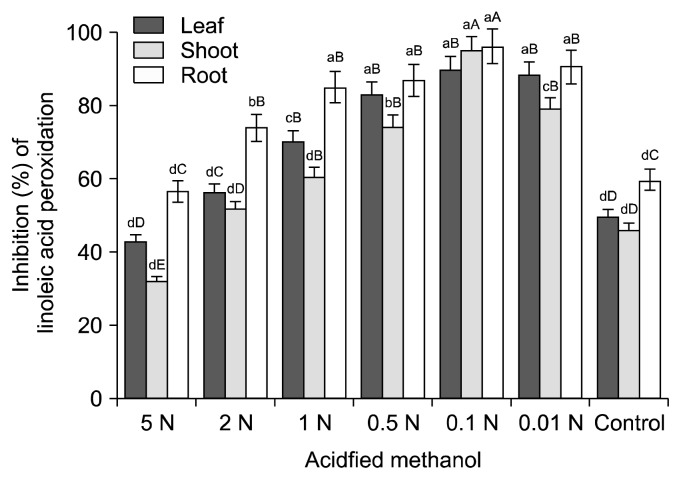
Fig. 2.
Antioxidant potential offered by T. portulacastrum hydrolysate in linoleic acid peroxidation system. Means with capital and small letters indicate variation (P<0.05) among solvent acidification levels and plant parts, respectively.
Table 2.
Pearson’s correlation between yield, TP, and antioxidant activities
| Root | Shoot | Leaf | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Yield | TP | DPPH | Inh | Yield | TP | DPPH | Inh | Yield | TP | DPPH | Inh | |
| TP | 0.821* | 0.695* | 0.849* | |||||||||
| 0.000 | 0.000 | 0.000 | ||||||||||
| DPPH | −0.016ns | 0.491* | −0.201ns | −0.171ns | −0.386ns | −0.725* | ||||||
| 0.945 | 0.02 | 0.360 | 0.459 | 0.474 | 0.000 | |||||||
| Inh | 0.829* | 0.904* | 0.118ns | 0.456* | 0.800* | −0.672* | 0.734* | 0.962* | −0.306ns | |||
| 0.000 | 0.000 | 0.612 | 0.038 | 0.000 | 0.001 | 0.000 | 0.000 | 0.177 | ||||
| RP | 0.297ns | 0.904* | −0.330ns | 0.389ns | 0.371ns | 0.682* | −0.794* | 0.939* | 0.397ns | 0.572* | −0.767* | 0.604* |
| 0.191 | 0.000 | 0.145 | 0.082 | 0.098 | 0.001 | 0.000 | 0.000 | 0.075 | 0.007 | 0.000 | 0.004 | |
TP, total phenolics; DPPH, DPPH radical scavenging activity of hydrolysates; Inh, inhibition of linoleic acid peroxidation; RP, reducing power. ns: Not significant.
Significant at P<0.05.
No previous reports describing the reducing power of T. portulacastrum plant parts were available for comparison. However, the FRP values of T. portulacastrum reported in this study are comparable with previously published values from certain Brazilian vegetables (29) and fruits (21), but lower than values from medicinal plants investigated by Sultana et al. (30).
Antioxidant activity against linoleic acid peroxidation
The potential of T. portulacastum hydrolysates to inhibit the peroxidation of linoleic acid in vitro was assessed using the thiocyanate method (14). Linoleic acid, a poly-unsaturated fatty acid, is highly susceptible to autoxidation, which leads to the formation of peroxides. These peroxides convert oxidized Fe2+ to Fe3+. The latter of the two then forms a complex with SCN−, which can be measured spectrophotometrically. The final concentration of the formed complex is inversely related to the antioxidant activity of hydrolysate. The extent of linoleic acid peroxidation was assessed after a 96-h incubation period. Ascorbic acid was used as positive control for this assay.
All hydrolysate fractions (root, shoot, and leaf) obtained with acidified methanol and aqueous methanol extracts (i.e., control) exhibited appreciable inhibition of linoleic acid peroxidation (43.26~89.98%). As shown in Fig. 2, the percent inhibition varied by plant part and solvent acidification level. Overall, hydrolysates obtained using 0.1 N acidified methanol were more effective at preventing the peroxidation of linoleic acid than other acid concentrations and aqueous methanol. Among the plant parts investigated, the root of T. portulacastrum produced hydrolysates offering a substantially greater linoleic acid peroxidation inhibition potential for all solvent acidification levels, followed by leaf fractions and shoot fractions.
The considerably higher linoleic acid peroxidation inhibition potential of the hydrolysates obtained with the 0.1 N acidified methanol solvent is thought to be due to the presence of an optimal concentration of liberated phenolics. This was confirmed by a number of previous studies (31,32) and a statistical analysis of the data, which revealed a significant correlation (Table 2) between TP and the inhibition potential of extracts.
DPPH radical scavenging activity
DPPH, a stable organic free radical with a deep violet color, is frequently used for the assessment of anti-oxidant activity (12,33). Upon accepting an electron, a yellow coloration that can be quantified spectrophotometrically is produced. As the concentration of phenolic compounds or the degree of hydroxylation of the phenolic compounds increases, the yellow color produced becomes more intense (34). T. portulacastrum weed hydrolysates were evaluated at six different concentrations (1, 10, 100, 1,000, 2,000, and 5,000 μg/mL) to assess their DPPH radical scavenging potential. The data revealed that the DPPH radical scavenging capacity of T. portulacastrum hydrolysates increases linearly with concentration (R2>50%). Therefore, the minimum inhibitory concentration (IC50) was calculated. T. portulacastrum stem hydrolysates produced with 0.1 N acidified methanol were the most effective at quenching DPPH radicals (28.87±1.33 mg/mL), followed by root hydrolysates (40.02±0.55 mg/mL) and leaf hydrolysates (54.50±4.03 mg/mL) (Table 3). The results of present investigation revealed that the recovery of phenolic compounds with retained antioxidant potential is greater with an optimally acidified extraction than it is with an aqueous methanol extraction, which is a more frequently used for phenolic extraction. The substantial level of antioxidant activity observed in this study may be related to the altered hydrolytic potential of acidified methanol, which allowed for the liberation of glycosidically entrapped plant phenolics. It should be noted that 0.1 N acidification was sufficient to increase antioxidant potential. Further increases in acidity level enhanced extraction yield but lowered the antioxidant potential of the hydrolysates, indicating that severe acidic conditions may deteriorate plant phenolics.
Table 3.
DPPH radical scavenging activity of T. portulacastrum hydrolysates obtained with acidified methanol
| Acidified methanol (N) | Plant parts | ||
|---|---|---|---|
|
| |||
| Root | Shoot | Leaf | |
| 5 | 163.95±4.16dB | 116.98±1.13aB | 190.74±4.27dB |
| 2 | 380.43±3.42dE | 201.47±0.84aE | 192.91±4.15dE |
| 1 | 134.61±4.34dD | 230.70±3.05aD | 257.95±3.43dD |
| 0.5 | 417.23±3.35dD | 48.17±4.16aD | 179.32±5.56dD |
| 0.1 | 40.02±0.55dA | 28.87±1.33aA | 54.50±4.03dA |
| 0.01 | 116.97±0.76dC | 311.61±1.02aC | 198.56±4.26dC |
| Control (80% aqueous methanol) | 120.78±3.11dB | 112.76±1.57aB | 190.89±3.53dB |
Values are mean±SD of the minimum inhibitory concentration (IC50).
IC50 was determined by in vitro experiments that were conducted in triplicate.
Superscripted capital and small letters indicate significant variation between solvent fractions used and plant parts investigated, respectively.
CONCLUSION
The results of the present analysis revealed that optimally acidified methanol is ideal for enhanced liberation of antioxidant phenolics for food and pharmaceutical industries. Furthermore, the data revealed that T. portulacastrum is a rich source of potentially valuable anti-oxidants. Further work is necessary to determine nutraceutical and chemopreventive applications of T. portulacastrum. Among the plant parts tested, the root fraction of T. portulacastrum exhibited the highest concentration of phenolic compounds and the greatest anti-oxidant activity.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Balyan RS, Bhan VM. Germination of horse purslane (Trianthema portulacastrum) in relation to temperature, storage conditions, and seeding depths. Weed Sci. 1986;34:513–515. [Google Scholar]
- 2.Shivhare MK, Singour PK, Chaurasiya PK, Pawar RS. Trianthema portulacastrum Linn. (Bishkhapra) Pharmacogn Rev. 2012;6:132–140. doi: 10.4103/0973-7847.99947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharmila Banu G, Kumar G, Murugesan AG. Effect of ethanolic leaf extract of Trianthema portulacastrum L. on aflatoxin induced hepatic damage in rats. Indian J Clin Biochem. 2009;24:414–418. doi: 10.1007/s12291-009-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar G, Banu GS, Pappa PV, Sundararajan M, Pandian MR. Hepatoprotective activity of Trianthema portulacastrum L. against paracetamol and thioacetamide intoxication in albino rats. J Ethnopharmacol. 2004;92:37–40. doi: 10.1016/j.jep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Chatterjee M. Trianthema portulacastrum restores the antioxidant defense enzyme levels and hepatic biotransformation patterns in experimental rat hepatocarcinogenesis. Ital J Biochem. 1998;47:225–232. [PubMed] [Google Scholar]
- 6.Agostini F, Bertussi RA, Agostini G, Atti Dos Santos AC, Rossato M, Vanderlinde R. Supercritical extraction from vinification residues: fatty acids, α-tocopherol, and phenolic compounds in the oil seeds from different varieties of grape. Sci World J. 20122012:790486. doi: 10.1100/2012/790486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed M, Sorifa Akter MST, Eun JB. Optimization conditions for anthocyanin and phenolic content extraction form purple sweet potato using response surface methodology. Int J Food Sci Nutr. 2011;62:91–96. doi: 10.3109/09637486.2010.511167. [DOI] [PubMed] [Google Scholar]
- 8.Beejmohun V, Fliniaux O, Grand E, Lamblin F, Bensaddek L, Christen P, Kovensky J, Fliniaux MA, Mesnard F. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem Anal. 2007;18:275–282. doi: 10.1002/pca.973. [DOI] [PubMed] [Google Scholar]
- 9.Bimakr M, Rahman RA, Taip FS, Adzahan NM, Sarker MZ, Ganjloo A. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules. 2012;17:11748–11762. doi: 10.3390/molecules171011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrera C, Ruiz-Rodriguez A, Palma M, Barroso CG. Ultrasound assisted extraction of phenolic compounds from grapes. Anal Chim Acta. 2012;732:100–104. doi: 10.1016/j.aca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Aaby K, Ekeberg D, Skrede G. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agric Food Chem. 2007;55:4395–4406. doi: 10.1021/jf0702592. [DOI] [PubMed] [Google Scholar]
- 12.Ilahi I, Samar S, Khan I, Ahmad I. In vitro antioxidant activities of four medicinal plants on the basis of DPPH free radical scavenging. Pak J Pharm Sci. 2013;26:949–952. [PubMed] [Google Scholar]
- 13.Rababah TM, Banat F, Rababah A, Ereifej K, Yang W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J Food Sci. 2010;75:C626–C632. doi: 10.1111/j.1750-3841.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 14.Gousiadou C, Gotfredsen CH, Matsa M, Hadjipavlou-Litina D, Skaltsa H. Minor iridoids from Scutellaria albida ssp. albida. Inhibitory potencies on lipoxygenase, linoleic acid lipid peroxidation and antioxidant activity of iridoids from Scutellaria sp. J Enzyme Inhib Med Chem. 2013;28:704–710. doi: 10.3109/14756366.2012.672415. [DOI] [PubMed] [Google Scholar]
- 15.Raudonis R, Raudone L, Jakstas V, Janulis V. Comparative evaluation of post-column free radical scavenging and ferric reducing antioxidant power assays for screening of antioxidants in strawberries. J Chromatogr A. 2012;1233:8–15. doi: 10.1016/j.chroma.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Keegstra K. Plant cell walls. Plant Physiol. 2010;154:483–486. doi: 10.1104/pp.110.161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar R, Singh S, Singh OV. Bioconversion of ligno-cellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol. 2008;35:377–391. doi: 10.1007/s10295-008-0327-8. [DOI] [PubMed] [Google Scholar]
- 18.Dashtban M, Maki M, Leung KT, Mao C, Qin W. Cellulase activities in biomass conversion: measurement methods and comparison. Crit Rev Biotechnol. 2010;30:302–309. doi: 10.3109/07388551.2010.490938. [DOI] [PubMed] [Google Scholar]
- 19.Ajila CM, Brar SK, Verma M, Tyagi RD, Godbout S, Valéro JR. Extraction and analysis of polyphenols: recent trends. Crit Rev Biotechnol. 2011;31:227–249. doi: 10.3109/07388551.2010.513677. [DOI] [PubMed] [Google Scholar]
- 20.Anwar F, Latif S, Przybylski R, Sultana B, Ashraf M. Chemical composition and antioxidant activity of seeds of different cultivars of mungbean. J Food Sci. 2007;72:S503–S510. doi: 10.1111/j.1750-3841.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 21.Sultana B, Hussain Z, Asif M, Munir A. Investigation on the antioxidant activity of leaves, peels, stems bark, and kernel of mango (Mangifera indica L.) J Food Sci. 2012;77:C849–C852. doi: 10.1111/j.1750-3841.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- 22.Ali-Emmanuel N, Moudachirou M, Akakpo JA, Quetin-Leclercq J. Treatment of bovine dermatophilosis with Senna alata, Lantana camara and Mitracarpus scaber leaf extracts. J Ethnopharmacol. 2003;86:167–171. doi: 10.1016/s0378-8741(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 23.Muanda F, Kone D, Dicko A, Soulimani R, Younos C. Phytochemical composition and antioxidant capacity of three malian medicinal plant parts. Evid Based Complement Alternat Med. 20112011:674320. doi: 10.1093/ecam/nep109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anwar F, Kalsoom U, Sultana B, Mushtaq M, Mehmood T, Arshad HA. Effect of drying method and extraction solvent on the total phenolics and antioxidant activity of cauliflower (Brassica oleracea L.) extracts. Int Food Res J. 2013;20:653–659. [Google Scholar]
- 25.Ahn CB, Shin TS, Seo HK, Je JY. Phenolic composition and antioxidant effect of aqueous extract of Arisaema cum Bile, the oriental herb medicine, in human fibroblast cells. Immunopharmacol Immunotoxicol. 2012;34:661–666. doi: 10.3109/08923973.2011.649289. [DOI] [PubMed] [Google Scholar]
- 26.Komes D, Belščak-Cvitanović A, Horžić D, Rusak G, Likić S, Berendika M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem Anal. 2011;22:172–180. doi: 10.1002/pca.1264. [DOI] [PubMed] [Google Scholar]
- 27.Apak R, Güçlü K, Demirata B, Özyürek M, Çelik SE, Bektaşoğlu B, Berker KI, Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C, Sun C, Chen K, Li X. Flavonoids, phenolics, and antioxidant capacity in the flower of Eriobotrya japonica Lindl. Int J Mol Sci. 2011;12:2935–2945. doi: 10.3390/ijms12052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiveron AP, Melo PS, Bergamaschi KB, Vieira TMFS, Regitano-d’Arce MAB, Alencar SM. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int J Mol Sci. 2012;13:8943–8957. doi: 10.3390/ijms13078943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez-Suarez JM, Tulipani S, Díaz D, Estevez Y, Romandini S, Giampieri F, Damiani E, Astolfi P, Bompadre S, Battino M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem Toxicol. 2010;48:2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Corral-Aguayo RD, Yahia EM, Carrillo-Lopez A, González-Aguilar G. Correlation between some nutritional components and the total antioxidant capacity measured with six different assays in eight horticultural crops. J Agric Food Chem. 2008;56:10498–10504. doi: 10.1021/jf801983r. [DOI] [PubMed] [Google Scholar]
- 33.Roy MK, Koide M, Rao TP, Okubo T, Ogasawara Y, Juneja LR. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: relationship between total polyphenol and individual catechin content. Int J Food Sci Nutr. 2010;61:109–124. doi: 10.3109/09637480903292601. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Gallego JI, López-Revuelta A, Hernández-Hernández A, Sardina JL, López-Ruano G, Sánchez-Yagüe J, Llanillo M. Comparative antioxidant capacities of quercetin and butylated hydroxyanisole in cholesterol-modified erythrocytes damaged by tert-butylhydroperoxide. Food Chem Toxicol. 2011;49:2212–2221. doi: 10.1016/j.fct.2011.06.014. [DOI] [PubMed] [Google Scholar]