Abstract
This study was designed to investigate the inhibitory effects of Polyopes lancifolia extract (PLE) on α-glucosidase activity, α-amylase activitiy, and postprandial hyperglycemia in streptozotocin (STZ)-induced diabetic mice. The results of this study revealed a marked inhibitory effect of PLE on α-glucosidase and α-amylase activities. The IC50s of PLE against α-glucosidase and α-amylase were 0.20 mg/mL and 0.35 mg/mL, respectively. PLE was a more effective inhibitor of α-glucosidase and α-amylase activities than acarbose, the positive control. The postprandial blood glucose levels of STZ-induced diabetic mice were significantly lower in the PLE treated group than in the control group. Moreover, PLE administration was associated with a decreased area under the curve for the glucose response in diabetic mice. These results indicate that PLE may be a potent inhibitor of α-glucosidase and α-amylase activities and may suppress postprandial hyperglycemia.
Keywords: Polyopes lancifolia, α-glucosidase, α-amylase, postprandial hyperglycemia, diabetic mice
INTRODUCTION
Diabetes mellitus is a serious, chronic metabolic disorder that is characterized by hyperglycemia (1). Postprandial hyperglycemia plays an important role in the development of diabetes and in the diabetic complications associated with micro- and macro-vascular diseases (2). Therefore, control of postprandial hyperglycemia is the most important factor for treating diabetes and preventing cardiovascular complications (3).
One of the therapeutic approaches to reducing postprandial hyperglycemia is the inhibition of intestinal glucose absorption by altering the activity of carbohydrate hydrolyzing enzymes, such as α-glucosidase and α-amylase, in digestive organs (4–6). Synthetic α-glucosidase and α-amylase inhibitors, such as acarbose, miglitol, and voglibose, are available to reduce high blood glucose levels. However, some of these synthetic agents can cause negative side effects, such as flatulence, abdominal cramps, vomiting, and diarrhea (7–10). Therefore, many studies have been performed to identify natural inhibitors of α-glucosidase and α-amylase that do not have adverse side effects.
Marine algae are known to contain an abundance of bioactive compounds that have great potential in the pharmaceutical, food, and biomedical industries. Polyopes lancifolia (Harvey) Kawaguchi et Wang is a type of red algae usually found off the coast of the Republic of Korea and Japan (11,12). According to several studies, red algae extracts have inhibitory effects on α-glucosidase (12,13) and hyaluronidase activities (14), an anti-inflammatory effect (15), and a protective effect against the induction of breast and colon cancers (16).
In a previous study, Kim et al. (12) demonstrated that bromophenol purified from Polyopes lancifolia may act as a natural α-glucosidase inhibitor. In addition, our group (17) has demonstrated positive diabetes-related effects of Polyopes lancifolia extracts (PLE) on endothelial cell function. However, there is presently no experimental data available exploring the effects of PLE on postprandial blood glucose levels. Therefore, in this study we investigated the effects of PLE on α-glucosidase and α-amylase activities. In addition, the effects of PLE on postprandial hyperglycemia in streptozotocin (STZ)-induced diabetic mice were investigated.
MATERIALS AND METHODS
Materials
Polyopes lancifolia (Harvey) Kawaguchi et Wang, a red algae, was collected along the coast of Jeju Island, Korea. The samples were washed three times with tap water to remove salt, epiphytes, and sand attached to the surface, then carefully rinsed with fresh water and freeze-dried. The dried sample was ground and sifted through a 50-mesh standard testing sieve. The sample was extracted with ten volumes of 80% methanol for 12 h three times at room temperature. The filtrate was then vacuum-evaporated to obtain the extract. After the PLE was thoroughly dried, the extract was stored in a deep freezer (−80°C).
Inhibition assay for in vitro α-glucosidase activity
The α-glucosidase inhibition assay was conducted by the chromogenic method described by Watanabe et al. (18) using a readily available yeast enzyme. Briefly, yeast α-glucosidase (0.7 units, Sigma, St. Louis, MO, USA) was dissolved in 100 mM phosphate buffer (pH 7.0) containing 2 g/L bovine serum albumin and 0.2 g/L NaN3 to form the enzyme solution. Five millimolar p-nitrophenyl-α-D-glucopyranoside was dissolved in the same buffer (pH 7.0) to form the substrate solution. Next, 50 μL of enzyme solution and 10 μL of sample dissolved in dimethylsulfoxide (5 mg/mL) were mixed in a well of a microtiter plate and the absorbance at 405 nm was measured with a microplate reader (zero time point). After incubation for 5 min, the substrate solution (50 μL) was added and the mixture was incubated for another 5 min at room temperature. Then, the increase in absorbance from the zero time point was measured. The inhibitory activities of varying concentrations of PLE were expressed as 100 minus the absorbance change of test compounds relative to the absorbance change of the control (%), where the test solution was replaced by the carrier solvent. The measurements were performed in triplicate and the IC50 value (i.e., the concentration of PLE that results in 50% inhibition of maximal activity) was determined.
Inhibition assay for in vitro α-amylase activity
The α-amylase inhibition assay was conducted as described for the α-glucosidase inhibition assay (18), except that porcine pancreatic amylase (100 units, Sigma) and p-nitrophenyl-α-D-maltopentoglycoside were used as the enzyme and substrate, respectively.
Experimental animals
Four-week-old, male Slc:ICR mice (Orient Bio Inc., Seongnam, Korea) were used. All animals were housed individually in a light (12-h on/12-h off) and temperature-controlled room with ad libitum access to pelleted food and water. After a 2 wk adjustment period, diabetes was induced as described below. All procedures were approved by the animal ethics committee of our university.
Induction of diabetes
To induce diabetes, mice were fasted for 18 h and then given a single intraperitoneal (i.p.) injection of 60 mg/kg STZ prepared in 0.1 M sodium citrate buffer (pH 4.5). Beginning one week after injection of STZ, fasting blood glucose levels were periodically measured using a glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Blood was obtained via tail bleed. Mice with fasting blood glucose values of 250 mg/dL or higher were included in the diabetic groups.
Measurement of blood glucose level
Normal mice and STZ-induced diabetic mice were fasted overnight (i.e., deprived of food for at least 12 h but allowed free access to water). After overnight fasting, normal and STZ-induced diabetic mice were each randomly divided into 3 groups of 7 mice (i.e., a total of 6 groups) and treated as follows: 1) control: mice received oral administration of soluble starch (2 g/kg body weight [BW]) alone; 2) PLE: mice received oral administration of starch with PLE (300 mg/kg BW); 3) acarbose: mice received oral administration of starch with acarbose (100 mg/kg BW). The PLE and acarbose doses were determined based on previous research (19,20). Blood samples were taken from the tail vein at 0 min, 30 min, 60 min, and 120 min after oral administration. Blood glucose was measured using a glucometer (Roche Diagnostics GmbH). Areas under the curve (AUC) of the glucose response were calculated using the trapezoidal rule (21).
Data and statistical analysis
The data are represented as the mean±standard deviation of triplicate experiments. The statistical analysis was performed using SAS software ver. 9.1 (SAS Institute Inc., Cary, NC, USA). Differences among groups were evaluated by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. P-values of less than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
The treatment goal for diabetic patients is to maintain a normal blood glucose level in both the fasting and the postprandial states. Postprandial hyperglycemia is the first metabolic abnormality to occur in diabetes mellitus (22). Thus, inhibition of pancreatic α-amylase activity or intestinal α-glucosidase activity is an effective strategy for the management of diabetes mellitus, as it retards the absorption of carbohydrates thereby controlling postprandial hyperglycemia (23). Several synthetic compounds have been tested in efforts to develop therapeutic agents for diabetes. However, these compounds generally are toxic or have undesirable side effects (10,19). Therefore, several recent studies have investigated the use of natural compounds to inhibit carbohydrate digestive enzyme activity without inducing adverse side effects. Marine algae are currently recognized as a good source of naturally-derived antidiabetic compounds. Kim et al. (12) noted that bromophenol compounds isolated from Polyopes lancifolia, a red algae, can inhibit the activity of α-glucosidase.
Inhibitory effect of PLE on in vitro α-glucosidase and α-amylase activities
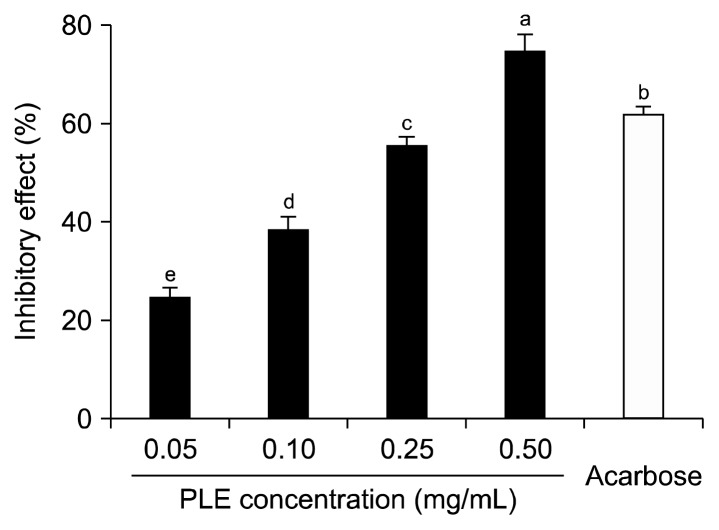
The inhibitory effect of PLE against α-glucosidase is shown in Fig. 1. PLE inhibited α-glucosidase activity in a dose-dependent manner by 24.67%, 38.41%, 55.56%, and 74.99% at PLE concentrations of 0.05 mg/mL, 0.10 mg/mL, 0.25 mg/mL, and 0.50 mg/mL, respectively. A 0.50 mg/mL concentration of acarbose, an α-glucosidase inhibitor used as an oral hypoglycemic agent, inhibited α-glucosidase activity by 62.03%. The α-glucosidase inhibitory activity of PLE was higher than that of the same concentration (i.e., 0.50 mg/mL) of acarbose.
Fig. 1.
Inhibitory activity of PLE on α-glucosidase. Each value is expressed as mean±SD in triplicate experiments. a–eValues with different letters are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. Acarbose (0.5 mg/mL) was used as the positive control. PLE, Polyopes lancifolia extract.
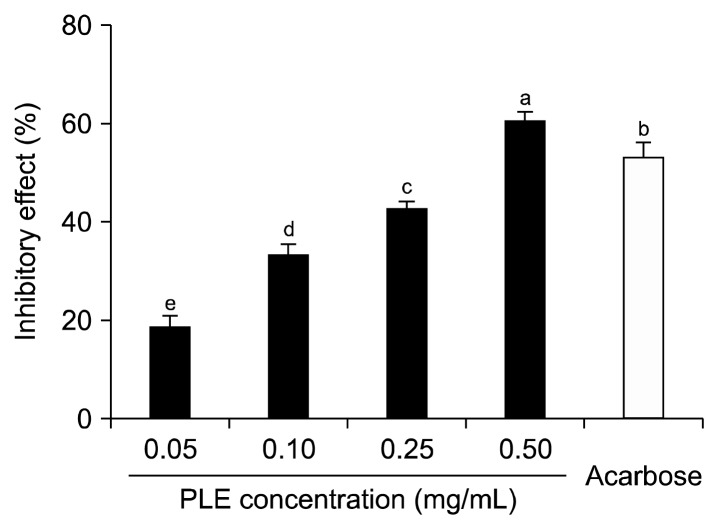
The inhibitory effect of PLE against α-amylase is shown in Fig. 2. PLE inhibited α-amylase activity by 18.97%, 33.17%, 42.70%, and 61.02% at PLE concentrations of 0.05 mg/mL, 0.10 mg/mL, 0.25 mg/mL, and 0.50 mg/mL, respectively. A 0.50 mg/mL concentration of acarbose inhibited enzyme activity by 53.40%. The α-amylase inhibitory activity of PLE was higher than that of the same concentration (i.e., 0.50 mg/mL) of acarbose.
Fig. 2.
Inhibitory activity of PLE on α-amylase. Each value is expressed as mean±SD in triplicate experiments. a–eValues with different letters are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. Acarbose (0.5 mg/mL) was used as the positive control. PLE, Polyopes lancifolia extract.
The IC50 values of PLE against α-glucosidase and α-amylase were 0.20 and 0.35 mg/mL, respectively. The IC50 values of PLE against α-glucosidase and α-amylase were lower than that of acarbose, suggesting that PLE has stronger inhibitory effects than the positive control (i.e., acarbose) (Table 1).
Table 1.
IC50 values of PLE on α-glucosidase and α-amylase
| Sample | IC50 (mg/mL)1) | |
|---|---|---|
|
| ||
| α-Glucosidase | α-Amylase | |
| PLE | 0.20±0.02* | 0.35±0.02* |
| Acarbose | 0.34±0.02 | 0.45±0.04 |
IC50 is the concentration of sample required for 50% inhibition.
Each value is expressed as mean±SD (n=3).
Value is significantly different from the positive control, acarbose at P<0.05.
PLE, Polyopes lancifolia extract.
α-amylase and α-glucosidase are key carbohydrate digestion enzymes. α-amylase catalyzes the hydrolysis of α-1,4-glycosidic linkages of starch, glycogen, and various oligosaccharides (24). α-glucosidase is located on the brush-border surface membrane of intestinal cells. α-glucosidase catalyzes the hydrolysis of disaccharides and oligosaccharides present in the lumen of the intestine; as a result, the glucose generated by α-glucosidase activity is readily available for intestinal absorption (25). The inhibition of α-amylase and α-glucosidase activities prevents the release of glucose from starch, thus reducing the absorption of glucose by the intestine (26). For this reason, the inhibition of these enzymes is considered to be an effective strategy for the management of postprandial blood glucose levels in diabetic patients, and scientists continue to seek effective and non-toxic inhibitors of α-glucosidase and α-amylase.
In this study, we investigated the inhibitory effect of PLE against α-glucosidase and α-amylase to elucidate the possible use of PLE as an anti-hyperglycemic agent. PLE had greater inhibitory effects against α-glucosidase and α-amylase than the commercial carbohydrate digestive enzyme inhibitor, acarbose. Previous work has revealed that Polyopes lancifolia contains bromophenol compounds (12). These polyphenolic compounds are known to form complexes with a variety of proteins (27). Notably, previous studies indicate that the hydroxyl groups of polyphenolic compounds may bind to active binding sites of the enzymes, resulting in the inhibition of enzyme activity (13,28). Thus, we hypothesize that bromophenol compounds in PLE may have an important role in the inhibition of α-glucosidase activity and α-amylase activity.
Effect of PLE on in vivo blood glucose levels
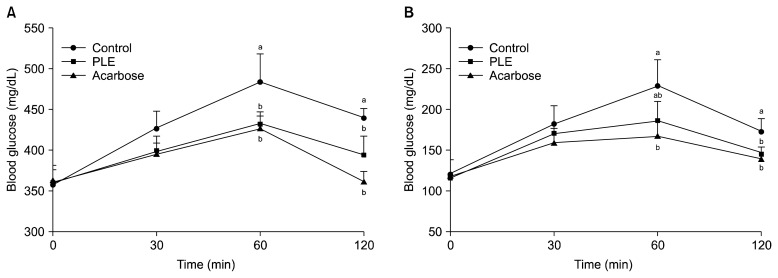
The effect of PLE on postprandial blood glucose levels was investigated in STZ-induced diabetic and normal mice. In diabetic mice, postprandial blood glucose levels of the PLE administered group were lower than those of the control group (Fig. 3A). The blood glucose level of the control group increased to 483.8 mg/dL at 60 min after a meal, and decreased thereafter. However, postprandial blood glucose levels were significantly lower (P<0.05) when diabetic mice were fed with PLE (400.2 mg/dL, 433 mg/dL, and 394 mg/dL at 30 min, 60 min, and 120 min, respectively). In normal mice, PLE significantly alleviated (P<0.05) postprandial hyperglycemia caused by starch. The peak postprandial blood glucose level was significantly decreased when starch with PLE was orally administered in normal mice (Fig. 3B). In diabetic mice, the AUC for the glucose response was lower for the PLE administration group (811.8±33.1 mg·h/dL) than for the control group (886.5±48.4 mg·h/dL) (Table 2).
Fig. 3.
Blood glucose levels after the administration of PLE in streptozotocin-induced diabetic mice (A) and normal mice (B). Control (distilled water), PLE (300 mg/kg), and acarbose (100 mg/kg) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of seven mice (n=42). a,bValues with different letters are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. PLE, Polyopes lancifolia extract.
Table 2.
Area under the curve (AUC) of postprandial glucose responses in normal and streptozotocin-induced diabetic mice
| Group1) | AUC (mg·h/dL) | |
|---|---|---|
|
| ||
| Normal mice | Diabetic mice | |
| Control | 381.2±47.4 | 886.5±48.4a |
| PLE | 327.4±29.9 | 811.8±33.1ab |
| Acarbose | 304.8±37.8 | 790.5±32.5b |
Control (distilled water), PLE (300 mg/kg), and acarbose (100 mg/kg) were co-administered orally with starch (2 g/kg).
Each value is expressed as mean±SD of seven mice (n=42).
Values with different letters are significantly different at P<0.05 as analyzed by Duncan’s multiple range test.
PLE, Polyopes lancifolia extract.
Postprandial hyperglycemia reduces insulin sensitivity (22,29) and insulin secretion due to the degradation of pancreas function (2), resulting in a deteriorated diabetic state. Also, postprandial hyperglycemia has been shown to increase the generation of free radicals, which stimulate prothrombotic pathways and induce vasoconstriction, leading to an increased risk for cardiovascular disease, a major cause of premature death in patients with diabetes (30). Therefore, the regulation of postprandial hyperglycemia is considered important in the treatment of diabetes and the prevention of cardiovascular complications. In this study, we investigated the anti-hyperglycemic effects of PLE in STZ-induced diabetic mice after administration of starch. Following PLE administration, postprandial blood glucose levels were significantly decreased in STZ-induced diabetic mice and normal mice. These results indicate that PLE may delay the absorption of dietary carbohydrates, thus suppressing the typical increase in postprandial blood glucose levels. Inoue et al. (31) reported that medication that flattens peak of postprandial blood glucose reduces the AUC of the blood glucose response. In this study, PLE reduced both the peak blood glucose level and the AUC.
In conclusion, our study indicates that the α-glucosidase and α-amylase inhibitory effects of PLE are responsible for PLE’s anti-hyperglycemic activity. PLE had a noticeable inhibitory effect against these enzymes. Furthermore, PLE may delay the absorption of dietary carbohydrates by the intestine, thus suppressing post-meal increases in blood glucose. These findings support the use of PLE as a nutraceutical to control diabetes and alleviate postprandial hyperglycemia. Further studies are needed to reveal the active compounds in PLE that are responsible for its hypoglycemic effects.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education, Science and Technology (grant number 2013027365).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Corry DB, Tuck ML. Protection from vascular risk in diabetic hypertension. Curr Hypertens Rep. 2002;2:154–159. doi: 10.1007/s11906-000-0075-2. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD. Postprandial hyperglycemia and α-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40:S51–S55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4.Clissold SP, Edwards C. Acarbose. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1998;35:214–243. doi: 10.2165/00003495-198835030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Saito N, Sakai H, Suzuki S, Sekihara H, Yajima Y. Effect of an alpha-glucosidase inhibitor (voglibose), in combination with sulphonylureas, on glycaemic control in type 2 diabetes patients. J Int Med Res. 1998;26:219–232. doi: 10.1177/030006059802600501. [DOI] [PubMed] [Google Scholar]
- 6.Raj Bhandari M, Jong-Anurakkun N, Hong G, Kawabata J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chem. 2008;106:247–252. [Google Scholar]
- 7.Lebovitz HE. Treating hyperglycemia in type 2 diabetes: new goals and strategies. Cleve Clin J Med. 2002;69:809–820. doi: 10.3949/ccjm.69.10.809. [DOI] [PubMed] [Google Scholar]
- 8.Carroll MF, Gutierrez A, Castro M, Tsewang D, Schade DS. Targeting postprandial hyperglycemia: a comparative study of insulinotropic agents in type 2 diabetes. J Clin Endocrinol Metab. 2003;88:5248–5254. doi: 10.1210/jc.2003-030649. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca V. Clinical significance of targeting postprandial and fasting hyperglycemia in managing type 2 diabetes mellitus. Curr Med Res Opin. 2003;19:635–641. doi: 10.1185/030079903125002351. [DOI] [PubMed] [Google Scholar]
- 10.Hanefeld M. The role of acarbose in the treatment of non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1998;12:228–237. doi: 10.1016/s1056-8727(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 11.Mineur F, De Clerck O, Le Roux A, Maggs CA, Verlaque M. Polyopes lancifolius (Halymeniales, Rhodophyta), a new component of the Japanese marine flora introduced to Europe. Phycologia. 2010;49:86–96. [Google Scholar]
- 12.Kim KY, Nguyen TH, Kurihara H, Kim SM. α-Glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J Food Sci. 2010;75:145–150. doi: 10.1111/j.1750-3841.2010.01629.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim KY, Nam KA, Kurihara H, Kim SM. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry. 2008;69:2820–2825. doi: 10.1016/j.phytochem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Shibata T, Fujimoto K, Nagayama K, Yamaguchi K, Nakamura T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int J Food Sci Technol. 2002;37:703–709. [Google Scholar]
- 15.Bergé JP, Debiton E, Dumay J, Durand P, Barthomeuf C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J Agric Food Chem. 2002;50:6227–6232. doi: 10.1021/jf020290y. [DOI] [PubMed] [Google Scholar]
- 16.Reddy BS, Sharma C, Mathews L. Effect of Japanese seaweed (Laminaria angustata) extracts on the mutagenicity of 7,12-dimethylbenz[a]anthracene, a breast carcinogen, and of 3,2′-dimethyl-4-aminobiphenyl, a colon and breast carcinogen. Mutat Res. 1984;127:113–118. doi: 10.1016/0027-5107(84)90011-3. [DOI] [PubMed] [Google Scholar]
- 17.Min SW, Han JS. Effect of Polyopes lancifolia extract on oxidative stress in human umbilical vein endothelial cells induced by high glucose. Prev Nutr Food Sci. 2013;18:38–44. doi: 10.3746/pnf.2013.18.1.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of α-glucosidase inhibitors from tochu-cha (Eucommia ulmoides) Biosci Biotechnol Biochem. 1997;61:177–178. doi: 10.1271/bbb.61.177. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Wen S, Kota BP, Peng G, Li GQ, Yamahara J, Roufogalis BD. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J Ethnopharmacol. 2005;99:239–244. doi: 10.1016/j.jep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Gholamhoseinian A, Fallah H, Sharifi far F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on α-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009;16:935–941. doi: 10.1016/j.phymed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS. Effect of Rhemanniae radix on the hyperglycemic mice induced with streptozotocin. J Korean Soc Food Sci Nutr. 2004;33:1133–1138. [Google Scholar]
- 22.Lebovitz HE. Postprandial hyperglycaemic state: importance and consequences. Diabetes Res Clin Pract. 1998;40:S27–S28. [PubMed] [Google Scholar]
- 23.Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 24.Prashanth D, Padmaja R, Samiulla DS. Effect of certain plant extracts on α-amylase activity. Fitoterapia. 2001;72:179–181. doi: 10.1016/s0367-326x(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 25.Hanefeld M, Schaper F. The role of alpha-glucosidase inhibitors (acarbose) In: Mogensen CE, editor. Pharmacotherapy of Diabetes: New Developments Improving Life and Prognosis for Diabetic Patients. Springer Science; New York, NY, USA: 2007. pp. 143–152. [Google Scholar]
- 26.Hara Y, Honda M. The inhibition of α-amylase by tea polyphenols. Agric Biol Chem. 1990;54:1939–1945. [Google Scholar]
- 27.Stern JL, Hagerman AE, Steinberg PD, Mason PK. Phlorotannin-protein interactions. J Chem Ecol. 1996;22:1877–1899. doi: 10.1007/BF02028510. [DOI] [PubMed] [Google Scholar]
- 28.Pierpoint WS. o-Quinones formed in plant extracts. Their reactions with amino acids and peptides. J Biochem. 1969;112:609–616. doi: 10.1042/bj1120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koivisto VA. Insulin therapy in type II diabetes. Diabetes Care. 1993;16:S29–S39. doi: 10.2337/diacare.16.3.29. [DOI] [PubMed] [Google Scholar]
- 30.Ceriello A, Davidson J, Hanefeld M, Leiter L, Monnier L, Owens D, Tajima N, Tuomilehto J International Prandial Glucose Regulation Study Group. Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutr Metab Cardiovasc Dis. 2006;16:453–456. doi: 10.1016/j.numecd.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Inoue I, Takahashi K, Noji S, Awata T, Negishi K, Katayama S. Acarbose controls postprandial hyperproinsulinemia in non-insulin dependent diabetes mellitus. Diabetes Res Clin Pract. 1997;36:143–151. doi: 10.1016/s0168-8227(97)00045-4. [DOI] [PubMed] [Google Scholar]