Abstract
Background
Even in high performing health systems, some hypertensive patients with diabetes have persistent poor blood pressure (BP) control. Medication nonadherence and lack of medication intensification contribute to this poor control. We examined whether the Adherence and Intensification of Medications (AIM) intervention, a targeted pharmacist-led intervention that combined state-of-the-art elements found in efficacy studies to lower BP, could improve BP among diabetes patients with persistent hypertension and poor refill adherence or insufficient medication intensification.
Methods and Results
We conducted a prospective, multi-site cluster randomized pragmatic trial with randomization of 16 primary care teams at five medical centers (3 Veterans Affairs [VA] and 2 Kaiser Permanente [KP]) to the AIM intervention or usual care. The primary outcome was the relative change in systolic blood pressure (SBP) measurements, comparing 1,797 eligible intervention team patients to 2,303 eligible control team patients, between the 6-months preceding and the 6-months following the 14-month intervention period. We examined shorter-term changes in SBP as a secondary outcome. In our primary analysis, the intervention group SBP change from 6-months prior to 6-months after the 14-month intervention period was approximately the same as the control group, declining approximately 9 mm Hg in both groups. SBP lowering occurred more rapidly among eligible intervention team patients, with mean SBPs 2.4 mm Hg lower (95% CI: −3.4 to −1.5; p<.001) immediately after the intervention than those achieved by eligible control patients.
Conclusions
The AIM program more rapidly lowered SBPs among eligible intervention patients, but there was no significant difference in blood pressure between intervention and control patients 6 months following the intervention period. These findings show the importance of rigorously evaluating in different real-life clinical settings programs found in efficacy trials to be effective before urging their widespread adoption in all settings.
Keywords: blood pressure, diabetes mellitus, trials, adherence, clinical inertia
Background
Good blood pressure (BP) control is an important clinical outcome in diabetes. In the UKPDS study, achieving mean systolic blood pressure (SBP) levels of 144 mm Hg led to an absolute risk reduction of 11% in diabetes complications over 10 years, an effect 3.5 times greater than intensive blood glucose control.1 While glycemic or cholesterol control has an incremental cost-effectiveness of $40-50,000 per quality-adjusted life-year (QALY), BP control saves almost $2000 per QALY.1-4
In part in response to these findings, BP control has improved in the United States among all patients5 and patients with diabetes.6 In high performing health care systems like the Veterans Affairs [VA] and Kaiser Permanente Northern California [KP] that have devoted significant resources and effort to improved risk factor control, BP control (percentage < 140/90) is now at least 80%,7, 8 compared to just 50% several years earlier.9 This achievement has been accomplished through population care management strategies, team-based programs, incentives and performance monitoring.10-13 Achieving even higher thresholds of BP control will likely be more difficult and costly and will require novel and complex interventions.
Patients with poorly controlled hypertension often have poor medication adherence or other issues contributing to lack of provider intensification of their medications.14-20 The most effective programs evaluated by efficacy trials in selected populations of volunteer subjects have included those led by nurse care managers or clinical pharmacists who generally are authorized to adjust medications.11, 21-31 However, the effectiveness of these interventions in routine practice, and specifically their ability to raise rates of BP control in ‘high performing systems’ (defined as those systems where blood pressure control is already at or above 80%), has not been well evaluated.
Accordingly, we designed a targeted pharmacist-led care management program, using the best evidence from efficacy trials to improve BP control among diabetes patients with persistent hypertension. Using electronic pharmacy prescribing and clinical data systems, clinical pharmacists proactively reached out to patients with uncontrolled hypertension and either poor adherence or no treatment changes in response to high BPs.16, 20, 32, 33 Supported by a computer application that provided up-to-date medication-specific refill information on each participant's anti-hypertensive and other diabetes medications, the pharmacists delivered tailored adherence counseling using motivational interviewing (MI)34-38 and medication management tailored for complex patients,11, 39 providing close follow-up once a behavioral or pharmacological change was initiated.29, 40 To evaluate the benefit of implementation of this program in real-life clinic settings, we conducted a stratified multi-site cluster randomized pragmatic trial41, 42 within clinic sites in two high-performing integrated health systems--Kaiser Permanente Northern California and the Department of Veterans Affairs-- with two-stage cluster sampling and additional stratification of the second stage of sampling within site by blood pressure levels.
Methods
Setting and Identification of Eligible Patients
The study protocol and methods are described in depth elsewhere.43 The study took place in the outpatient primary care clinics at three urban VA facilities in the Midwest and two KP facilities in California. All sites’ Institutional Review Boards approved the study. Diabetes patients were identified from electronic medical record data using a well-validated algorithm.43-45 Eligible diabetic patients had persistent poor BP control and poor refill adherence or insufficient medication intensification as defined in Appendix A and in the published protocol.32, 43, 46-48
The 14-month intervention period during which eligible subjects were identified and offered pharmacist encounters at 3 month intervals (time 0, 3, 6, 9, and 12 months) extended from August 2008 through September 2009. A subject could be eligible at just one or at all time periods (analyses corrected for clustering by patient).
Randomization of Primary Care Teams and Stratification and Randomization of Intervention Team Patients
We used two-stage cluster sampling, whereby we first selected all team clusters at each site and randomly assigned primary care teams within the five sites to treatment vs. control. 16 primary care teams were randomized for a total of 8 intervention and 8 control teams. Each team consisted of 5-28 primary care providers, their staff, and patients. Cluster randomization afforded a better opportunity than individual randomization to evaluate the real-world effectiveness of pharmacist-team interactions as they would occur with full implementation. Team-level randomization also minimized cross-over contamination due to pharmacist contact within teams. Randomization within site was done to allow us to stratify analyses by site, thus reducing the major source of cluster variation attributable to site of care.
In the second stage we randomly sampled subjects within each team for activation by assigning a priority order. First, patients were randomly selected from those patients with the highest SBPs (≥160 mm hg) during the sample selection period, then from the SBP 150-159 strata and then from the140-149 strata. The highest SBP strata received the first priority for patient outreach. The randomly ordered list of names was loaded into the computerized tool used by the pharmacists (the Medications Management Tool [MMT]). Pharmacists were instructed to contact patients in the order they appeared on their list. Any patient who the pharmacist attempted to contact, regardless of whether they were contacted or enrolled in the program, was included in the intervention group and considered “activated” and included in the intention-to-treat analyses. Thus, the activated subjects represented a stratified random sample of the eligible population on the intervention teams with the size of the sample determined by the capacity of the pharmacist resources dedicated at that site. All eligible patients in the control group were included in the analysis sample.
We used stratified two-stage sampling because we were not sure how many patients would be eligible on each team each quarter of the 14-month period the AIM pharmacists were in place, and the team sizes varied substantially across sites. If the pharmacists were able to activate all of the patients on some teams and not on others at different sites, our results would be affected by not only by the intervention itself but also the balance between capacity and number of eligible patients at any given site. By randomly prioritizing patients from the eligible pool for activation on the intervention team, we ensured that we maintained comparability between the intervention sample and the control subjects under conditions of adequate capacity to deliver the intervention.
Usual Care
Patients assigned to the usual care teams received standard health care services through their primary care provider, which in all sites included access to care manager and other non-AIM program clinical pharmacist services targeting diabetes patients with poor risk factor control. The study team had no contact with the usual care teams, nor did the AIM clinical pharmacists who worked exclusively with intervention team patients. At VA sites, providers on both intervention and usual care teams received quarterly reports of their diabetic patients who had poor BP control and adherence or intensification issues. At KP sites, these reports were not required. Instead, patients were eligible for contact by clinical pharmacists as part of KP's PHASE (Preventing Heart Attacks and Strokes Everyday) program for patients at high risk for CVD events. [www.permanente.net/healthyheart/] PHASE pharmacists and VA care managers received no training in Motivational Interviewing (MI), did not have access to the MMT or other IT tools providing adherence or intensification data, and provided briefer contacts with patients with less sustained follow-up.
Description of the AIM Intervention
Behavioral Counseling Training of Clinical Pharmacists
Each site had two full-time clinical pharmacist equivalents, three pharmacists at KP (2 were half-time) and two in VA who worked exclusively with patients on intervention teams. All pharmacists participated in an initial 3-day, interactive Motivational-Interviewing (MI) training that focused on patient-centered approaches to achieving health goals.49, 50 Pharmacists were provided with an outline (or ‘road map’) as a guide for structuring the flow of an intake encounter and as a tool to reinforce motivational interviewing principles and techniques. Booster training was provided during biweekly webinars among all participating pharmacists and study staff. At six months, an expert assessment of pharmacists’ MI techniques concluded that all pharmacists met or exceeded MI proficiency standards.51
Interactions between AIM pharmacists and intervention team primary care providers (PCPs)
Prior to the beginning of the intervention period, all PCPs on the intervention teams agreed that AIM pharmacists assigned to their teams could proactively reach out to eligible patients. Although the AIM pharmacists were authorized to make medication changes, at all sites the clinical pharmacists copied the participating patient's assigned PCP on all of that patient's clinical notes and alerted the PCP when one of that PCP's patients declined participation in the program, entered the program, or was discharged. Once a patient was on three anti-hypertensive medications, the clinical pharmacists were instructed to consult with the assigned PCP about or refer the patient back to the PCP for any additional anti-hypertensive medications.
Initial contact by the pharmacist
Before calling eligible intervention subjects, the pharmacist reviewed each patient's electronic medical record and information on medication-specific refill gaps and prior provider intensification supplied in the MMT, key components of which are described in detail in Appendix B and elsewhere.43 If a patient agreed to participate, a phone or in person intake encounter was scheduled, and a welcome packet was mailed with an introduction letter from the pharmacist and educational materials, including instructions for home monitoring and documents to record BPs and action plans. A patient was considered unreachable after five unsuccessful attempts. Once the pharmacists tried to call a patient on the list (“activated” them), whether or not the patient was reached or agreed to participate, that patient was considered a study participant and included in all analyses.52 Because the AIM program was considered a standard clinical program at all sites, participants received no monetary incentive for participating in the program and did not have to provide informed consent for participation.43
Encounters with the pharmacist
Encounters took place at the clinics and by phone. Office encounters ranged from 15% of all encounters at one site to over 60% at another site. Participants were encouraged to self-monitor and aided in obtaining home BP monitors. At the intake encounter, the pharmacist, supported by the road map and the MMT, assessed adherence to each prescribed BP, lipid and anti-hyperglycemic medication, explored barriers (e.g., cost, side-effects, forgetfulness) to adherence, and discussed recent BP, HbA1c, and LDL levels. At in-person encounters, a BP was measured by the pharmacist (or medical assistant at KP) as per JNC-7 protocols.53 Labs were ordered according to the provided treatment algorithms.43 The pharmacist then explored with the patient their goals and values and how taking medications affected these. If the patient faced barriers to adherence, the pharmacist worked with the patient to set a short-term action step.54, 55 If the patient reported no adherence problems, the pharmacist recommended and was authorized to make BP medication changes using the site- approved treatment algorithms. At the end of the encounter, the pharmacist summarized agreed-upon next steps and scheduled a follow-up encounter. All encounters were documented in the electronic medical record, and patients’ PCPs were copied on all notes. Follow-up encounters focused primarily on assessing medication adherence, progress on prior action plans, additional action planning, and, when appropriate, intensification of medications.
A patient was eligible for discharge when all medication adherence issues had been addressed; home or clinic BPs were at target [average <135/80 for VA and <130/80 for KP patients according to each institution's guidelines] or diastolic BP<60;56 or the patient was on maximum tolerated medications. In addition, patients were discharged if lost to follow-up (e.g., no showed for three scheduled encounters), enrolled for 6 months without achieving BP target with no progress, or declined further participation.
Patients who had been previously discharged but met eligibility criteria in subsequent quarters could reenter the program after a three-month window. Thirty-five participants reentered over the 14-month intervention period.
Outcomes and Analysis
The primary outcome was the relative change in SBP measurements between the 6-months preceding and the 6-months following the 14-month intervention period among all eligible control and intervention subjects regardless of participation in the intervention (i.e., an intention to treat analysis) (See Appendix C.) SBP measures came from the sites’ usual clinical care electronic databases excluding all BPs measured by the AIM pharmacists. To ensure that BPs taken by an AIM pharmacist were not included, we excluded all BPs on days a patient had an in-person encounter with the AIM pharmacist. BP values obtained in the ER, urgent care, inpatient, and surgery departments were also excluded.
In addition to 6 month follow-up, pre-specified secondary analyses examined shorter term changes in SBP. The sample selection period comprised the 9-month window used to determine a patient's eligibility for the study. The 1-month preparation period extended from the day the patient was determined to be eligible for the study to the quarter start date (i.e., the first date of possible activation). The activation period extended for 3-months after the quarter start date. During this period pharmacists were activating eligible patients sequentially from the provided stratified random sample list. The short-term follow-up periods - Quarters 1, 2, and 3 -followed the activation period. Each of these periods was also 3 months in length. The final period, also known as the long-term follow-up period, comprised BPs from the end of quarter 3 to March 31, 2010 (6 months after the intervention ended). Thus, patients eligible in later quarters had fewer short-term follow-up periods although the length of follow-up remained balanced across the intervention and control groups. (See Appendix D.) Control participants were assigned random activation dates by strata for analysis purposes to match the distribution of activation dates in the intervention group, although no specific actions resulted from activation.
The analysis was done with a 3-level multiple linear regression, with SBP measurements nested within subject within team to account for the clustering of patients within teams and the precision of BP measurement given differing numbers of BP measurements per subject. The analysis further accounted for the sampling design by including site and the BP eligibility strata in all models. All analyses were done using STATA 11.1 (Stata, College Station, Texas, 2010). As described in detail elsewhere,43 this study was powered to detect a 4 mm Hg difference with a power of 0.8 with only 2 observed blood pressures per person in the pre- and post- intervention measurement windows, under an assumption of an ICC of 0.02. We had an average of 4 observed BPs per person for each window, and the observed ICC at the team level after stratification by site was considerably less than 0.02. The target sample size for the power calculation was achieved.
Results
Baseline attributes of eligible subjects
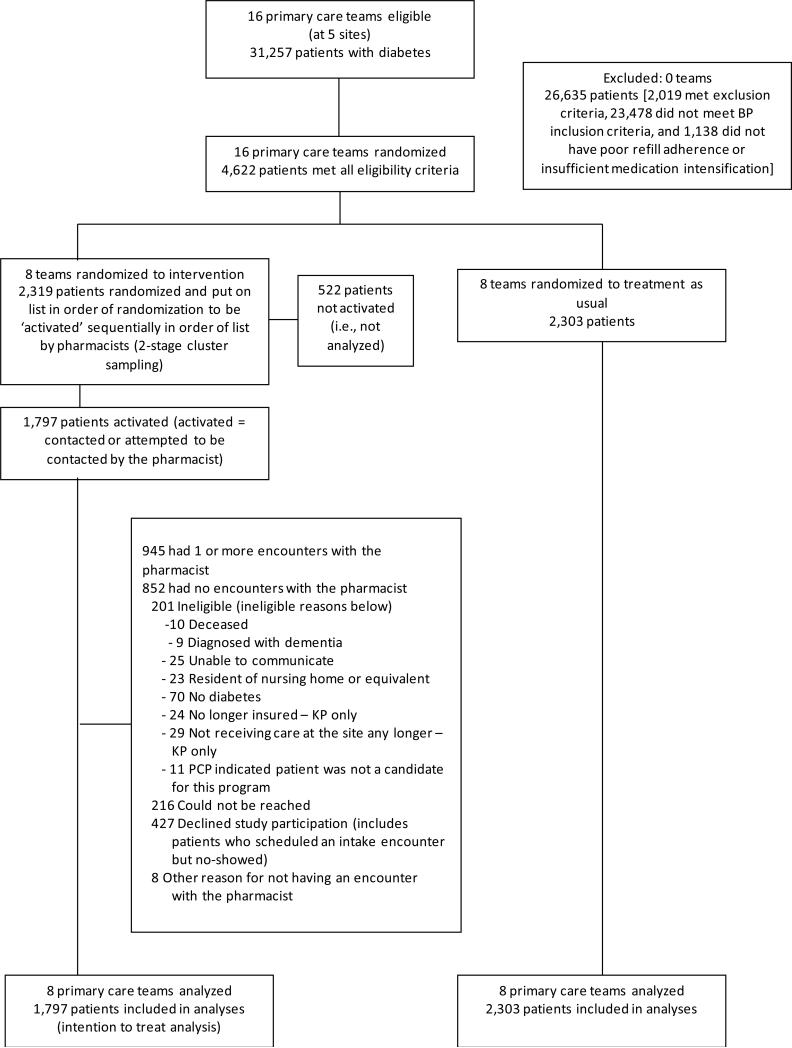
The CONSORT diagram in Figure 1 shows participant flow in the intervention. Table 1 shows that the baseline characteristics of eligible intervention and control patients were similar. Fifteen percent of diabetic patients were eligible for the intervention. Most of those excluded did not have persistent hypertension. There were no differences in age, race/ethnicity or gender between the 1797 intervention team patients whom the pharmacists tried to contact and the 522 whom they did not have time to try to contact.
Figure 1.
CONSORT diagram
Table 1.
Baseline Characteristics of Intervention and Control Team Patients
| Characteristics | Intervention (N=1,797) | Control (N=2,303) |
|---|---|---|
| N (%) or Mean ± SD | ||
| Age (years) on 8/1/08 | 65.3 ± 11.7 | 65.3 ± 12.1 |
| Male | 1,250 (70) | 1,582 (69) |
| Race | ||
| White | 928 (52) | 1,128 (49) |
| Black | 275 (15) | 353 (15) |
| Hispanic | 174 (10) | 257 (11) |
| Asian | 115 (6) | 180 (8) |
| Other | 61 (3) | 83 (4) |
| Unknown | 244 (14) | 302 (13) |
| Identified with adherence gap(s) >=20% | 1,059 (59) | 1,361 (59) |
| Most recent Systolic Blood Pressure in the last 3 months (mm Hg)* | 157 ±12 | 156 ± 13 |
| Mean Systolic Blood Pressure over last 9 months (mm Hg)* | 154 ± 10 | 153 ± 12 |
| Most recent Diastolic Blood Pressure in the last 3 months (mm Hg)* | 79 ± 11 | 79 ± 12 |
| Mean Diastolic Blood Pressure over last 9 months (mm Hg)* | 79 ± 9 | 78 ± 11 |
| Most recent Hemoglobin A1c in the last 12 months (%) | 7.4 ±1.6 | 7.4 ±1.6 |
| Most recent LDL cholesterol in the last 12 months (mg/dL) | 94 ±33 | 95 ±34 |
| On insulin** | 479 (27) | 614 (27) |
| On a statin** | 1,134 (63) | 1,478 (64) |
| On a moderate or higher dose statin** | 992 (55) | 1,257 (55) |
| Moderate or higher doses BP classes** | 1.8 ± 1.4 | 1.8 ± 1.3 |
| Classes of antihypertensive medications** | 2.4 ±1.5 | 2.3 ±1.5 |
| Primary care visits in the past 12 months | 4.9 ±4.9 | 4.9 ±5.3 |
Weighting based on blood pressure strata groups (140-149, 150-159, and 160+). A greater proportion of participants in the intervention group were in the higher stratas because of our activation protocol. We weighted the participants in the lower stratas of the intervention so that the distribution for intervention and control would be similar.
Refill documented within 120 days of eligible date
Health care utilization and intervention engagement during study period
Table 2 shows that there were no differences in health services utilization between eligible intervention and control patients during the 14-month intervention period. Intervention patients were more likely than control patients to undergo medication changes during the 6 month period following the quarter start date, although both groups had high rates of medication changes.
Table 2.
Other resource use during the 14-month intervention period (July 2008-August 2009)
| Intervention (N=1,797) | Control (N=2,303) | ||
|---|---|---|---|
| N (%) or Mean ± SD | P Value | ||
| Hospitalized in VA or KP facility | 227 (13) | 300 (13) | 0.71 |
| Primary Care Visits | 4.6 ± 5.9 | 4.3 ± 6.1 | 0.10 |
| Had ER Visit | 434 (24) | 532 (23) | 0.43 |
| Proportion of patients with BP medication changes* | 69.7% | 63.0% | <.01 |
Calculated as a change (increase in dose, decrease in dose, adding of a class, dropping of a class, or switching of a medication within the same class) in BP medications during the six-month period following the quarter start date
Table 3 presents information on mean and median number and frequency of pharmacist encounters among the 945 eligible intervention team patients who had at least one encounter with AIM pharmacists. Participants had a median of 3.8 pharmacist encounters and a median of 9 weeks follow-up during their enrollment in the program. Their intake encounter averaged 50 minutes and follow-up encounters averaged 27 minutes. 60.8% of all encounters took place by phone. 69% of all patients were discharged with a target BP.
Table 3.
Description of Intervention Processes
| N (%) or Mean ± SD | |
|---|---|
| Pharmacist level | |
| Patients activated over the entire intervention period | 1797 |
| Patients having at least one encounter with the pharmacist | 945 (52.6% of activated) |
| Patient level | |
| Encounters during 1st enrollment, (median) | 3.8 ± 3.2 (3) |
| Days enrolled during 1st enrollment, (median) | 62 ±71 (41) |
| Enrolled in the program more than once during intervention | 35 (3.7) |
| Encounters over entire intervention period, (median) | 3.9 ±3.3 (3) |
| Days enrolled over entire intervention period, (median) | 64 ± 71 (42) |
| Discharged at the 1st encounter | 184 (19.5) |
| Reasons for discharge (examining a patient's first discharge in the program) | |
| Had a target BP (Clinic or home) | 650 (68.8) |
| Lost to follow-up (e.g., no-showed for 3 encounters) or enrolled for 6+ months and no further progress was being made | 97 (10.3) |
| Program ended (i.e., 14 month intervention ended) | 74 (7.8) |
| Declined further participation | 50 (5.3) |
| DBP<60 | 46 (4.9) |
| On maximum medications | 22 (2.3) |
| DBP<60 and on maximum medications | 2 (0.2) |
| Other | 4 (0.4) |
| Encounter level | |
| Length of intake encounters (minutes), (median)* | 50.2 ± 7.9 (52) |
| Length of follow-up encounters (minutes), (median)* | 26.9 ± 4.7 (28) |
| Phone encounters (not Office) | 2241 (60.8) |
From pharmacist daily logs; collected during four different weeks over the intervention period
Team-Level Changes in SBP over the 14- Month Intervention Period and Six Months After
In our primary analysis, the intervention group SBP change from the 6 calendar months prior to vs. 6-months after the 14-month intervention was not different from control group, declining 8.9 mm Hg in the intervention group as compared to a 9.0 mm Hg for the control group (difference of 0.18 [ -0.77, 1.13]). There were no differences in mean A1c and LDL levels between intervention and control teams after the end of the intervention period (examining a 12 month period): LDL mean values of 89.1 mg/dl (31.1) on intervention teams vs. 87.8 mg/dl (32.9) on control teams and A1c mean of 7.4% (1.4) and 7.6% (1.6) on control teams.
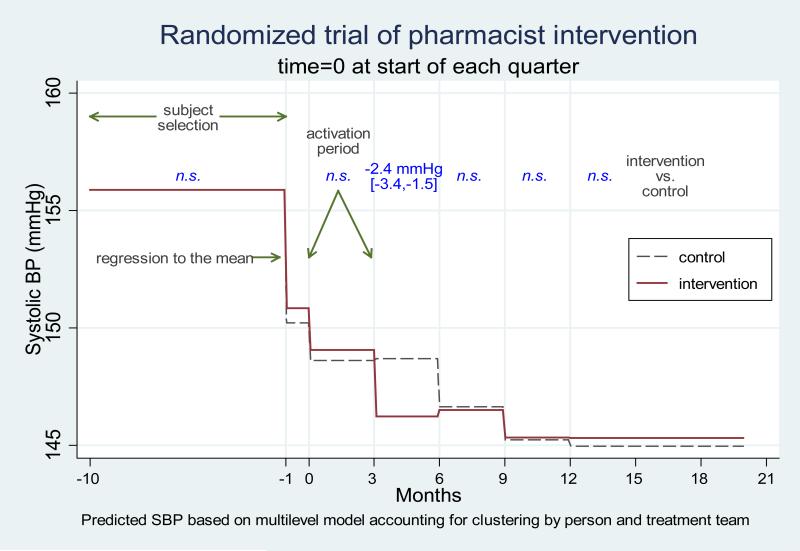
Figure 2 reports the results of our secondary analyses examining short-term SBP changes, examining 3-month intervals through the study period calculated before and after the first date of the quarter in which each participant was activated (see Appendix D). Control participants improved at a slower rate. By the end of quarter 1, the period after the quarter in which patients were activated, mean SBPs had dropped 7.2 mm Hg in the control group compared to 9.7 mm Hg in the intervention group (difference of 2.4 mm Hg [1.5, 3.4]; p<0.001). By six months and throughout the remainder of follow-up, eligible control team patients’ mean SBP were indistinguishable from those of intervention group participants.
Figure 2.
Predicted Changes in Systolic Blood Pressures Per Quarter Between Eligible Intervention and Usual Care Teams in Multi-level Models
Table 4 illustrates the observational cohort results comparing those who agreed to participate (i.e., activated intervention patients who had at least one encounter with a pharmacist) to those who did not get the intervention (i.e., activated intervention patients who did not have an encounter with the pharmacist AND all control patients). There were more medication changes among those who accepted the intervention as compared to the non-treated group. The intervention participants who had at least one encounter achieved a maximal SBP improvement of approximately 4 mm Hg greater than the intervention participants with no encounters. This difference also disappeared as the control group approached the same level of control over time.
Table 4.
Medication changes 6 months following the quarter start date (observational results from “as treated analysis”)*
| Treated (N=945) | Non-treated (N=3,155) | |
|---|---|---|
| % | % | |
| Any change | 81.2 | 61.6 |
| Increase dose | 33.6 | 19.6 |
| Add class | 57.3 | 38.1 |
| Switch drug | 6.5 | 3.9 |
| Drop class | 15.1 | 17.6 |
| Decrease dose | 6.1 | 3.9 |
These results should not be interpreted as being from the randomized trial, they are the observational cohort results comparing those accepting the intervention (treated = activated intervention patients who had an encounter) to those who did not get the intervention (Non-treated = activation intervention patients who did not have an encounter AND all control patients).
Discussion
In this team-level pragmatic randomized trial providing targeted adherence counseling and medication management to diabetes patients with persistent hypertension in two high-performing integrated healthcare systems, we sought to respond to the Institute of Medicine's call to evaluate state of-the-art approaches to improve quality of care in real-life clinical practice.42, 57 Integrated delivery systems provide health care to over 40 million Americans, and with the spread of Accountable Care Organizations (ACOs), increasing numbers of patients will be receiving care in systems that have virtual integration.58,59 We designed AIM to incorporate elements of previously successful interventions and apply them to a high-risk population with diabetes, hypertension and presumed medication adherence or management problems.60 We used evidence-based algorithms for medication intensification and provided pharmacists with objective data on participants’ refill gaps and training in evidence-based MI approaches to assist their adherence assessments, yet found similar levels of SBP reduction among intervention and usual care patients six months after the end of the intervention period. Therefore, the answer to our primary question is that at least within higher performing healthcare systems additional monetary and staff resources devoted to state of the art interventions cannot be counted on to improve BP control beyond usual care and may simply add to greater polypharmacy in intervention subjects.
Intervention implementation was successful. Participants had an average of 4 encounters totaling several hours over 9 weeks. Patients who met with AIM pharmacists had more rapid intensification of their medications. The intervention was effective in reducing BP, as both during and immediately after receiving the intervention activated intervention team patients on average had clinically and statistically significant 2.4 mm Hg lower SBPs than control team patients. Furthermore, this average decline in BP includes the 47% of activated team patients who did not participate at all in the program. A 2.4 mm Hg difference in SBP if sustained could translate to a 6-8% reduction in stroke mortality and a 4-5% reduction in CHD mortality.61 Despite this earlier and more rapid decline in BP among the intervention patients, patients on the control teams experienced a similar decline about 3 months behind the intervention group.
Other existing programs contributed to BP lowering among this high-risk population on control teams. In both systems, rates of meeting BP performance measures among diabetic patients were routinely reported, and nurse care managers were available to all PCPs for follow-up on BP control issues. In the VA physician performance bonuses were tied in part to achieving BP control goals. At the KP sites, PHASE pharmacists were also reaching out to diabetes patients with poor risk factor control on the control teams for brief interventions. While lower than among activated intervention team patients, high rates of treatment intensification and medication changes occurred among eligible control team patients during the study period. It is also possible that the AIM intervention caused better than “usual care” in the control group. At the VA, providers on the control team were also provided with quarterly reports that listed their patients who would have been eligible for AIM, along with their adherence and intensification data. However, the literature does not suggest that individual provider audit and feedback alone is particularly effective.62
In the intervention arm, only 53% of subjects had a pharmacist encounter. Higher rates of participation might have led to a more substantial initial improvement and a detectable longer term effect. However, it is hard to conceive of a way to get higher levels of participation in a real-world setting than using proactive outreach with specially trained pharmacists who were members of the teams already providing primary care to these patients. Of potential concern, once the program ended at 14 months, all of the subjects were returned to usual care during the 6 month follow-up period used in our primary outcome analysis. Results from other recent trials have reinforced that short-term gains in risk factor control often fail to persist if there is no maintenance after program completion.29,63 However, in our study the lack of longer term difference did not appear to be a result of deterioration of control in the intervention group, but rather continued improvement in the control group, suggesting that usual care or regression to the mean for a cohort of patients selected on the basis of elevated BPs accounted for the lack of effect, not the absence of maintenance.
The study findings reinforce the importance of carefully testing the effectiveness of interventions with known efficacy in real-world practice before broad implementation. It also demonstrates the fallacy of using uncontrolled pre-post data to justify expenditures on clinical programs, as without controls our intervention would have seemed very successful, lowering SBP by almost 10 mm Hg. We collected qualitative information to explore in greater depth factors that influenced actual delivery of the intervention and both facilitators and barriers to success.52, 64 Most prior evaluations of pharmacist-led interventions shown to improve blood pressure included only volunteer clinical trial subjects, used research clinicians during the intervention, assessed BP outcomes measured as part of the study, and compared these BP outcomes of intervention and control patients made immediately after the patient participated in the intervention. In contrast, we measured the ability to translate these findings into practice by focusing on the team level, employing team-based pharmacists, assessing BP outcomes through BPs taken during routine clinic care, and evaluating the impact on the entire target population, including all those activated to receive the intervention, whether or not they were successfully reached by or ever had an encounter with the pharmacist. In this manner, we sought to provide information most relevant to health center leaders who need to decide whether to invest resources to implement interventions.
If our intervention indeed successfully deployed elements found to be most consistently effective in the experimental literature then there are four possible conclusions. The first is that we need different interventions that translate more effectively into routine clinical practice. For example, we might move from the focus on physician clinical inertia and provider-intensive clinical redesign to empower the patient to do their own intensification as suggested by a successful recent UK clinical efficacy trial that allowed patients to make a limited number of intensification steps themselves without interaction with the health care system.65 A second possibility is that we are not able to improve the control of those with persistent hypertension because we can't identify them accurately. Emphasizing the imprecision of routine clinical BP measurement, data from the recent PROGRESS trial showed that “Six months after BP was stabilized on treatment, if SBP was measured as having increased by >10 mm Hg, six of those measurements would be false positives for every true increase of ≥10 mm Hg.”66 Other recent studies have also highlighted the risk of misclassification based on clinic or home BPs alone. 67, 68 If using an average of recent routine clinical BP measurements to identify eligible patients results in targeting many patients who do not in fact have elevated BP, then it is not surprising to fail to find a sustained improvement in BP over and above usual care. A third possibility is that the 47% of eligible patients who did not participate in the program represent the small “intractable” group of patients, in whom BP control is essentially impossible, owing to either biological or psychosocial factors. And finally, the greater medication intensification in the intervention teams without correspondingly greater sustained improved SBPs relative to the usual care group suggests that the intervention was more effective in increasing medications than in improving medication adherence.
In summary, in these two high performing health-care systems that have achieved high levels of BP control a state of the art intensive pharmacist-led program did not provide sustained incremental benefit for the small target group of “fall-outs”. Indeed, the systems that have been put into place to achieve the impressive 80% rates of “control” may be demonstrating best achievable practices. While these programs improve control for some patients during a given time period, other patients fall out of control during that same time period. As long as current usual care practices and incentives remain in place, these systems may be nearing their near maximal safe control rates for the diabetic population. Our study emphasizes how difficult it is to move the control barometer once high rates of control are achieved and suggests that clinical inertia alone is not what is preventing us from reaching optimal control in all our patients.69 Our study further reinforces the importance of rigorously evaluating in different real-life clinical settings programs found in efficacy trials to be effective before urging their widespread adoption in all settings.
Supplementary Material
Acknowledgements
The study team thanks our extraordinary clinical pharmacists, as well as their supervisors, at KP and VA. Many thanks also to Rob Holleman for his meticulous data management and analysis. We also thank our advisory team members Dr. Pam Reeves, Dr. Adam Tremblay, Dr. Fran Cunningham, Dr. Steve Bernstein, Dr. John Piette, Dr. Jim Chan, and Mr. Tom Stavenger. Dr. Rod Hayward made valuable contributions to an earlier draft of this manuscript.
Primary Funding Sources
Department of Veterans Affairs (VA) Health Services Research and Development (HSR&D) SDP 06-128 and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 5 R18 DK076622
Footnotes
Clinical trial registration: www.clinicaltrials.gov (NCT00495794)
Conflicts of Interests
None
References
- 1.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 2.The ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. NEJM. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The CDC Diabetes Cost-effectiveness Group Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287(19):2542–2551. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P, Engelgau MM, Norris SL, Gregg EW, Narayan KM. Application of economic analysis to diabetes and diabetes care. Ann Intern Med. 2004;140(11):972–977. doi: 10.7326/0003-4819-140-11-200406010-00039. [DOI] [PubMed] [Google Scholar]
- 5.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 6.Preis SR, Pencina MJ, Hwang SJ, D'Agostino RB, Sr, Savage PJ, Levy D, Fox CS. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120(3):212–220. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. [December 20, 2011];CCHRI Report on Quality:2010. 2010 from http://tenfold.biz/report_card.php?group=HMO&report_year=2010.
- 8.United States Department of Veteran Affairs [September 15, 2011];Quality of Care. 2010 from http://www.qualityofcare.va.gov/reports/graph.cfm?CFID=4064909&CFTOKEN=28917425.
- 9.Kerr EA, Gerzoff RB, Krein SL, Selby JV, Piette JD, Curb JD, Herman WH, Marrero DG, Narayan KM, Safford MM, Thompson T, Mangione CM. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med. 2004;141(4):272–281. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- 10.Van Herck P, De Smedt D, Annemans L, Remmen R, Rosenthal MB, Sermeus W. Systematic review: Effects, design choices, and context of pay-for-performance in health care. BMC Health Serv Res. 2010;10:247. doi: 10.1186/1472-6963-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169(19):1748–1755. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;(3):CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 13.Kerr EA, Fleming B. Making performance indicators work: experiences of US Veterans Health Administration. BMJ. 2007;335(7627):971–973. doi: 10.1136/bmj.39358.498889.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolen SD, Samuels TA, Yeh HC, Marinopoulos SS, McGuire M, Abuid M, Brancati FL. Failure to intensify antihypertensive treatment by primary care providers: a cohort study in adults with diabetes mellitus and hypertension. J Gen Intern Med. 2008;23(5):543–550. doi: 10.1007/s11606-008-0507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28(2):337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heisler M, Hogan MM, Hofer TP, Schmittdiel JA, Pladevall M, Kerr EA. When more is not better: treatment intensification among hypertensive patients with poor medication adherence. Circulation. 2008;117(22):2884–2892. doi: 10.1161/CIRCULATIONAHA.107.724104. [DOI] [PubMed] [Google Scholar]
- 17.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148(10):717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- 18.Krein SL, Hofer TP, Holleman R, Piette JD, Klamerus ML, Kerr EA. More than a pain in the neck: how discussing chronic pain affects hypertension medication intensification. J Gen Intern Med. 2009;24(8):911–916. doi: 10.1007/s11606-009-1020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuels TA, Bolen S, Yeh HC, Abuid M, Marinopoulos SS, Weiner JP, McGuire M, Brancati FL. Missed opportunities in diabetes management: a longitudinal assessment of factors associated with sub-optimal quality. J Gen Intern Med. 2008;23(11):1770–1777. doi: 10.1007/s11606-008-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmittdiel JA, Uratsu CS, Karter AJ, Heisler M, Subramanian U, Mangione CM, Selby JV. Why don't diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23(5):588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bex SD, Boldt AS, Needham SB, Bolf SM, Walston CM, Ramsey DC, Schmelz AN, Zillich AJ. Effectiveness of a hypertension care management program provided by clinical pharmacists for veterans. Pharmacotherapy. 2011;31(1):31–38. doi: 10.1592/phco.31.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Bosworth HB, Olsen MK, Gentry P, Orr M, Dudley T, McCant F, Oddone EZ. Nurse administered telephone intervention for blood pressure control: a patient-tailored multifactorial intervention. Patient Educ Couns. 2005;57(1):5–14. doi: 10.1016/j.pec.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, Adams MB, Svetkey LP, Reed SD, Li Y, Dolor RJ, Oddone EZ. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med. 2009;151(10):687–695. doi: 10.1059/0003-4819-151-10-200911170-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt JS, Siemienczuk J, Pape G, Rozenfeld Y, MacKay J, LeBlanc BH, Touchette D. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23(12):1966–1972. doi: 10.1007/s11606-008-0791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson SH, Majumdar SR, Tsuyuki RT, Lewanczuk RZ, Spooner R, Johnson JA. Effect of adding pharmacists to primary care teams on blood pressure control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2011;34(1):20–26. doi: 10.2337/dc10-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobari H, Arimoto T, Shimojo N, Yuhara K, Noda H, Yamagishi K, Iso H. Physician- pharmacist cooperation program for blood pressure control in patients with hypertension: a randomized-controlled trial. Am J Hypertens. 2010;23(10):1144–1152. doi: 10.1038/ajh.2010.127. [DOI] [PubMed] [Google Scholar]
- 27.Weber CA, Ernst ME, Sezate GS, Zheng S, Carter BL. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170(18):1634–1639. doi: 10.1001/archinternmed.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, Carrell D, Tyll L, Larson EB, Thompson RS. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299(24):2857–2867. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JK, Grace KA, Taylor AJ. Effect of a Pharmacy Care Program on Medication Adherence and Persistence, Blood Pressure, and Low-Density Lipoprotein Cholesterol: A Randomized Controlled Trial. JAMA. 2006;296(21):2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 30.Planas LG, Crosby KM, Mitchell KD, Farmer KC. Evaluation of a Hypertension Medication Therapy Management Program in Patients with Diabetes. J Am Pharm Assoc. 2009;49(2):164–168. doi: 10.1331/JAPhA.2009.08164. [DOI] [PubMed] [Google Scholar]
- 31.Magid DJ, Ho PM, Olson KL, Brand DW, Welch LK, Snow KE, Lambert-Kerzner AC, Plomondon ME, Havranek EP. A multimodal blood pressure control intervention in 3 healthcare systems. American Journal of Managed Care. 2011;17(4):e96–e103. [PubMed] [Google Scholar]
- 32.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selby JV, Uratsu CS, Fireman B, Schmittdiel JA, Peng T, Rodondi N, Karter AJ, Kerr EA. Treatment intensification and risk factor control: toward more clinically relevant quality measures. Med Care. 2009;47(4):395–402. doi: 10.1097/mlr.0b013e31818d775c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anstiss T. Motivational interviewing in primary care. J Clin Psychol Med Settings. 2009;16(1):87–93. doi: 10.1007/s10880-009-9155-x. [DOI] [PubMed] [Google Scholar]
- 35.Greaves CJ, Middlebrooke A, O'Loughlin L, Holland S, Piper J, Steele A, Gale T, Hammerton F, Daly M. Motivational interviewing for modifying diabetes risk: a randomised controlled trial. Br J Gen Pract. 2008;58(553):535–540. doi: 10.3399/bjgp08X319648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden A, Butterworth SW, Prochaska JO. Motivational interviewing-based health coaching as a chronic care intervention. J Eval Clin Pract. 2010;16(1):166–174. doi: 10.1111/j.1365-2753.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- 37.Rubak S, Sandbaek A, Lauritzen T, Borch-Johnsen K, Christensen B. General practitioners trained in motivational interviewing can positively affect the attitude to behaviour change in people with type 2 diabetes. One year follow-up of an RCT, ADDITION Denmark. Scand J Prim Health Care. 2009;27(3):172–179. doi: 10.1080/02813430903072876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubak S, Sandboek A, Lauritzen T, Christensen B. Motivational interviewing: A systematic review and meta-analysis. British Journal of General Practice. 2005;55:305–312. [PMC free article] [PubMed] [Google Scholar]
- 39.Chisholm-Burns MA, Kim Lee J, Spivey CA, Slack M, Herrier RN, Hall-Lipsy E, Graff Zivin J, Abraham I, Palmer J, Martin JR, Kramer SS, Wunz T. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48(10):923–933. doi: 10.1097/MLR.0b013e3181e57962. [DOI] [PubMed] [Google Scholar]
- 40.Carter BL, Doucette WR, Franciscus CL, Ardery G, Kluesner KM, Chrischilles EA. Deterioration of blood pressure control after discontinuation of a physician-pharmacist collaborative intervention. Pharmacotherapy. 2010;30(3):228–235. doi: 10.1592/phco.30.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 42.Ware JH, Hamel MB. Pragmatic trials--guides to better patient care? N Engl J Med. 2011;364(18):1685–1687. doi: 10.1056/NEJMp1103502. [DOI] [PubMed] [Google Scholar]
- 43.Heisler M, Hofer TP, Klamerus ML, Schmittdiel J, Selby J, Hogan MM, Bosworth HB, Tremblay A, Kerr EA. Study protocol: the Adherence and Intensification of Medications (AIM) study--a cluster randomized controlled effectiveness study. Trials. 2010;11:95. doi: 10.1186/1745-6215-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krein SL, Bingham CR, McCarthy JF, Mitchinson A, Payes J, Valenstein M. Diabetes treatment among VA patients with comorbid serious mental illness. Psychiatr Serv. 2006;57(7):1016–1021. doi: 10.1176/ps.2006.57.7.1016. [DOI] [PubMed] [Google Scholar]
- 45.Krein SL, Heisler M, Piette JD, Butchart A, Kerr EA. Overcoming the Influence of Chronic Pain on Older Patients’ Difficulty With Recommended Self-Management Activities. Gerontologist. 2007;47(1):61–68. doi: 10.1093/geront/47.1.61. [DOI] [PubMed] [Google Scholar]
- 46.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, Canning C, Platt R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37(9):846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Grant RW, Devita NG, Singer DE, Meigs JB. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26(5):1408–1412. doi: 10.2337/diacare.26.5.1408. [DOI] [PubMed] [Google Scholar]
- 48.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 49.Resnicow K, DiIorio C, Soet JE, Ernst D, Borrelli B, Hecht J. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol. 2002;21(5):444–451. [PubMed] [Google Scholar]
- 50.Rollnick S, Mason P, Butler C. Health behavior change: a guide for practicioners. Churchill Livongstone; Edinburgh and New York: 2008. [Google Scholar]
- 51.Resnicow K. 1-PASS coding system for motivational interviewing: introduction and scoring. Rollins School of Public Health, Emory University; 2002. pp. 1–7. [Google Scholar]
- 52.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Amer J Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee.The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 54.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174–180. doi: 10.1016/j.pec.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Handley M, MacGregor K, Schillinger D, Sharifi C, Wong S, Bodenheimer T. Using action plans to help primary care patients adopt healthy behaviors: a descriptive study. J Am Board Fam Med. 2006;19(3):224–231. doi: 10.3122/jabfm.19.3.224. [DOI] [PubMed] [Google Scholar]
- 56.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma Disputed: Can Aggressively Lowering Blood Pressure in Hypertensive Patients with Coronary Artery Disease Be Dangerous? Ann Intern Med. 2006;144(12):884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 57.Institute of Medicine Committee on Enhancing Federal Healthcare Quality Programs CJEJ . Leadership by example: coordinating government roles in improving health care quality. National Academies Press; Washington, DC: 2003. [Google Scholar]
- 58.Enthoven AC. Integrated Delivery Systems: the cure for fragmentation. Am J Manag Care. 2009;15:S284–S290. [PubMed] [Google Scholar]
- 59.Integrated Healthcare Networks (IHN) Market Overview. Trumbull, CT: 2011. Knowledge Source: Health Care Market Information. [Google Scholar]
- 60.Timbie JW, Hayward RA, Vijan S. Diminishing efficacy of combination therapy, response-heterogeneity, and treatment intolerance limit the attainability of tight risk factor control in patients with diabetes. Health Serv Res. 2010;45(2):437–456. doi: 10.1111/j.1475-6773.2009.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288(15):1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 62.Walsh JM, McDonald KM, Shojania KG, Sundaram V, Nayak S, Lewis R, Owens DK, Goldstein MK. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44(7):646–657. doi: 10.1097/01.mlr.0000220260.30768.32. [DOI] [PubMed] [Google Scholar]
- 63.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 64.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, Kaambwa B, Banting M, Bryan S, Little P, Williams B, Hobbs FD. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376(9736):163–172. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 66.Keenan K, Hayen A, Neal BC, Irwig L. Long term monitoring in patients receiving treatment to lower blood pressure: analysis of data from placebo controlled randomised controlled trial. BMJ. 2009;338:b1492. doi: 10.1136/bmj.b1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FD, Deeks JJ, Heneghan C, Roberts N, McManus RJ. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. doi: 10.1136/bmj.d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med. 2011;154:781–788. doi: 10.7326/0003-4819-154-12-201106210-00005. [DOI] [PubMed] [Google Scholar]
- 69.Giugliano D, Esposito K. Clinical inertia as a clinical safeguard. JAMA. 2011;305(15):1591–1592. doi: 10.1001/jama.2011.490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.