Abstract
Purpose of Review
One of the major obstacles in fully understanding HIV transmission comes from the impracticality of studying transmission in humans. Because of this encumbrance, the early phases of HIV transmission and systemic dissemination are poorly understood. In order to fully comprehend these critical steps in HIV infection, animal models must be devised to accurately reflect HIV’s mode of action. This review seeks to highlight the essential nature of modeling HIV transmission in nonhuman primates.
Recent Findings
Recently it was discovered that HIV infection is established in newly infected recipients by a single or few transmitted/founder variants. This has reshaped how animal modeling is conducted with researchers currently recapitulating a physiologically relevant, low-titer infection. Pertinent animal models have been established for the most common routes of infection, including rectal, vaginal, and penile transmission; models for intravenous and oral transmission are still in developmental stages.
Summary
These limited dose models now accurately reflect HIV transmission in humans, and provide a realistic experimental platform for vaccine development and other intervention strategies which can be used to inform vaccine development in humans. Using information obtained in NHP and human trials, it is conceivable to envision effective prevention modalities in the near future.
Keywords: transmission, NHP models, acute infection, mucosa, SIV
Introduction
HIV transmission in humans is a complex biological process confounded by the difficulty of identifying and characterizing primary HIV infections. These obstacles are further complicated by socioeconomic demographics, stigmatism of sexual practices, and the social fridge nature of these at-risk populations. Nonhuman primate (NHP) models have been developed to examine key features of HIV disease that otherwise would be challenging or impossible to determine in humans. In order to most accurately model HIV transmission in NHP, we must first identify what is known about HIV transmission in humans then determine what key aspects of transmission are or can be faithfully reproduced in this model. NHP species not naturally infected with simian immunodeficiency virus (SIV) have been shown to be an excellent model of HIV disease by accurately reproducing immune activation, CD4 depletion, and significant viral replication when infected with SIV from other naturally infected monkeys. Although this model has been used for years, SIV transmission studies in NHP have recently been modified to incorporate new findings from HIV transmission studies. At its most basic level, HIV transmission is caused by the exposure of mucosal surfaces or the blood compartment to infectious virus. Epidemiological studies have identified a number of key parameters detailing the risks of HIV transmission which include (i) identifying the most probable behaviors conducive to infection and estimating the infection rate per unprotected exposure to be 1:5 to 1:3,000 (depending on behavior and site of exposure) [1], (ii) determining that transmission is more likely to occur from partners with primary HIV infection due to unknown infection status, higher viral load, and potentially a more fit virus [2–4], (iii) identifying preexisting sexually transmitted diseases that increase probability of infection [5–7], and (iv) proving that male circumcision reduces infection rate by over 60% [8–10]. Understanding how humans are exposed to infectious virus and how often these events lead to productive infection is crucial to creating a NHP model that most accurately mirrors HIV infection.
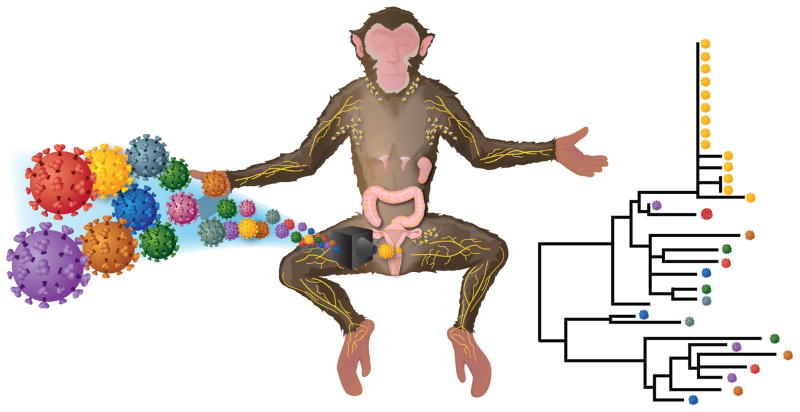
One essential concept in HIV transmission predicted by epidemiological studies and proven by molecular and mathematical studies involves a genetic bottleneck between donor and recipient. In general, a genetic bottleneck is the reduction in genetic diversity of a population with only relatively few lineages surviving some otherwise catastrophic event. While this new population is by definition reduced, the extent or completeness of the reduction is variable. Since HIV is found as a quasi-species with varying degrees of diversity associated with the time since infection, each infected individual represents a population of virus. The genetic bottleneck during transmission from a diverse population has been known imprecisely for years with chronic patients being defined as containing a heterogeneous viral population and most primary infections as being homogeneous. Recently, we and others have discovered that during transmission, genetic diversity is reduced so drastically that the vast majority of infected individuals are productively infected with a single genetic unit [11–15]. Additionally, patients infected with one or few variants represent over 95% of all HIV infections in these studies suggesting a very low infectious dose at time of transmission, a significant host barrier to new infections or both. A key technological advance allowing for the exact enumeration of infecting variants is single genome amplification (SGA), which is a limiting dilution PCR where cDNA or DNA is diluted so that the majority of reactions have only a single template. The benefits of SGA over standard bulk sequencing or cloning and then sequencing includes proportional representation of the viral population, a lack of in vitro recombination, and no Taq induced errors [13,16,17]. This remarkably simple technical advance provided the opportunity to elucidate fundamental questions in viral transmission including: (i) enumerating transmitted/founder variants [11–15], (ii) identifying and molecularly cloning the exact nucleotide sequence of these genomes [13,18], (iii) computationally modeling early viral diversification [19–22] (iv) identifying increased variants in men-who-have-sex-with-men and intravenous-drug-users [14,23–25], (v) determining the earliest anti-viral responses both innate and humoral [26–28], and (vi) defining the phenotype of transmitted viruses which includes the requirement for CCR5 [13,18,29,30]. However, there are limitations in studying HIV transmission, which include an inability to sample relevant tissues immediately following exposure, an inability to genetically characterize or quantify the donor’s virus at the time of transmission, and to unambiguously identify the route of exposure. These obstacles make an accurate NHP model an essential tool to fully understanding HIV transmission (Fig. 1).
Figure 1. Model of the viral genetic-bottleneck following mucosal challenge.
Despite exposing animals mucosally with a large number of genetically distinguishable variants, the systemic dissemination of a single genetic unit can be obtained in NHP models thereby recapitulating HIV-1 infection. Genetic analyses of variants that are transmitted to the inoculum allows for enumerating the number of variants systemically replicating and potentially identifying unique features of these lineages. However, a precise molecular description of how this genetic constraint is accomplished is still largely unknown with the earliest events of viral infection still within a metaphorical black-box. Additional experiments are needed to assess the various contributions of anatomic and other host barriers to infection and current NHP models provide the necessary sensitivity and tissue availability to describe these early events.
NHP Models of Transmission
There are many NHP models available for research. The fundamental criterion for a successful model is whether or not it recapitulates HIV infection. For mucosal infections in rhesus macaques, infection with SIV or chimeric SHIV can recapitulate the key features of HIV transmission if investigators are willing to invest time and resources. Various challenge sites and viruses can be used to initiate infection and model viral transmission including the earliest events in establishing infection in a new host. As for the sites of infection, they can be categorized as mucosal or intravenous. While the intravenous challenge model is frequently used for convenience, it is rarely utilized to model intravenous infections in humans. Mucosal transmissions are modeled most frequently using intrarectal or intravaginal challenge. Recently, there have been advances in modeling male genital track infection to better recapitulate heterosexual HIV risk. Single genome amplification has been used as a means to more precisely determine the infectious dose necessary to infect animals with a minimal number of variants, thereby reflecting the most important feature of HIV infection—low infectious titer [31–41]. Furthermore, titrating virus to a limiting dose is possible without the benefits of enumerating founder variants (i.e. using cloned virus stocks) but requires significant animal testing [42]. Finally, using a low-dose, repeated challenge with an infection rate of approximately 20%, allows for a reduced founder population and can be performed in a reasonable time frame [37,41,43]. Regardless of route of challenge, using a single challenge titered-dose provides the opportunity for few but often multiple transmitted/founder variants while a repetitive low-dose challenge often results in productive infection of a single transmitted/founder variant perfectly modeling the vast majority of HIV infections (Table 1).
Table 1.
Characteristics of various modes of transmission
| Route of Transmission | Epithelial Barrier | Target Cell Availability | Key Advantages | Limitations | Selected References | |
|---|---|---|---|---|---|---|
| Rectal | Columnar | Abundant | Most widely used infection route, consistent infection rate, requires limited dose, primary site of infection in developed countries | Frequently overdosed, atraumatic model only | [31,32,41] | |
| Female Genital Tract |
Vagina/Ectocervix Endocervix |
Squamous Columnar |
Abundant Limited |
Widely used infection route, modeling the most frequent mode of infection globally | Inconsistent infection rate, typically includes monitoring menstrual cycle, requires significant inoculum dose | [33,44,45] |
| Male Genital Tract |
Foreskin Urethra |
Keratinized Squamous Columnar |
Limited Limited |
Recently developed as a transmission model, frequent site of male infections | Technically challenging to perform, requires significant inoculum dose | [34,46–48] |
| Oral | Squamous & Columnar | Variable | Understudied, but important site of transmission | Logistically challenging to establish model | [49,50] | |
| Non Mucosal | No Barrier | Abundant | Consistent infection rate, simple to administer | Typically not used to model transmission | -- | |
Rectal
HIV infection resulting from unprotected anal intercourse (UAI) is the most common route of disease transmission in many developed countries and represents the highest risk mode of sexual HIV transmission at 1:20 – 1:300 infections per exposure [1]. The rectum is lined with only a single layer of protective columnar epithelium as a barrier between infected blood or semen and the lymphoid-rich mucosal tissue [44]. Intestinal mucosal tissues contain the largest reservoir of activated CD4+ and CCR5+ cells in the body (particularly in the lamina propria and within lymphoid aggregates) and represents an environment prime for viral infection. Rectal infection is the most commonly utilized NHP model for its consistency and as an authentic site of HIV infection. Previous models of rectal transmission have relied upon high dose challenges in order to ensure infection, yet recent findings have shown that low dose challenges in NHPs provide a more accurate model for the route of HIV transmission and dissemination throughout the body [31,32]. In studies using a single challenge, the goals are to infect most if not all animals with as few variants as possible. Based on a Poisson distribution, it is apparent that at a dose required to infect all animals in a study, the majority of animals will not be infected with a single variant, but most likely infected with 3–6 unique variants [31]. To ensure a single variant infection, a limited dilution challenge is necessary so that the majority of animals are uninfected (i.e. ~20% infection rate) [41]. These models provided for a limited transmitted/founder population, which revealed a delay in detectable viral load in animals infected with one or few variants. Furthermore, the infecting virus originated in multiple sites within the phylogenetic tree suggesting an unbiased selection of variants. This infection route using single or repetitive titered challenges accurately reflects HIV transmission via UAI and should be used for studies aimed at identifying the key early events surrounding transmission and systemic dissemination.
Female Genital Tract
Heterosexual transmission of HIV via the vagina is the most prevalent mode of disease transmission globally, despite the fact that it has a comparatively low risk per exposure rate estimated at 1:200 – 1:2000 [1]. In female genital tract transmission, virus carried in semen or blood contacts both the vagina and the cervix with a mucosal barrier consisting of a single layer of columnar epithelium (in the endocervix) or squamous nonkeritinized epithelium in the vagina or ectocervix [44]. As with rectal challenges, until recently, vast excess quantities of virus were used to challenge animals vaginally. These studies focused on the endocervix for convenience, but may have been interpreted by others as the major or only site of infection [45]. Recently, a lower dosed challenge model has been adopted which better imitates HIV in animals [33]. In this study, the number of transmitted/founder variants was determined in animals challenges intravaginally at either 105 or 103 infectious units. Although there was greater variation in the number of variants vaginally compared to rectal challenges, there was clearly a dose effect and some animals at the lower dose showed evidence of productive infection with a single variant. Additional studies using a low, titered dose should provide additional insight into where in the female genital tract infection originates and how infection disseminates systemically.
Male Genital Tract
Penile HIV transmission occurs during homo- or hetero- sexual intercourse during which virus is transferred from infectious cervicovaginal or rectal secretions. Putatively, infection can occur either at the foreskin (which presents an epithelial barrier of squamous, poorly keratinized cells) or in the penile urethra (in which stratified, columnar epithelium acts as a barrier to transmission) [46]. Until recently, this route of transmission as an NHP model was not well developed. A new emphasis on relevant models has led to the development of two NHP models for penile transmission [34,47]. Here researchers expose penis to infectious virus by dipping a flaccid penis into infectious virus or by forming a cup with the foreskin and applying virus. Productive infection was limited to a single viral variant per animal using a repeated challenge model [34]. Recently, this model has been used in an adenovirus-vector based vaccine efficacy study recapitulating a clinical trial humans [48]. Additional studies are necessary to elucidate the initial sites of productive infection and routes of systemic dissemination following male genital tract exposure.
Oral
Oral transmission of HIV occurs primarily during the exposure to infected breastmilk, maternal vaginal secretions intrapartum, infected blood, or semen. For infants, infection can occur from mouth through the upper gastrointestinal tract. For adults, it is unlikely that infectious virus can survive in an acidic stomach and infection is more likely to occur in mouth or esophagus. Importantly, tonsils are highly active secondary lymphoid tissue and are a likely site of viral infection. Depending on the exact site of viral transmission, there are various barriers to infection and target cell availability. Currently, there are limited NHP models dedicated to oral transmission [49,50]. Additional studies will be needed to expand our understanding of oral transmission.
Non Mucosal Infections
HIV infection via blood contact occurs during direct blood to blood contact (i.e. sharing contaminated needles), or in utero when virus from the mother crosses the placental barrier. Direct intravenous infection is commonly used to infect animals, but not specifically to model this route of HIV transmission. In bypassing a mucosal barrier, intravenous infections eliminate the genetic bottleneck and alters the dynamics of viral replication and systemic dissemination. Little effort is made in this model to limit transmitted/founder variants found in most human patients [23,24], but doing so could provide useful information as to the sites and timing of early viral replication.
Optimally Using NHP Models
Having an authentic model of HIV transmission is useful in understanding transmission itself, but it is also essential for determining the correct method to challenge animals in vaccine trials, therapeutic interventions, passive antibody studies, and in viral reservoir studies. High-dose intravenous challenge is an unrealistic model for vaccine intervention because it excludes the mucosal barrier and alters the dynamics of viral dissemination [32]. Even high-dose mucosal challenges are potentially unnecessarily stringent for vaccine efficacy studies. Many investigators improved the challenge portion of vaccine efficacy studies by challenging animals with a single, limited dose or by repeated exposure at a fraction of that dose [35–41,48,51–58]. For example, Barouch et al. and Hansen et al. utilized a repetitive low-dose intrarectal challenge (described in [31,59]) as a bases for a heterologous challenge in a successful adenovirus-virus-based vaccine study [43] and cytomegalovirus-vector-based vaccine study [52]. Furthermore, Vaccari et al. specifically tested the notion that vaccine efficacy was correlated to challenge dose and found partial vaccine effect in low-dose challenged animals but no vaccine effect in animals challenged at a 10 fold higher dose [39]. It is clear that challenging animals at too high a dose does not allow for accurate assessment of vaccine potential. However, it is uncertain if vaccine strategies that are successful in a low-dose NHP model will translate to humans, but our current opinion is that it will. Furthermore, therapeutic intervention studies and passive antibody protection studies have been used for years, but have recently been altered to limit founder variants of the chimeric HIVenv with a SIV-backbone (SHIV) virus [60–62]. Additional insights could be obtained using this more relevant transmission model.
Although the transmission event itself could be defined as productive infection of a single cell, transmission typically includes the time of exposure through establishment of productive, systemic dissemination. This period of time is also known as the eclipse phase not because the virus is dormant, but because the virus is undetectable in peripheral blood. Importantly, it is during this time that the infecting virus is at its most vulnerable. Regardless of the route of infection, plasma viremia represents the accumulation of thousands of rounds of exponential replication in a new host starting with as few as one virus to billions of viruses found throughout the host. The length of the eclipse phase is likely dependent on the route of exposure, the replicative capacity of the infecting virus, the host response and the inflammation state of the host. Furthermore, compared to HIV’s eclipse phase (7–21 days [13,20]), the time to viremia via mucosal challenges ranges from 3–9 days in nonhuman primates [31,63]. While the actual time to detectable plasma viremia is variable and dependent on dose, route, viral strain, and potentially other non-viral constituents in the inoculum [64], this period represents our best window of opportunity for intervention (i.e. the virus’s Achilles heel) because viral reservoirs are not yet fully established and viral replication is anatomically limited. Once viral reservoirs that can persist for the life of the patient are established in sufficient quantities, this small window to interdict is shut. Importantly, it is not well known how rapidly intervention must be initiated to prevent infection. However, early antiretroviral treatment in post-exposure prophylaxis studies and in latency studies suggest that reservoirs establishment sufficient to maintain infection is approximately 3–5 days post exposure [65–71]. Additional studies are necessary to more accurately determining the timing of reservoir establishment in animals infected with a limited number of transmitted/founder variants.
Conclusions
The first tenant of creating any animal model should be Primum non nocere, “first, do no harm”. Unfortunately some investigators believe that animals models can and often mislead human trials [72]. We submit that authentically reproducing HIV infection comes from robust and thoughtful animal modeling. The most compelling rationale for using models appropriately originates in a NHP study that predicts the STEP trial failure but was unfortunately performed in retrospect [51]. When new evidence is generated by NHP/SIV or human/HIV researchers, it behooves us to adapt and modify as necessary. The NHP/SIV field has actively and successfully incorporated new knowledge obtained in HIV transmission studies into new models of transmission. These improved models allow for productive infection of one or few variants challenged at multiple mucosal sites thereby recapitulating the essence of HIV transmission. We hope that many human/HIV researchers will acknowledge these improved models and allow NHP research to inform human clinical trials. Overall we are in a position to address several fundamental research questions including: (i) identifying the source of transmitted virus (cell-associated or cell-free), (ii) the mechanism for CXCR4-tropic exclusion or CCR5-tropic selection, (iii) the means used to breach the epithelial barrier (transcytosis, breaks or abrasions, or simply navigating between cells), (iv) the amount and inhibitory potential of mucus at various sites of infection, (v) the number of variants transmitted and replicating only locally versus variants identified systemically, (vi) the dynamics and routes of systemic dissemination, and (vii) the quantity and rapidity of long-term viral reservoir establishment. Greater understanding of these essential questions will require both NHP research and studies in HIV infected humans, and successfully addressing these questions will provide the necessary underpinning for future intervention strategies.
Key Points.
HIV infection is established in newly infected recipients by a single or few transmitted/founder variants.
Animal modeling is conducted with researchers currently recapitulating a physiologically relevant, low-titer infection.
Pertinent animal models have been established for the most common routes of infection, including rectal, vaginal, and penile transmission.
Vaccine and other intervention studies have the modified challenge approach to recapitulate HIV infection.
Acknowledgments
This work was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We would also like to acknowledge Allen Kane and Joseph Meyer at Scientific Publications, Graphics & Media at SAIC-Frederick, Inc., for help with artwork.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Shaw GM, Hunter E. Cold Spring Harbor perspectives in medicine. 2012. HIV transmission; p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS. Preventing sexual transmission of HIV. Clin Infect Dis. 2007;45 (Suppl 4):S287–292. doi: 10.1086/522552. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haaland RE, Hawkins PA, Salazar-Gonzalez J, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS pathogens. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. The Lancet infectious diseases. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 11.Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearney M, Maldarelli F, Shao W, et al. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J Virol. 2009;83:2715–2727. doi: 10.1128/JVI.01960-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Bar KJ, Wang S, et al. High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS pathogens. 2010;6:e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. The Journal of experimental medicine. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer S, Kearney M, Maldarelli F, et al. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol. 2005;43:406–413. doi: 10.1128/JCM.43.1.406-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar-Gonzalez JF, Bailes E, Pham KT, et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol. 2008;82:3952–3970. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Ochsenbauer C, Edmonds TG, Ding H, et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. Journal of virology. 2012;86:2715–2728.a. doi: 10.1128/JVI.06157-11. In this study, 10 transmitted/founder HIV-1 clade B viruses were molecularly cloned and biologically characterized. It was observed that these T/F viruses were CCR5-tropic but not macrophage-tropic and that these clones may be further used to elucidate virus-host interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer W, Ganusov VV, Giorgi EE, et al. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One. 2010;5:e12303. doi: 10.1371/journal.pone.0012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HY, Giorgi EE, Keele BF, et al. Modeling sequence evolution in acute HIV-1 infection. Journal of theoretical biology. 2009;261:341–360. doi: 10.1016/j.jtbi.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood N, Bhattacharya T, Keele BF, et al. HIV evolution in early infection: selection pressures, patterns of insertion and deletion, and the impact of APOBEC. PLoS Pathog. 2009;5:e1000414. doi: 10.1371/journal.ppat.1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song H, Pavlicek JW, Cai F, et al. Impact of immune escape mutations on HIV-1 fitness in the context of the cognate transmitted/founder genome. Retrovirology. 2012;9:89. doi: 10.1186/1742-4690-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar KJ, Li H, Chamberland A, et al. Wide variation in the multiplicity of HIV-1 infection among injection drug users. Journal of virology. 2010;84:6241–6247. doi: 10.1128/JVI.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masharsky AE, Dukhovlinova EN, Verevochkin SV, et al. A substantial transmission bottleneck among newly and recently HIV-1-infected injection drug users in St Petersburg, Russia. J Infect Dis. 2010;201:1697–1702. doi: 10.1086/652702. [DOI] [PubMed] [Google Scholar]
- 25.Brumme ZL, John M, Carlson JM, et al. HLA-associated immune escape pathways in HIV-1 subtype B Gag, Pol and Nef proteins. PLoS One. 2009;4:e6687. doi: 10.1371/journal.pone.0006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar KJ, Tsao CY, Iyer SS, et al. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS pathogens. 2012;8:e1002721. doi: 10.1371/journal.ppat.1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. The Journal of experimental medicine. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asmal M, Hellmann I, Liu W, et al. A signature in HIV-1 envelope leader peptide associated with transition from acute to chronic infection impacts envelope processing and infectivity. PLoS One. 2011;6:e23673. doi: 10.1371/journal.pone.0023673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Gnanakaran S, Bhattacharya T, Daniels M, et al. Recurrent signature patterns in HIV-1 B clade envelope glycoproteins associated with either early or chronic infections. PLoS pathogens. 2011;7:e1002209. doi: 10.1371/journal.ppat.1002209. Using single genome analysis of samples obtained from recently infected or chronically infected HIV-1 clade B patients, the authors were able to identify a signature peptide sequence in the Env protein which may allow for positive selection during transmission. Additionally, patterns of viral mutations were identified which may reflect common immune evasion pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Keele BF, Li H, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. Journal of virology. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keele BF, Li H, Learn GH, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. The Journal of experimental medicine. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone M, Keele BF, Ma ZM, et al. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. Journal of virology. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Ma ZM, Keele BF, Qureshi H, et al. SIVmac251 is inefficiently transmitted to rhesus macaques by penile inoculation with a single SIVenv variant found in ramp-up phase plasma. AIDS research and human retroviruses. 2011;27:1259–1269. doi: 10.1089/aid.2011.0090. This study is the first example of modeling penile transmission of SIV in rhesus macaques. Single genome analysis and sequencing were used to identify and enumerate founder varients in acutely infected HIV-1 patients, and this information was used to refine the penile transmission NHP model such that it accurately recapitulated penile HIV-1 transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolton DL, Song K, Wilson RL, et al. Comparison of systemic and mucosal vaccination: impact on intravenous and rectal SIV challenge. Mucosal immunology. 2012;5:41–52. doi: 10.1038/mi.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenizia C, Keele BF, Nichols D, et al. TRIM5alpha does not affect simian immunodeficiency virus SIV(mac251) replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. Journal of virology. 2011;85:12399–12409. doi: 10.1128/JVI.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Patel V, Jalah R, Kulkarni V, et al. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1215393110. This group used DNA prime and inactivated virus as vaccine. Heterologous challenge was performed with weekly challenges of SIVmacE660. All control animals were infected after 11 challenges while two vaccinated animals remained uninfected after 14 challenges. This vaccine protocol was able to achieve effective cellular and humoral immune responses that delayed heterologous SIVsmE660 infection and to provided long-term control of viremia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Pegu P, Vaccari M, Gordon S, et al. Antibodies with High Avidity to the gp120 Envelope Protein in Protection from Simian Immunodeficiency Virus SIVmac251 Acquisition in an Immunization Regimen That Mimics the RV-144 Thai Trial. Journal of virology. 2013;87:1708–1719. doi: 10.1128/JVI.02544-12. Here the authors recapitulate the partially successful RV-144 Thai Trial in NHP by challenging animals rectally at a dose sufficient to infect with a few transmitted/founder variants. Sera in protected animals had higher avidity antibodies to gp120 (specifically the V1/V2) than unprotected animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Vaccari M, Keele BF, Bosinger SE, et al. Protection afforded by an HIV vaccine candidate in macaques dependent on the dose of SIVmac251 challenge exposure. Journal of virology. 2013 doi: 10.1128/JVI.02863-12. This paper highlights the importance of challenge dosage for HIV vaccine development using NHP models, demonstrating that vaccine efficacy is observed in low-dose challenges while any protection provided by the vaccine became negligable in high-dose challenges. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson NA, Keele BF, Reed JS, et al. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. Journal of virology. 2009;83:6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Xiao P, Patterson LJ, Kuate S, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. Journal of virology. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. In this study, macaques were treated with replicating adenovirus type 5 host range mutant HIV/SIV recombinant priming followed by envelope protein boosting to elicit cellular and humoral immunity. Animals subsequently challenged repeatedly via intrarectal route demonstrated reduced peak viremia compared to controls and and correlation of vaccine-induced sIgA titers with delayed acquisition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. In this study, the authors present data demonstrating the identification of a vaccine against neutralization-resistant SIV which protects against acquisition of infection, rather than simply modulating virological action post-infection. This is the first development of a vaccine which prevents neutralization-resistant SIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. This review describes the anantomic and other host barriers to transmission using the female genital tract and male genital tract as mucosal transmission models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinh MH, Okocha EA, Koons A, et al. Expression of structural proteins in human female and male genital epithelia and implications for sexually transmitted infections. Biology of reproduction. 2012;86:32. doi: 10.1095/biolreprod.111.094789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Yeh WW, Rao SS, Lim SY, et al. The TRIM5 gene modulates penile mucosal acquisition of simian immunodeficiency virus in rhesus monkeys. Journal of virology. 2011;85:10389–10398. doi: 10.1128/JVI.00854-11. This study uses NHP models of penile SIV transmission to demonstrate the importance of the TRIM5 genotype in permitting or restricting infectivity. Animals bearing the TRIM5-restrictive genotype demonstrated markedly higher levels of SIV resistance compared to those harboring a TRIM5-permissive genotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **48.Qureshi H, Ma ZM, Huang Y, et al. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. Journal of virology. 2012;86:2239–2250. doi: 10.1128/JVI.06175-11. This study recapitulates the results of the Phase IIb Step Trial of an HIV vaccine in NHP models. The authors demonstrate that rhesus macaques immunized with a Ad5-based SIV gag/pol/nef vaccine following low-dose penile exposure to SIVmac251 actually enhances susceptibility to SIV, rather than decreasing it; these results mirror what was observed in the human Step trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Giavedoni LD, Chen HL, Hodara VL, et al. Impact of mucosal inflammation on oral simian immunodeficiency virus transmission. Journal of virology. 2013;87:1750–1758. doi: 10.1128/JVI.02079-12. Here the authors demonstrate that there is no correlation between increased susceptibility to SIV and moderate gingivitis when rhesus macaques were challenged orally, though animals with gingivitis did exhibit elevated levels of cytokines in the oral mucosa and plasma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Reeves RK. Functional perturbation of classical natural killer and innate lymphoid cells in the oral mucosa during SIV infection. Frontiers in immunology. 2012;3:417. doi: 10.3389/fimmu.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds MR, Weiler AM, Piaskowski SM, et al. A trivalent recombinant Ad5 gag/pol/nef vaccine fails to protect rhesus macaques from infection or control virus replication after a limiting-dose heterologous SIV challenge. Vaccine. 2012;30:4465–4475. doi: 10.1016/j.vaccine.2012.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **52.Hansen SG, Ford JC, Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. Here the authors report the identification of a highly effective SIV vaccine containing rhesus cytomegalovirus vectors which establishes persistant, high-frequency, SIV-specific effector memory T-cell responses at sites of SIV replication, and can stringently control SIV infection early after mucosal challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai L, Kwa SF, Kozlowski PA, et al. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine. 2012;30:1737–1745. doi: 10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reece JC, Alcantara S, Gooneratne S, et al. Trivalent live attenuated Influenza-SIV vaccines: efficacy and evolution of CTL escape in macaques. Journal of virology. 2013 doi: 10.1128/JVI.02645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strbo N, Vaccari M, Pahwa S, et al. Cutting Edge: Novel Vaccination Modality Provides Significant Protection against Mucosal Infection by Highly Pathogenic Simian Immunodeficiency Virus. Journal of immunology. 2013 doi: 10.4049/jimmunol.1202655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaccari M, Halwani R, Patterson LJ, et al. Antibodies to gp120 and PD-1 Expression on Virus-Specific CD8+ T-cells in Protection from Simian AIDS. Journal of virology. 2013 doi: 10.1128/JVI.02686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh WW, Brassard LM, Miller CA, et al. Envelope variable region 4 is the first target of neutralizing antibodies in early simian immunodeficiency virus mac251 infection of rhesus monkeys. Journal of virology. 2012;86:7052–7059. doi: 10.1128/JVI.00107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurupati R, Tuyishime S, Kossenkov AV, et al. Correlates of relative resistance against low-dose rectal simian immunodeficiency virus challenges in peripheral blood mononuclear cells of vaccinated rhesus macaques. Journal of leukocyte biology. 2012 doi: 10.1189/jlb.0612287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature medicine. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burton DR, Hessell AJ, Keele BF, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. Journal of virology. 2012;86:718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Q, Ruone S, Switzer WM, et al. Limited SHIV env diversification in macaques failing oral antiretroviral pre-exposure prophylaxis. Retrovirology. 2012;9:40. doi: 10.1186/1742-4690-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishimura Y, Martin MA. The acute HIV infection: implications for intervention, prevention and development of an effective AIDS vaccine. Current opinion in virology. 2011;1:204–210. doi: 10.1016/j.coviro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **64.Del Prete GQ, Scarlotta M, Newman L, et al. Comparative Characterization of Transfection- and Infection-Derived SIV Challenge Stocks for In Vivo Non-Human Primate Studies. Journal of virology. 2013 doi: 10.1128/JVI.03507-12. In this study, the authors highlight the differences between transfection and infection-derived SIV challenge stocks. While stocks prepared by both methods displayed similar infectivity in vitro, transfection-derived stocks possess higher overall virus content and markedly lower virion-associated Env than infection-derived stocks. These results indicate that there may be underappreciated features of SIV in vivo challenge stock which may affect early infection events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sellier P, Mannioui A, Bourry O, et al. Antiretroviral treatment start-time during primary SIV(mac) infection in macaques exerts a different impact on early viral replication and dissemination. PLoS One. 2010;5:e10570. doi: 10.1371/journal.pone.0010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clements JE, Gama L, Graham DR, et al. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Current opinion in HIV and AIDS. 2011;6:37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kader M, Hassan WM, Eberly M, et al. Antiretroviral therapy prior to acute viral replication preserves CD4 T cells in the periphery but not in rectal mucosa during acute simian immunodeficiency virus infection. Journal of virology. 2008;82:11467–11471. doi: 10.1128/JVI.01143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. Journal of virology. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *69.Malzahn J, Shen C, Caruso L, et al. Effect of early anti-retroviral therapy on the pathogenic changes in mucosal tissues of SIV infected rhesus macaques. Virology journal. 2012;9:269. doi: 10.1186/1743-422X-9-269. This study examines the effect of early ART on the early stages of HIV/SIV replication and pathogenesis in the gastrointestinal tract. The authors found that early ART could not effectively inhibit SIV replication, nor could it reduce immune activation in the intestinal tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heneine W, Kashuba A. HIV Prevention by Oral Preexposure Prophylaxis. Cold Spring Harbor perspectives in medicine. 2012;2:a007419. doi: 10.1101/cshperspect.a007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Lerma JG, Heneine W. Animal models of antiretroviral prophylaxis for HIV prevention. Current opinion in HIV and AIDS. 2012;7:505–513. doi: 10.1097/COH.0b013e328358e484. [DOI] [PubMed] [Google Scholar]
- 72.Girard MP, Plotkin SA. HIV vaccine development at the turn of the 21st century. Current opinion in HIV and AIDS. 2012;7:4–9. doi: 10.1097/COH.0b013e32834ddc96. [DOI] [PubMed] [Google Scholar]