Abstract
Abnormal synaptic plasticity has been implicated in the cognitive deficits seen in schizophrenia, where alterations have been found in neurotransmission, signaling and dendritic dynamics. Rapid rearrangement of the actin cytoskeleton is critical for plasticity and abnormalities of molecular regulators of this process are candidates for understanding mechanisms underlying these changes in schizophrenia. The myristoylated, alanine-rich C-kinase substrate (MARCKS) is crucial for many roles associated with synaptic plasticity, including facilitation of neurotransmission, dendritic branching and in turn cognitive function. Accordingly, we hypothesized that this protein is abnormally expressed or regulated in schizophrenia. We measured protein expression of MARCKS by Western blot analysis in postmortem samples of dorsolateral prefrontal cortex (DLPFC) from elderly schizophrenia patients (N=16) and a comparison group (N=20). We also assayed phosphorylated-MARCKS (pMARCKS), given the role of phosphorylation in reversing membrane association by MARCKS. We found decreased expression of both MARCKS and pMARCKS in schizophrenia. Altered myristoylation may be a mechanism that explains this down-regulation of MARCKS, so we also assayed expression of the two isoforms of the key myristoylation enzyme, NMT, and an enzymatic inhibitor of this enzyme, NMT-inhibitor protein (NIP71) by Western blotting in these same subjects. Expression did not change between groups for these proteins, suggesting a mechanism other than myristoylation is responsible for decreased MARCKS expression in schizophrenia. These data suggest a potential mechanism underlying aspects of altered synaptic plasticity observed in schizophrenia.
Keywords: Synaptic plasticity, cytoskeleton, neurotransmission, myristoylation, dendritic dynamics
1.0 Introduction
Cognitive dysfunction is a core feature of schizophrenia and is thought to be due to abnormalities in synaptic plasticity, where alterations have been found in trafficking, signaling, receptor localization, neurotransmission, and dendritic dynamics (Aoto et al., 2013; Arnsten et al., 2012; Glausier and Lewis, 2013; Jia et al., 2013). These synaptic processes all share the requirement for rapid rearrangement of the actin cytoskeleton at the synapse. The myristoylated, alanine-rich C-kinase substrate (MARCKS) is a novel protein that is widely distributed in the nervous system and well-known as an actin binding protein associated with dynamic cytoskeletal restructuring. MARCKS is important for a myriad of events at the plasma membrane; its roles in trafficking, regulation of the cell cycle, cellular motility, memory, dendritic morphology, and secretion all depend on dynamic rearrangement of the actin cytoskeleton (Aderem, 1992; Arbuzova et al., 1997; Calabrese and Halpain, 2005; Sheu et al., 1993). Studies have shown that alterations in MARCKS can impair learning and memory, neurotransmission, dendritic branching, and synaptogenesis (Calabrese and Halpain, 2005; McNamara et al., 2005; Okuda et al., 2010; Sasaki, 2003), abnormalities of all of which have been associated with schizophrenia.
MARCKS regulates actin dynamics through a myristoyl-electrostatic switch mechanism that creates a reversible association with biological membranes or phospholipid vesicles (McLaughlin and Aderem, 1995; Seykora et al., 1996). MARCKS membrane association is vital for many of its functional roles; its crosslinking of F-actin, lateral sequestration of phosphatidylinositol 4, 5-bisphosphate (PI(4,5)P2), proximity to kinases, and PKC-mediated vesicle transport from the Golgi, all require MARCKS membrane adsorption (Arbuzova et al., 2002; McLaughlin et al., 2002; Radau et al., 2000; Sasaki, 2003; Spizz and Blackshear, 2001; Wang et al., 2001; Wang et al., 2002). Regulating and reversing MARCKS membrane association is through phosphorylation and/or Ca2+-calmodulin (CaM) binding to the effector domain (ED) of MARCKS (Arbuzova et al., 1997; Hartwig et al., 1992) following a depolarization-induced Ca2+ influx (Wang et al., 1988). The reversible membrane association of MARCKS and its role initiating calcium-dependent changes through dynamic rearrangement of the cytoskeleton are necessary for CNS development, balanced synaptic functioning and modification of brain circuitry.
Given the role of MARCKS in regulating cytoskeletal dynamics that are associated with synaptic abnormalities seen in schizophrenia, we hypothesized that this key protein is abnormally expressed and regulated in schizophrenia. Accordingly, in this study we measured protein expression of MARCKS in postmortem brain from elderly schizophrenia patients and a comparison group. To further characterize the regulation of this protein, we assayed phosphorylated-MARCKS (pMARCKS), as well as the two isoforms of the key myristoylation enzyme, N-myristoyltransferase (NMT), and an enzymatic inhibitor of this enzyme, NMT-inhibitor protein (NIP71).
2.0 Experimental/Materials and methods
2.1 Human subjects
Autopsy-obtained samples of dorsolateral prefrontal cortex (DLPFC, Brodmann areas 9/46) from schizophrenia and comparison subjects (Table 1) were obtained from the Mount Sinai Medical Center brain collection as previously described (Powchik et al., 1998; Rubio et al., 2012). Next of kin consent was obtained for each subject. The medical history of each subject was reviewed extensively. Subjects with previous drug or alcohol abuse, coma greater than 6 hours, or suicide were excluded from study. Neuropathological examination of all subjects was conducted, and none used for study had evidence of any degenerative diseases, including Alzheimer’s disease. Subjects with schizophrenia all met DSM-III-R criteria for this illness, had documented psychosis before the age of 40, at least 10 years of hospitalization for schizophrenia, and were diagnosed by two clinicians. Comparison subjects were free of any psychiatric or neurological disorders.
Table 1.
Su bject Demographics
| Schizophrenia | Comparison | |
|---|---|---|
| N | 16 | 20 |
| Sex | 9M/7F | 11M/9F |
| Tissue pH | 6.5 ± 0.2 | 6.6 ± 0.3 |
| PMI (hours) | 15.5 ± 6.0 | 8.0 ± 5.9 |
| Age (years) | 78.2 ± 10.5 | 77.1 ± 10.4 |
Abbreviatio ns: F, female; M, m ale; PMI, p ostmortem interval Values presented as means ± standard deviation.
2.2 Antipsychotic treated rats
Animal studies and procedures were performed according to UAB guidelines and approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (250g) were housed in pairs during the 9 month course of study. Treatment was either sesame oil (vehicle, N=10) or haloperidol decanoate (28.5mg/kg, N=10) administered via intramuscular injection every 3 weeks, for a total of 12 injections (Harte et al., 2005; Kashihara et al., 1986). The animals were sacrificed by decapitation, and brains were immediately harvested; the right frontal cortex was dissected on wet ice, snap frozen and stored at −80°C.
2.3 Sample preparation
Tissue samples were reconstituted in cold 5mM Tris-HCl pH 7.5, 0.32M sucrose with a protease inhibitor tablet and a phosphatase inhibitor tablet (Complete Mini, EDTA-free and PhosSTOP both from Roche Diagnostics, Mannheim Germany). A Power Gen 125 (Thermo Fisher Scientific, Rockford, Illinois) homogenizer was used at speed setting 5 for 60 seconds. Protein concentration was determined using a BCA protein assay kit (Thermo Scientific, Rockford, Illinois). After homogenization, samples were stored at −80°C until used for assay.
2.4 Western blotting
Tissue homogenates were thawed on ice, denatured at 70°C for 10min under reducing conditions, and stored at −20°C until use. Samples were loaded in duplicate onto NuPAGE 4-12% Bis-Tris 1mm, 17 well gels (Invitrogen, Carlsbad, CA) and transferred to 0.45μm nitrocellulose membranes using a BioRad Semi-Dry Transblotter (Hercules, CA). Membranes were blocked for 1 hour at room temperature in LiCor blocking buffer (Lincoln, NE) before being probed with the primary antibody diluted in LiCor blocking buffer + 0.1% Tween-20, under the conditions indicated in Table 2. All antibodies were optimized for each protein to determine ideal conditions within the linear range of detection for the assay, and that the primary antibody was present in excess (Table 2). All antibodies were used for both human and rat experiments with the exception of MARCKS. In this case, the antibody used for the human blots had inadequate cross-reactivity with the rat protein, and accordingly we used a different antibody for those blots. Additionally, two different valosin-containing protein (VCP) antibodies from separate host species were used; this was necessary to permit simultaneous quantification of both the protein of interest and VCP as an intralane normalizing control on the same blots. VCP has been shown to not be altered in subjects with schizophrenia in multiple brain areas and has been used as an intralane control in previous studies (Bauer et al., 2009; Stan et al., 2006). We used VCP in this work given that its molecular weight was sufficiently different from proteins we planned to study, thus could be used for each study experiment.
Table 2.
Antibodies Used for Western Blotting
| Antibody | Species | Dilution | Incubation | Company |
|---|---|---|---|---|
| Total MARCKS | Mouse | 1:500 | 16hr 4°C | Abgent, San Diego, CA |
| Total MARCKS* | Rabbit | 1:500 | 16hr 4°C | Abcam, Cambridge, MA |
| pMARCKS (S152/156) | Rabbi | 1:250 | 16hr 4°C | Cell Signaling, Danvers, MA |
| NMT1 | Rabbit | 1:250 | 16hr 4°C | Abgent, San Diego, CA |
| NMT2 | Mouse | 1:250 | 16hr 4°C | BD Biosciences, San Jose, CA |
| NIP71 (HSC70) | Rabbit | 1:3000 | 16hr 4°C | Novus, Littleton, CO |
| VCP | Mouse | 1:25,000 | 1hr RT | Abcam, Cambridge, MA |
| VCP | Rabbit | 1:25,000 | 1hr RT | Abcam, Cambridge, MA |
Abbreviations: MARCKS, myristoylated, alanine-rich C kinase substrate; NMT, N-myristoyltransferase; NIP71, NMT protein inhibitor; RT, room temperature; VCP, valosin-containing protein.
Antibody used for rat studies
Membranes were washed 5X in cold Tris-buffered Saline + 0.05% Tween-20 (TBST) for 5 minutes each before being probed in IR-dye labeled secondary antibody diluted in LiCor blocking buffer + 0.1% Tween-20. Membranes were again washed 5X in cold TBST for 5 minutes, then twice in MilliQ water before being scanned with the LiCor Odyssey imager, and then stored in MilliQ at 4°C.
2.5 Data Analysis
LiCor Odyssey 3.0 analytical software (Lincoln, NE) was used to determine the expression of each protein. Integrated Intensity values were first normalized to the same-lane value of VCP (Bauer et al., 2009; Stan et al., 2006), then the duplicate values were averaged for each subject. All data were analyzed by one-way ANOVA using Statistica software (Statsoft, Tulsa, OK). Integrated intensity values for VCP were not different between schizophrenia and comparison groups, consistent with previous reports (Bauer et al., 2009; Stan et al., 2006). Correlation analyses indicated that no dependent measures were correlated with age, pH, or PMI. For all statistical tests, α=0.05.
3.0 Results
Both total MARCKS (F(1,34)=7.79, p=0.009) and pMARCKS (F(1,34)=4.85, p=0.035) were decreased in schizophrenia relative to the comparison group (Figure 1). To determine if the fraction of MARCKS that is phosphorylated is altered in schizophrenia, the ratio of protein expression of pMARCKS to total MARCKS was calculated. No significant difference in this ratio was found between schizophrenia and comparison subjects (Figure 2). In rats treated chronically with haloperidol, treatment did not affect the expression in frontal cortex of either total MARCKS or pMARCKS (Figure3).
Figure 1.
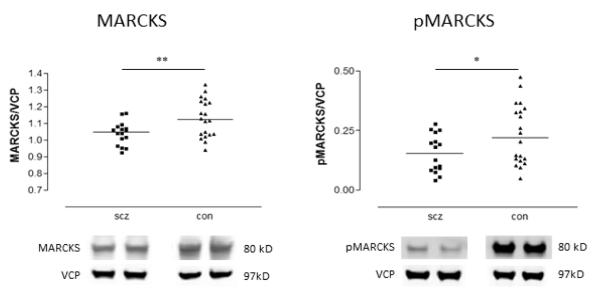
Expression of MARCKS and pMARCKS in DLPFC from patients with schizophrenia (scz) and comparison (con) subjects. Data are expressed for each subject as the ratio of signal intensity for protein of interest divided by the intensity of valosin-containing protein (VCP) determined in the same lane from the same blot. Data are mean values of this ratio from duplicate samples. Both MARCKS and pMARCKS are reduced in schizophrenia. p<0.05*, p<0.01**.
Figure 2.
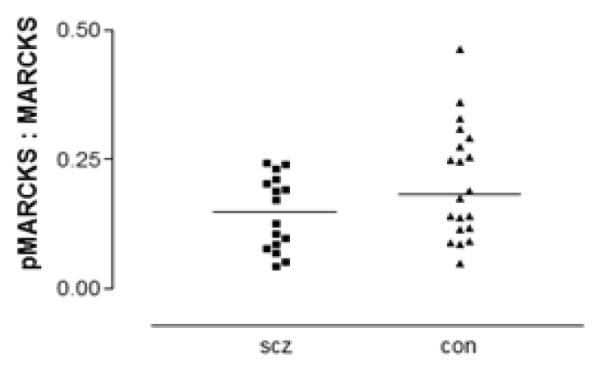
Ratio of pMARCKS to total MARCKS in DLPFC in schizophrenia (scz) and comparison (con) subjects. The fraction of total MARCKS that is phosphorylated is not different between groups.
Figure 3.
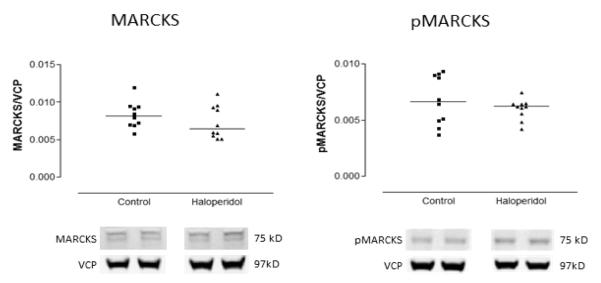
Expression of MARCKS and pMARCKS in frontal cortex from adult male rats treated chronically with haloperidol decanoate (28.5mg/kg/3 weeks for 9 months) or vehicle (sesame oil), expressed as a ratio of signal intensity for each target protein to intensity of labeling for VCP. Each data point represents the mean value from duplicate lanes for each animal. Haloperidol treatment did not change expression of either MARCKS or pMARCKS.
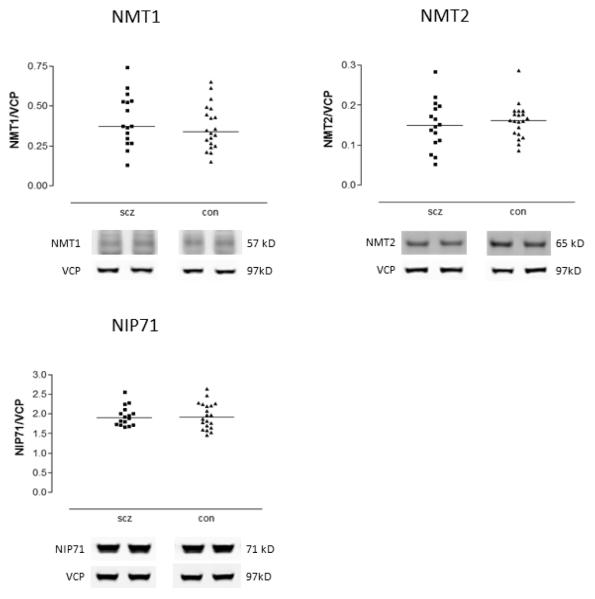
Next, we assayed expression of the two isoforms of the key myristoylation enzyme, NMT1 and NMT2, to explore possible mechanisms to explain the observation of decreased MARCKS in schizophrenia. Neither NMT1 nor NMT2 protein levels were changed in schizophrenia (Figure 4). To further characterize this pathway, we also measured an endogenous inhibitor of NMT, NIP71, in these same subjects. As in the case of the NMT isoforms, no changes were seen in the levels of this protein between schizophrenia and comparison subjects (Figure 4).
Figure 4.
Expression of NMT1, NMT2, and NIP71 in DLPFC from schizophrenia (scz) and comparison (con) subjects. Data are expressed for each subject as means from duplicate values first normalized to same-lane value of VCP. Expression did not differ between groups for any of these three proteins.
4.0 Discussion
Abnormal cytoskeletal responses to synaptic stimuli may underlie multiple abnormalities associated with the pathophysiology of schizophrenia. MARCKS is an actin binding protein critical for dynamic cytoskeletal restructuring, thus we hypothesized that this key protein may be abnormally expressed or regulated in schizophrenia. We found decreased expression of both MARCKS and pMARCKS in the DLPFC in schizophrenia. On the other hand, we found no changes in the expression of the myristoylation enzymes NMT1 and NMT2, or the enzyme inhibitor protein NIP71. These deficits may underlie abnormalities of synaptic plasticity seen in schizophrenia (Aoto et al., 2013; Arnsten et al., 2012; Glausier and Lewis, 2013; Jia et al., 2013).
Schizophrenia is associated with multiple neurotransmitter systems which converge to produce synaptic dysfunction. MARCKS is central to these neurotransmitter pathways and is a potential molecular mechanism underlying synaptic plasticity, given the mediatory role of MARCKS in translating Ca2+ dependent kinase activity into dynamic cytoskeletal restructuring (Leenders and Sheng, 2005; Ramakers et al., 1999). Localized to axon terminals, dendritic spines and glial processes (Ouimet et al., 1990; Ramakers et al., 1999), MARCKS is highly expressed during development, and remains high in adulthood in neuronal populations with high degrees of neuroplasticity; these include the hippocampus, amygdala and multiple cortical regions (McNamara et al., 2005; McNamara and Lenox, 1997; Ouimet et al., 1990; Ramakers et al., 1999). Decreased MARCKS expression is consistent with changes in Ca2+ induced vesicular transport and synaptic vesicle cycling, via MARCKS interactions with PKC, PI(4,5)P2, and cellular membranes (Horn, 1998; Rose et al., 2001; Sasaki, 2003; Walaas and Sefland, 2000; Yang et al., 2002). Loss of MARCKS and consequent sequestration of PI(4,5)P2 at the cell membrane has been shown to impair cognition and LTP in mice (Trovo et al., 2013). Altered synaptic responses from dysregulated kinase-dependent MARCKS-PI(4,5)P2 mediated processes could underlie changes in the regulation of the neurotransmitter pathways implicated in schizophrenia.
Synaptic transmission is facilitated by actin-rich dynamic dendrites that alter shape, growth and retraction of spines in response to stimuli (Amaral and Pozzo-Miller, 2009). Dendritic morphology has been found to be abnormal in schizophrenia (Glausier and Lewis, 2013; Penzes et al., 2011). MARCKS has been shown to play a role in maintenance of spine morphology, filopodia formation, and dendritic branching (Calabrese and Halpain, 2005; Li et al., 2008; Matus, 2005). MARCKS-deficient mice have abnormalities in cortical lamination, increased ventricular volume, and decreased brain size (Stumpo et al., 1995). Taken together these data suggest that the decreased MARCKS protein expression we found in schizophrenia could be associated with abnormalities of diminished dendritic morphology (Garey, 2010; Glausier and Lewis, 2013), decreased grey matter (Glahn et al., 2008; Honea et al., 2008), and alterations in cortical volume (Fatemi and Folsom, 2009; Northoff et al., 1999) seen in schizophrenia. A recent study found that MARCKS transcription is regulated by Dysbindin (Okuda et al., 2010), which has been implicated as a susceptibility gene in schizophrenia (Papaleo et al., 2012) and involved in synaptic glutamate release and cognitive function (Chen et al., 2008; Jentsch et al., 2009; Saggu et al., 2013). Dysbindin dysfunction could be associated with abnormal regulation of MARCKS-dependent actin reorganization.
MARCKS and pMARCKS have also been found to be changed in other psychiatric conditions associated with abnormal synaptic plasticity (Duman, 2013). MARCKS expression and its phosphorylation are abnormal in suicide (Le-Niculescu et al., 2013; Pandey et al., 2003), and pMARCKS levels decrease with lithium treatment (Fitzgerald et al., 2010; Szabo et al., 2009). Interestingly, the polysialylated neural cell adhesion molecule (PSA-NCAM) has been shown to be altered in the dorsolateral prefrontal cortex in schizophrenia and in the amygdala in major depression (Gilabert-Juan et al., 2012; Varea et al., 2012). A recent study found that interaction of MARCKS and extracellular polysialic acid (PSA) is involved in neurite outgrowth (Theis et al., 2013). PSA glycosylates neural cell adhesion molecule (NCAM); the polysialylated form, PSA-NCAM, plays an important role in synaptic plasticity and learning (Kochlamazashvili et al., 2010; Senkov et al., 2012; Varea et al., 2012). Given that PSA-NCAM is restricted to interneurons in frontal cortex (Gascon et al., 2007; Gomez-Climent et al., 2011; Guirado et al., 2013; Rutishauser, 2008), decreased MARCKS expression in that subpopulation of cells could affect PSA-NCAM-mediated membrane interactions and in turn the modulation of inhibitory cortical circuits. Altered MARCKS may thus contribute to the dendritic changes seen in both schizophrenia and depression.
MARCKS membrane interactions require a myristoyl-electrostatic switch mechanism facilitated by N-myristoylation and a highly basic effector domain (ED) (McLaughlin and Aderem, 1995; Murray et al., 2002; Murray et al., 1997; Vergeres et al., 1995). Myristoylation targets the subcellular location of MARCKS, thereby facilitating its actin-binding activity (Calabrese and Halpain, 2005; Ramsden and Vergeres, 1999; Tapp et al., 2005). Given the decreased expression of MARCKS we found, we sought to determine if this was as a result of abnormalities of the key myristoylating enzymes in brain. Therefore we measured the two isoforms of the key myristoylating enzyme, NMT, and the NMT inhibitor NIP71. We found protein expression of these was not changed in schizophrenia. These data suggest that a mechanism other than myristoylation is responsible for the down-regulation of MARCKS expression seen in schizophrenia.
The decreased MARCKS expression in schizophrenia could be due to an assay limitation; the antibodies we used may not detect pools of demyr-, unmyr-, or cleaved MARCKS. PKC phosphorylation of MARCKS protects it from cathepsin proteolysis (Spizz and Blackshear, 2001) and potential dendritic loss (Graber et al., 2004); decreased pMARCKS may suggest a larger pool of cleaved MARCKS undetected by our assay. Thus we sought to determine if the percentage of MARCKS phosphorylation differed in schizophrenia, and found the fraction of total MARCKS that is phosphorylated was not changed, further supporting our finding that MARCKS expression is reduced in schizophrenia.
There are several limitations to this work and all postmortem brain studies in schizophrenia. Our subjects were aged, and these results may not generalize to younger subjects, although we predict that similar changes in this plasticity-related protein will be found across the lifespan. This study was also limited to frontal cortex, thus it is not yet known if this change is restricted to this region or is found in other areas of brain. Finally, the effects of chronic antipsychotic treatment are always of concern in these types of studies. Although lithium has been shown to alter MARCKS expression (Lenox et al., 1996; Pandey et al., 2003; Pandey et al., 2002; Watson and Lenox, 1996), these patients were not receiving this medication at the time of death. Most had received antipsychotics close to the time of death, however, but rats chronically treated with haloperidol did not exhibit this change in MARCKS expression. Thus, we feel it is likely that these changes are due to the illness and not antipsychotic treatment. Finally, although pH was well matched between diagnostic groups, PMI was appreciably longer in the schizophrenia patients than the comparison subjects. Neither MARCKS nor pMARCKS expression was significantly correlated with PMI (which was normally distributed) in the total sample (r=−0.27 for MARCKS and r=−0.23 for pMARCKS), or in just the schizophrenia group (r=0.37 and 0.33, respectively) or the comparison subjects (r=−0.26 and −0.25, respectively).
In summary, we found decreased MARCKS and pMARCKS in the frontal cortex in schizophrenia. Abnormal MARCKS expression is consistent with altered synaptic morphology and plasticity mediated by dysregulated cytoskeletal dynamics. These data suggest abnormalities of MARCKS as a possible mechanism underlying the altered synaptic plasticity observed in schizophrenia.
Acknowledgment
None
Funding This work was supported by MH53327 (JMW), and MH64673 and MH66392 (VH).
Footnotes
Conflict of interest All authors declare that they have no conflicts of interest.
Contribution ALP and JHMW designed the study. ALP performed the experiments and statistical analyses, and wrote the first draft of the manuscript. VH provided the human tissue. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Amaral MD, Pozzo-Miller L. The dynamics of excitatory synapse formation on dendritic spines. Cellscience. 2009;5(4):19–25. [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Sudhof TC. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell. 2013;154(1):75–88. doi: 10.1016/j.cell.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. The Biochemical journal. 2002;362(Pt 1):1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuzova A, Wang J, Murray D, Jacob J, Cafiso DS, McLaughlin S. Kinetics of interaction of the myristoylated alanine-rich C kinase substrate, membranes, and calmodulin. The Journal of biological chemistry. 1997;272(43):27167–27177. doi: 10.1074/jbc.272.43.27167. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. Journal of neural transmission. 2009;116(4):487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48(1):77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, Guo N, Huang HP, Xiong W, Zheng H, Zuo PL, Zhang CX, Li W, Zhou Z. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. The Journal of cell biology. 2008;181(5):791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Remodeling chromatin and synapses in depression. Nature medicine. 2013;19(3):267. doi: 10.1038/nm.3125. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophrenia bulletin. 2009;35(3):528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Barkus C, Feyder M, Wiedholz LM, Chen YC, Karlsson RM, Machado-Vieira R, Graybeal C, Sharp T, Zarate C, Harvey-White J, Du J, Sprengel R, Gass P, Bannerman D, Holmes A. Does gene deletion of AMPA GluA1 phenocopy features of schizoaffective disorder? Neurobiology of disease. 2010;40(3):608–621. doi: 10.1016/j.nbd.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. Journal of anatomy. 2010;217(4):324–333. doi: 10.1111/j.1469-7580.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Kiss JZ. Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons. Brain research reviews. 2007;56(1):101–118. doi: 10.1016/j.brainresrev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibanez JM, Crespo C, Nacher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neuroscience letters. 2012;530(1):97–102. doi: 10.1016/j.neulet.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Climent MA, Guirado R, Castillo-Gomez E, Varea E, Gutierrez-Mecinas M, Gilabert-Juan J, Garcia-Mompo C, Vidueira S, Sanchez-Mataredona D, Hernandez S, Blasco-Ibanez JM, Crespo C, Rutishauser U, Schachner M, Nacher J. The polysialylated form of the neural cell adhesion molecule (PSA-NCAM) is expressed in a subpopulation of mature cortical interneurons characterized by reduced structural features and connectivity. Cerebral cortex. 2011;21(5):1028–1041. doi: 10.1093/cercor/bhq177. [DOI] [PubMed] [Google Scholar]
- Graber S, Maiti S, Halpain S. Cathepsin B-like proteolysis and MARCKS degradation in sub-lethal NMDA-induced collapse of dendritic spines. Neuropharmacology. 2004;47(5):706–713. doi: 10.1016/j.neuropharm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castillo-Gomez E, Liberia T, Rovira-Esteban L, Varea E, Crespo C, Blasco-Ibanez JM, Nacher J. The Dendritic Spines of Interneurons Are Dynamic Structures Influenced by PSA-NCAM Expression. Cerebral cortex. 2013 doi: 10.1093/cercor/bht156. [DOI] [PubMed] [Google Scholar]
- Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophrenia research. 2005;75(2-3):303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biological psychiatry. 2008;63(5):465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G. Visual imprinting and the neural mechanisms of recognition memory. Trends in neurosciences. 1998;21(7):300–305. doi: 10.1016/s0166-2236(97)01219-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(12):2601–2608. doi: 10.1038/npp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia JM, Zhao J, Hu Z, Lindberg D, Li Z. Age-dependent regulation of synaptic connections by dopamine D2 receptors. Nature neuroscience. 2013;16(11):1627–1636. doi: 10.1038/nn.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashihara K, Sato M, Fujiwara Y, Harada T, Ogawa T, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biological psychiatry. 1986;21(7):650–656. doi: 10.1016/0006-3223(86)90126-5. [DOI] [PubMed] [Google Scholar]
- Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, Gerardy-Schahn R, Engel AK, Feig L, Semyanov A, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(11):4171–4183. doi: 10.1523/JNEUROSCI.5806-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, Winiger E, Bhosrekar S, Shankar G, Radel M, Bellanger E, Duckworth H, Olesek K, Vergo J, Schweitzer R, Yard M, Ballew A, Shekhar A, Sandusky GE, Schork NJ, Kurian SM, Salomon DR, Niculescu AB., 3rd Discovery and validation of blood biomarkers for suicidality. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AG, Sheng ZH. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacology & therapeutics. 2005;105(1):69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenox RH, McNamara RK, Watterson JM, Watson DG. Myristoylated alanine-rich C kinase substrate (MARCKS): a molecular target for the therapeutic action of mood stabilizers in the brain? The Journal of clinical psychiatry. 1996;57(Suppl 13):23–31. discussion 32-23. [PubMed] [Google Scholar]
- Li H, Chen G, Zhou B, Duan S. Actin filament assembly by myristoylated alanine-rich C kinase substrate-phosphatidylinositol-4,5-diphosphate signaling is critical for dendrite branching. Molecular biology of the cell. 2008;19(11):4804–4813. doi: 10.1091/mbc.E08-03-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. MARCKS for maintenance in dendritic spines. Neuron. 2005;48(1):4–5. doi: 10.1016/j.neuron.2005.09.013. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends in biochemical sciences. 1995;20(7):272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annual review of biophysics and biomolecular structure. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hussain RJ, Simon EJ, Stumpo DJ, Blackshear PJ, Abel T, Lenox RH. Effect of myristoylated alanine-rich C kinase substrate (MARCKS) overexpression on hippocampus-dependent learning and hippocampal synaptic plasticity in MARCKS transgenic mice. Hippocampus. 2005;15(5):675–683. doi: 10.1002/hipo.20089. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Lenox RH. Comparative distribution of myristoylated alanine-rich C kinase substrate (MARCKS) and F1/GAP-43 gene expression in the adult rat brain. J Comp Neurol. 1997;379(1):48–71. [PubMed] [Google Scholar]
- Murray D, Arbuzova A, Honig B, McLaughlin S. The role of electrostatic and nonpolar interactions in the association of peripheral proteins with membranes. Curr Top Membr. 2002;52:277–307. [Google Scholar]
- Murray D, Ben-Tal N, Honig B, McLaughlin S. Electrostatic interaction of myristoylated proteins with membranes: simple physics, complicated biology. Structure. 1997;5(8):985–989. doi: 10.1016/s0969-2126(97)00251-7. [DOI] [PubMed] [Google Scholar]
- Northoff G, Waters H, Mooren I, Schluter U, Diekmann S, Falkai P, Bogerts B. Cortical sulcal enlargement in catatonic schizophrenia: a planimetric CT study. Psychiatry research. 1999;91(1):45–54. doi: 10.1016/s0925-4927(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Okuda H, Kuwahara R, Matsuzaki S, Miyata S, Kumamoto N, Hattori T, Shimizu S, Yamada K, Kawamoto K, Hashimoto R, Takeda M, Katayama T, Tohyama M. Dysbindin regulates the transcriptional level of myristoylated alanine-rich protein kinase C substrate via the interaction with NF YB in mice brain. PloS one. 2010;5(1):e8773. doi: 10.1371/journal.pone.0008773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, Wang JK, Walaas SI, Albert KA, Greengard P. Localization of the MARCKS (87 kDa) protein, a major specific substrate for protein kinase C, in rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10(5):1683–1698. doi: 10.1523/JNEUROSCI.10-05-01683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR, Tamminga C. Altered expression and phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) in postmortem brain of suicide victims with or without depression. Journal of psychiatric research. 2003;37(5):421–432. doi: 10.1016/s0022-3956(03)00047-5. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, SridharaRao J, Ren X, Janicak PG, Sharma R. Protein kinase C and phospholipase C activity and expression of their specific isozymes is decreased and expression of MARCKS is increased in platelets of bipolar but not in unipolar patients. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26(2):216–228. doi: 10.1016/S0893-133X(01)00327-X. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, Weinberger DR. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Molecular psychiatry. 2012;17(1):85–98. doi: 10.1038/mp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nature neuroscience. 2011;14(3):285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, Davis KL. Postmortem studies in schizophrenia. Schizophrenia bulletin. 1998;24(3):325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- Radau B, Otto A, Muller EC, Westermann P. Protein kinase C alpha-dependent phosphorylation of Golgi proteins. Electrophoresis. 2000;21(13):2684–2687. doi: 10.1002/1522-2683(20000701)21:13<2684::AID-ELPS2684>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ramakers GM, McNamara RK, Lenox RH, De Graan PN. Differential changes in the phosphorylation of the protein kinase C substrates myristoylated alanine-rich C kinase substrate and growth-associated protein-43/B-50 following Schaffer collateral long-term potentiation and long-term depression. Journal of neurochemistry. 1999;73(5):2175–2183. [PubMed] [Google Scholar]
- Ramsden JJ, Vergeres G. Nonelectrostatic contributions to the binding of MARCKS-related protein to lipid bilayers. Archives of biochemistry and biophysics. 1999;371(2):241–245. doi: 10.1006/abbi.1999.1451. [DOI] [PubMed] [Google Scholar]
- Rose SD, Lejen T, Zhang L, Trifaro JM. Chromaffin cell F-actin disassembly and potentiation of catecholamine release in response to protein kinase C activation by phorbol esters is mediated through myristoylated alanine-rich C kinase substrate phosphorylation. The Journal of biological chemistry. 2001;276(39):36757–36763. doi: 10.1074/jbc.M006518200. [DOI] [PubMed] [Google Scholar]
- Rubio MD, Haroutunian V, Meador-Woodruff JH. Abnormalities of the Duo/Ras-Related C3 Botulinum Toxin Substrate 1/p21-Activated Kinase 1 Pathway Drive Myosin Light Chain Phosphorylation in Frontal Cortex in Schizophrenia. Biological psychiatry. 2012;71(10):906–914. doi: 10.1016/j.biopsych.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nature reviews. Neuroscience. 2008;9(1):26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Saggu S, Cannon TD, Jentsch JD, Lavin A. Potential molecular mechanisms for decreased synaptic glutamate release in dysbindin-1 mutant mice. Schizophrenia research. 2013;146(1-3):254–263. doi: 10.1016/j.schres.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y. New aspects of neurotransmitter release and exocytosis: Rho-kinase-dependent myristoylated alanine-rich C-kinase substrate phosphorylation and regulation of neurofilament structure in neuronal cells. Journal of pharmacological sciences. 2003;93(1):35–40. doi: 10.1254/jphs.93.35. [DOI] [PubMed] [Google Scholar]
- Senkov O, Tikhobrazova O, Dityatev A. PSA-NCAM: synaptic functions mediated by its interactions with proteoglycans and glutamate receptors. The international journal of biochemistry & cell biology. 2012;44(4):591–595. doi: 10.1016/j.biocel.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Seykora JT, Myat MM, Allen LA, Ravetch JV, Aderem A. Molecular determinants of the myristoyl-electrostatic switch of MARCKS. The Journal of biological chemistry. 1996;271(31):18797–18802. doi: 10.1074/jbc.271.31.18797. [DOI] [PubMed] [Google Scholar]
- Sheu FS, McCabe BJ, Horn G, Routtenberg A. Learning selectively increases protein kinase C substrate phosphorylation in specific regions of the chick brain. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(7):2705–2709. doi: 10.1073/pnas.90.7.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizz G, Blackshear PJ. Overexpression of the myristoylated alanine-rich C-kinase substrate inhibits cell adhesion to extracellular matrix components. The Journal of biological chemistry. 2001;276(34):32264–32273. doi: 10.1074/jbc.M103960200. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain research. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo DJ, Bock CB, Tuttle JS, Blackshear PJ. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(4):944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Machado-Vieira R, Yuan P, Wang Y, Wei Y, Falke C, Cirelli C, Tononi G, Manji HK, Du J. Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of mania. Neuropharmacology. 2009;56(1):47–55. doi: 10.1016/j.neuropharm.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp H, Al-Naggar IM, Yarmola EG, Harrison A, Shaw G, Edison AS, Bubb MR. MARCKS is a natively unfolded protein with an inaccessible actin-binding site: evidence for long-range intramolecular interactions. The Journal of biological chemistry. 2005;280(11):9946–9956. doi: 10.1074/jbc.M414614200. [DOI] [PubMed] [Google Scholar]
- Theis T, Mishra B, von der Ohe M, Loers G, Prondzynski M, Pless O, Blackshear PJ, Schachner M, Kleene R. Functional role of the interaction between polysialic acid and myristoylated alanine-rich C kinase substrate at the plasma membrane. The Journal of biological chemistry. 2013;288(9):6726–6742. doi: 10.1074/jbc.M112.444034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovo L, Ahmed T, Callaerts-Vegh Z, Buzzi A, Bagni C, Chuah M, Vandendriessche T, D’Hooge R, Balschun D, Dotti CG. Low hippocampal PI(4,5)P(2) contributes to reduced cognition in old mice as a result of loss of MARCKS. Nature neuroscience. 2013;16(4):449–455. doi: 10.1038/nn.3342. [DOI] [PubMed] [Google Scholar]
- Varea E, Guirado R, Gilabert-Juan J, Marti U, Castillo-Gomez E, Blasco-Ibanez JM, Crespo C, Nacher J. Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. Journal of psychiatric research. 2012;46(2):189–197. doi: 10.1016/j.jpsychires.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Vergeres G, Manenti S, Weber T, Sturzinger C. The myristoyl moiety of myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein is embedded in the membrane. The Journal of biological chemistry. 1995;270(34):19879–19887. doi: 10.1074/jbc.270.34.19879. [DOI] [PubMed] [Google Scholar]
- Walaas SI, Sefland I. Modulation of calcium-evoked [3H]noradrenaline release from permeabilized cerebrocortical synaptosomes by the MARCKS protein, calmodulin and the actin cytoskeleton. Neurochemistry international. 2000;36(7):581–593. doi: 10.1016/s0197-0186(99)00159-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. The Journal of biological chemistry. 2001;276(7):5012–5019. doi: 10.1074/jbc.M008355200. [DOI] [PubMed] [Google Scholar]
- Wang J, Gambhir A, Hangyas-Mihalyne G, Murray D, Golebiewska U, McLaughlin S. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. The Journal of biological chemistry. 2002;277(37):34401–34412. doi: 10.1074/jbc.M203954200. [DOI] [PubMed] [Google Scholar]
- Wang JK, Walaas SI, Greengard P. Protein phosphorylation in nerve terminals: comparison of calcium/calmodulin-dependent and calcium/diacylglycerol-dependent systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8(1):281–288. doi: 10.1523/JNEUROSCI.08-01-00281.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DG, Lenox RH. Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. Journal of neurochemistry. 1996;67(2):767–777. doi: 10.1046/j.1471-4159.1996.67020767.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang X, Sumners C, Raizada MK. Obligatory role of protein kinase Cbeta and MARCKS in vesicular trafficking in living neurons. Hypertension. 2002;39(2 Pt 2):567–572. doi: 10.1161/hy0202.103052. [DOI] [PubMed] [Google Scholar]