Abstract
BACKGROUND
d-Lactic acidosis in infants fed lactic acid bacteria-containing products is a concern.
METHODS
The primary objective of this non-inferiority trial was to compare urinary d-lactic acid concentrations during the first 28 days of life in infants fed formula containing Lactobacillus reuteri (1.2 × 106 colony forming units (CFU)/ml) with those fed a control formula. The non-inferiority margin was set at a two-fold increase in d-lactic acid (0.7 mmol/mol creatinine, log-transformed). Healthy term infants in Greece were enrolled between birth and 72 hours of age, and block randomized to a probiotic (N = 44) or control (N = 44) group. They were exclusively fed their formulae until 28 days of age and followed up at 7, 14, 28, 112, and 168 ± 3 days. Anthropometric measurements were taken at each visit and tolerance recorded until 112 days. Urine was collected before study formula intake and at all visits up to 112 days and blood at 14 days.
RESULTS
d-Lactic acid concentration in the probiotic group was below the non-inferiority margin at 28 days: treatment effect −0.03 (95% confidence interval [CI]: [−0.48 to 0.41]) mmol/mol creatinine but was above the non-inferiority margin at 7 and 14 days—treatment effect 0.50 (95% CI: [0.05–0.96]) mmol/mol creatinine and 0.45 (95% CI: [0.00–0.90]) mmol/mol creatinine, respectively. Blood acid excess and pH, anthropometry, tolerance, and adverse events (AEs) were not significantly different between groups.
CONCLUSION
Intake of L. reuteri-containing formula was safe and did not cause an increase in d-lactic acid beyond two weeks.
Keywords: Lactobacillus reuteri, d-lactic acid, acidosis, infant
Introduction
The l-isomer of lactic acid, formed by the anaerobic reduction of pyruvate to lactate by l-lactate dehydrogenase, is the exclusive lactic acid produced in humans. d-Lactic acid is produced endogenously in humans in much smaller quantities than l-lactic acid via the mitochondrial methylglyoxal pathway.1,2 Other important sources of d-lactate in humans are either via consumption of fermented products or conversion of carbohydrates or l-lactate into d-lactate by lactic acid-producing bacteria in the gut.2,3
About 70% of l-lactic acid in humans is eliminated from the body by the liver through the l-lactate dehydrogenase-catalyzed oxidation to pyruvate, which is then converted either to glucose or, less often, to acetyl-CoA and ultimately CO2.4 Skeletal and cardiac muscle tissues are responsible for the metabolism of about 20% of the l-lactic acid, and about 5% or less is excreted by the kidneys.4
d-Lactic acid metabolism in humans is believed to occur inefficiently because they (as other mammals) lack d-lactate dehydrogenase. Although a putative mammalian gene encoding d-lactate dehydrogenase was reported by Flick and Konieczny,5 to date there are no reports of the identification of the enzyme in humans. Furthermore, because l-lactate dehydrogenase is stereo-specific, d-lactate is oxidized at a much slower rate of about 20–30% of the rate of l-lactate in humans.3,6 On the other hand, some have argued that d-lactate is metabolized efficiently in humans via catalysis by the mitochondrial d-2-hydroxyacid-dehydrogenase.2,7
Most probiotic bacterial species currently used in supplementing human nutrition belong to the Lactobacillus and Bifidobacterium genera. Among the lactobacilli, many produce d-lactic acid either via the d-lactate dehydrogenase-catalyzed reduction of pyruvate to d-lactic acid or by the isomerization of l-lactic acid to d-lactic acid by dl-lactate racemase. This has led to concerns about d-lactic acid accumulation in the blood. The occurrence of d-lactic acidosis in humans is caused by the fermentation of undigested carbohydrates by bacteria in the colon, although this has been reported almost exclusively in patients with short bowel syndrome. The resulting acidic environment further favors selective growth of acid-resistant bacteria and thus lactobacillus overgrowth.2,3,6
To date no case of d-lactic acidosis because of intake of d-lactate-producing Lactobacillus has been documented in healthy infants. However, some authors have hypothesized that because infants do not have fully developed organs the rate of d-lactate clearance, either by the liver or the kidney, may not be rapid enough to prevent d-lactic acid buildup following intake of Lactobacillus-containing products. This has led to concerns of d-lactic acidosis in infants.3,8
Lactobacillus reuteri, a probiotic bacterium isolated from humans, has been associated with health benefits and has shown to be safe for use in healthy infants.8,9 The main objective of the current study was to address the questions raised about the potential for d-lactic acidosis in infants fed d-lactic acid-producing probiotics. To this aim, this study compared urinary d-lactate concentrations in healthy infants fed L. reuteri-containing formula with those fed a control formula without probiotic supplementation.
Participants and Methods
Trial design
This was a prospective, multi-center, randomized (1:1 ratio) double blind non-inferiority trial aimed at testing the hypothesis that an infant formula containing the probiotic L. reuteri was not inferior to a control formula (without probiotic) with respect to urinary d-lactate concentration (ie urinary d-lactate concentration was not greater in the probiotic group than in the control group). The study was performed in Athens, Greece between May 2010 and July 2011. Two centers participated in this trial: Department of Neonatology, “Helena Venizelou” Maternity Hospital and Alexandra General Hospital.
Although the study was initially designed as a single-center study with recruitment of infants ≤24 hours of age, some difficulties in recruiting sufficient numbers of infants led to a protocol amendment to include recruitment from an additional hospital and included infants up to 72 hours of age.
The study was reviewed and approved by the Institutional Review Board/Institutional Ethical Committee of each hospital before its commencement. The study was explained to infants’ parents/legal representatives, who signed a written informed consent document before their infants were randomized into the two formula groups.
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Participants
Study participants were recruited from mothers admitted and delivered in the two study centers. Only infants whose parents/legal guardians had decided to exclusively feed them formula at the time of recruitment and had signed the informed consent document were assessed for eligibility to enter the trial. Those fulfilling all the inclusion criteria and having none of the exclusion criteria were enrolled in the study. Inclusion criteria were being healthy, being born at term (≥37 weeks), being ≤72 hours old at the time of enrollment, being under the care of a pediatrician or other qualified healthcare professional, and having had ≥1 post-natal visit with a healthcare provider. Infants were excluded from the study if they had any chromosomal or any other major congenital abnormality, had any significant pre- or post-natal disease, had been treated with antibiotics, or were participating in another study. Additionally, infants were excluded from the study if their mothers had taken probiotic supplements during the last trimester of pregnancy or had taken any antibiotics during the last 14 days of pregnancy, or if their families were not expected to be able to comply with the study protocol.
Study formula
The control and probiotic formulae used in the study were based on the same commercially available starter infant formula intended for infants up to six months of age. They contained protein, carbohydrates, fats, vitamins, and minerals in quantities suitable for infants in the target age group. The only difference between the two formulas was that the probiotic formula contained 1.2 × 106 colony forming units (CFU) of L. reuteri (strain DSM-17938) per milliliter of formula. The study formulae had similar taste and color, and were packaged in similar cans that were coded by the manufacturer. Parents, investigators, and study staff were all blinded to the identity of the formulae.
Study procedure and outcome measures
The primary outcome was urine d-lactate concentration at the 7-, 14-, and 28-day visits. Secondary outcomes were urine d-lactate concentration at the 112-day visit; urine l-lactate and total (l + d)-lactate concentrations, and the ratio of d- to l-lactate at the 7-, 14-, and 28-day visits; blood acid excess and pH; stool bacterial counts; anthropometry (weight, length, head circumference, and body mass index [BMI]); sleep patterns; duration of crying; gastrointestinal tolerance (frequency of spitting up and vomiting, stool characteristics, and frequency of flatulence); and occurrence of adverse events (AEs).
Eligible infants were enrolled at 0–72 hours following birth and their demographic characteristics, mode of delivery, number of siblings, and baseline weight and length measurements were recorded. The first urine samples were taken at the hospital during the first 72 hours following birth before any study formula intake had commenced. Infants were randomly assigned to receive one of the two study formulae, and their caretakers were provided with cans of their assigned formulas along with instructions on their preparation. Infants were to be exclusively fed their assigned formulas ad libitum from the time of enrollment until 28 days of age.
Follow-up visits to the study site took place at 7, 14, 28, 112, and 168 ± 3 days of age. At enrollment, caregivers were given instructions on keeping a diary where they recorded daily volume of formula intake (and intake of any other nutrition or medication); stool frequency and consistency; frequency of spitting up, vomiting, or flatulence; sleeping and crying patterns; and the occurrence of AEs. Daily diaries were completed for the three days preceding each visit up to the 112-day visit. The study investigators evaluated the records kept in diaries at each visit.
Protocol deviations were defined as attending visits outside of the three-day window of the scheduled visit, use of antibiotics during the first 28 days of life, and consumption of any non-study formula for more than two consecutive days during the first 28 days.
Analysis of urine samples
Urine samples were collected at each visit except the last. On the morning of the day of visit, parents attached sterile plastic urine collection bags on their infants before the first feeding initiated. The bags were removed at the study center, and 6-mL urine aliquots were placed into cryotubes and frozen immediately at −40°C until further analysis. Urinary d- and l-lactate concentrations, and creatinine concentrations were determined at the Centre Hospitalier Universitaire Vaudois (Lausanne, Switzerland) as described previously.10 Both d- and l-lactate concentrations were normalized per mole creatinine (mmol lactate/mol creatinine).
Blood pH was measured directly from the arterial blood samples, and blood acid excess was calculated indirectly from the same blood sample taking into account PaCO2 measurement, using an algorithm.
Bacterial quantification
On the 14- and 112-day visits, parents collected 5 g stool samples from their infants into sterile tubes within 30 minutes of emission. Samples were placed in aluminum bags that were maintained under anaerobic conditions using an AnaeroGen packet (Oxoid, Hampshire, UK) and stored at 4°C until transport to the study site, which occurred within 10 hours of collection. At the study site, samples were processed immediately as follows: for L. reuteri quantification, approximately 1 g of stool was suspended in 1 ml of Ringer or phosphate buffered saline solution containing 10% glycerol, and the suspension was frozen at −40°C until further analysis. For all other bacterial quantifications, 0.5 g aliquots of stool were stored at −40°C without any other treatment until further analysis.
L. reuteri was cultivated and quantified by polymerase chain reaction (PCR, performed by Advanced Analytical Technologies, Piacenza, Italy). Bifidobacteria, lactobacilli, Enterobacteriaceae, and Clostridium difficile were quantified by fluorescence in situ hybridization (FISH, performed by BioVisible, the Netherlands) or as follows: fecal samples were pulverized using a cryoPREP device (Covaris, USA), and total DNA extracted using QIAamp DNA stool mini kit (QIAGEN, Germany) following the manufacturer’s instructions, except for additional mechanical disruption steps (11 × 45 seconds) using a FastPrep apparatus and Lysing Matrix B tubes (MP Biochemicals, USA). DNA amplification was performed using two sets of primers targeting the hypervariable regions V1–V3 (V123) and V4–V6 (V456) of the 16S rRNA gene. For amplification of the V123 region, a mixture of forward primers, designed according to Hamady et al11 and one reverse primer was used:
V123 forward primers V123-1—5′CTATGCGCCTTGCCAGCCCGCTCAG TCAGAGTTTGATYMTGGCTCAG V123-2—5′CTATGCGCCTTGCCAGCCCGCTCAG TCAGGGTTCGATTCTGGCTCAG V123-3—5′CTATGCGCCTTGCCAGCCCGCTCAG TCAGAGTTTGATCCTGGCTTAG V123-4—5′CTATGCGCCTTGCCAGCCCGCTCAG TCAGAATTTGATCTTGGTTCAG
V123 reverse primer 5′CGTATCGCCTCCCTCGCG CCATCAGNNNNNNNNGGTTACCGCGGCT GCTGGCAC.
The forward and reverse primers for the V4–V6 (V456) region were as follows:
V456 forward primer 5′CTATGCGCCTTGCCAGCCC GCTCAGGCCRRCACGAGCTGACGAC
V456 reverse primer 5′CGTATCGCCTCCCTCGCGC CATCAGNNNNNNNNAGGCCAGCAGCCGCG GTAA.
Italicized sequences indicate adapters for Roche 454 FLX Titanium sequencing, underlined sequences indicate the linkers, NNNNNNNN sequences designate the sample-specific eight-base barcodes used to tag each PCR product, and bold sequences correspond to broadly conserved 16S rRNA gene regions. V123 forward primers 1, 2, 3, and 4 were combined in 4:1:1:1 ratio. Each PCR amplification reaction contained 2 μL of DNA extract, 50 μM dNTPs, 200 nM forward primer (mix of forward primers for the V123 region), 200 nM reverse primer, 1 × expand high fidelity reaction buffer, and 2.5 U of expand high fidelity enzyme blend (Roche Applied Science, Switzerland). PCR conditions were 94°C for two minutes followed by 25 cycles of 94°C for 30 seconds, 49°C for 30 seconds, and 72°C for one minute, and a final elongation at 72°C for seven minutes. Amplification products were purified, pooled in equimolar amounts, and sequenced using a 454 FLX Titanium technology (Microsynth AG, Balgach, Switzerland). Raw data were analyzed using the Quantitative Insights Into Microbial Ecology (QIIME) software package12 with default parameter settings, except that no barcode correction was allowed, and reverse primers were removed if present. Chimera detection and removal was performed using ChimeraSlayer based on reference alignment from Greengenes (as provided in QIIME 1.2) and default parameters. Samples described by less than 200 sequencing reads were excluded from the analysis. Quality-filtered sequencing reads were analyzed using the Uclust method at a similarity threshold of 97% identity for operational taxonomic units (OTUs) clustering. OTUs were assigned into the Bergey’s bacterial taxonomy using RDP Classifier with a confidence value threshold of 60%. Total bacterial counts were determined by the FISH method.
Anthropometric measurements
At each visit, weight, length, and head circumference measurements were recorded. Infants were weighed to the nearest 10 g. Recumbent length was measured using length boards with at least two clinical staff present to ensure body was fully extended and feet flexed. Head circumference was measured using a non-elastic, plastic-coated measuring tape approximately 2.5 cm above the eye brows, at the largest measurement of the head circumference. Both length and head circumference measurements were taken repeatedly to the nearest 1 mm until consecutive measurements were within 5 mm, and the medians of the two consecutive measurements were recorded.
Evaluation of digestive tolerance
Spitting up was defined as non-projectile emission of small volumes of milk shortly after feeding, and vomiting was defined as projectile or non-projectile emission of relatively large volumes of stomach content. Each was assessed by the frequency of occurrence. Stool characteristics were assessed by stool frequency (number of outputs during a 24-hour period) and the predominant consistency (hard/lumpy, formed, soft/creamy, or loose/watery), and the occurrence of flatulence was assessed by its presence or absence. Sleep patterns were assessed by the duration of sleep and the frequency of awakenings at night, and crying was assessed by its duration.
Sample size calculation
Sample size was calculated using data on urinary d-lactate concentrations in healthy infants reported by Haschke-Becher et al.13 As data were skewed, they were log-transformed and the mean and standard deviation (SD) values estimated from the three groups in the study. The non-inferiority margin was set using the empirical assumption of a significant difference of a two-fold increase in urinary d-lactate concentration ie log(2) ≈0.7 mmol/mol creatinine. The mean and SD of the d-lactate concentrations in the study by Haschke-Becher et al were 2.25 mmol/mol creatinine and 1.46 mmol/mol creatinine, respectively.13 With a two-sided test at a 5% significance level and 80% power, 35 infants had to be included in each arm. Assuming a 20% drop-out/non-compliance rate, 44 infants had to be enrolled.
Randomization
Block randomization (block sizes of 2, 4, 6, or 8) with stratification by gender and delivery mode (vaginal or cesarean) was performed using R version 2.12.0.
Statistical analyses
Only data from infants who completed the study until the 28-day visit were analyzed [intention-to-treat (ITT), population]. None of the infants had any major protocol deviations, and therefore a per protocol (PP) population was not relevant. Data were summarized as mean and SD or median and interquartile range (IQR). Codes were broken after the blinded statistical analysis of data up to day 28.
d-Lactate concentrations were log-transformed and data presented as mean and SD, and differences between groups and 95% confidence intervals (CIs) calculated. Data between groups were compared by analysis of covariance (ANCOVA) adjusting for baseline urinary d-lactate concentration. Non-inferiority was tested in a hierarchical manner starting with the 28-day data. At each stage, non-inferiority was established if the upper bound of the 95% CI was below the non-inferiority margin of 0.7 mmol/mol creatinine.
Owing to the asymmetry of the data and the presence of outliers, l-lactate and total lactate concentrations, and d-lactate/l-lactate ratio, data were log-transformed and analyzed using the Hodges-Lehmann estimator on the differences from baseline. Differences in blood acid excess and pH between groups were tested using a linear model.
Bacterial counts were left-censored, considering the minimum bacterial counts observed as the detection limit. P-values were calculated by Fisher’s exact test between the two treatment groups. Because of data asymmetry and the presence of outliers, stool bacterial counts were analyzed using the Hodges–Lehmann estimator.
Growth data were analyzed using a linear mixed model accounting for repeated measures. The frequencies of spitting up, vomiting, and flatulence were analyzed using Poisson regression model fitted to the number of each episode occurring at each visit. The average daily number of stools was compared between groups using a mixed effect Poisson regression model. For stool consistency, the odds ratios (OR) for liquid and hard stools were determined and Fisher’s exact test was used to compare between groups. Sleep duration and crying frequencies were aggregated from the three-day records and analyzed using the Hodges–Lehmann estimator.
The R statistical package (version 2.12.0) was used in all analyses.
AEs
AEs were defined as untoward occurrences in infants who received any of the study formulas regardless of causality. These were illnesses, or signs or symptoms, including any abnormal laboratory findings, occurring or worsening during the study. AEs were coded using the World Health Organization International Classification of Diseases and were assessed by investigators who classified them as serious or non-serious and who established their relationship to study formula intake.
Results
Study population, demographics, and baseline characteristics
A total of 88 infants were enrolled and 44 randomized into each formula group. In all, 17 infants dropped out before the 28-day visit and were excluded from the ITT analysis (Fig. 1). An additional 9 infants dropped out before the 112-day visit; thus, data from 62 infants were available at the end of the study (Fig. 1). The reasons for dropping out from the control group were transportation problems (n = 4), moving away (n = 2), diarrhea (n = 1), constipation (n = 1), eczema (n = 1), loss to follow-up (n = 1), and other reasons (n = 4). In the probiotics group, the reasons for dropping out were moving away (n = 1), diarrhea (n = 1), constipation (n = 2), eczema (n = 1), loss to follow-up (n = 5), and other reasons (n = 2).
Figure 1.
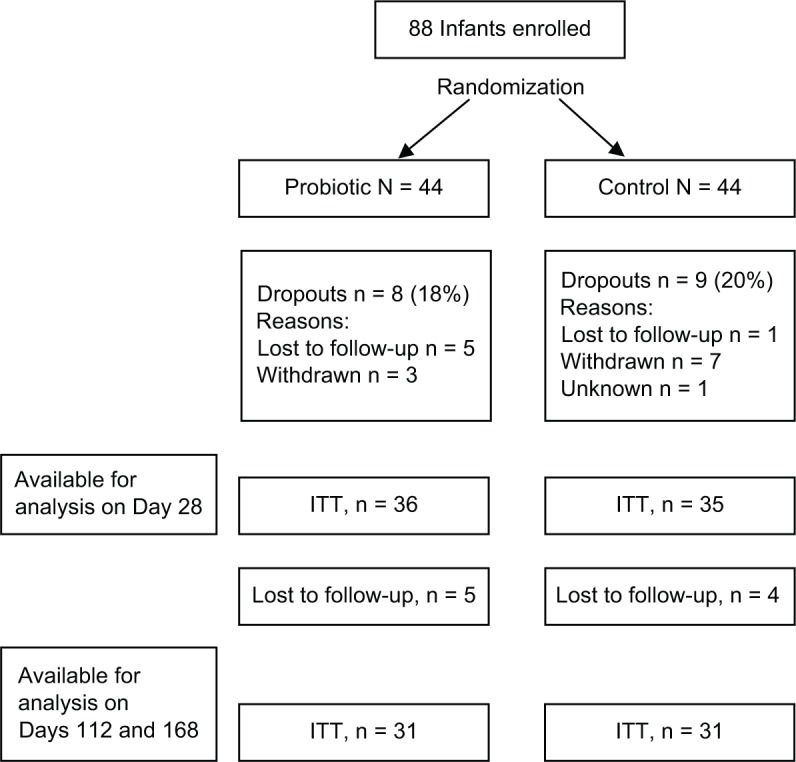
Flowchart of infants participating in the study.
Abbrevation: ITT, intention-to-treat.
Demographics and baseline characteristics of infants were balanced between the two formula groups (Table 1). Even though the baseline d-lactate concentration was slightly higher in the probiotics group than in the control group, it was not statistically significant (P = 0.853).
Table 1.
Baseline characteristics of infants in the study, intention-to-treat.
| CHARACTERISTICS | PROBIOTIC N = 36 | CONTROL N = 35 |
|---|---|---|
| Male, n (%) | 16 (44) | 17 (49) |
| Gestational age, weeks median (IQR) | 38.0 (38.0, 39.0) | 37.5 (39.0, 39.0) |
| Delivery mode | ||
| Vaginal, n (%) | 13 (36) | 14 (40) |
| Cesarean, n (%) | 23 (64) | 21 (60) |
| Weight, kg, median (IQR) | 2.95 (2.74, 3.30) | 3.04 (2.81, 3.23) |
| Length, cm, median (IQR) | 48.5 (47.5, 50.1) | 49.0 (47.5, 50.0) |
| Baseline d-lactate concentration*, mean (SD) | −0.02 (1.07) | 0.04 (1.33) |
Values are log-transformed data.
Abbreviations: IQR, inter quartile age; SD, standard deviation.
Formula intake and compliance
Mean ± SD daily formula intake during the first 28 days was 549.34 ± 156.86 mL in the probiotics group (n = 36) and 565.38 ± 153.62 mL in the control group (n = 31). None of the infants changed formula or started taking complementary food for greater than or equal to two consecutive days before the 28-day visit.
d-Lactate concentrations
For the test group, there was an increase in urinary d-lactate concentrations at day 7 and then a steady decrease up to day 28 (Table 2). Urinary d-lactate concentrations at 7, 14, and 112 days were higher in the probiotic group than in the control group (Table 2). The upper bound of the 95% CI of the difference in d-lactate concentrations between the two groups at 7 and 14 days was above the non-inferiority margin of 0.7 mmol/mol creatinine (Table 2). However, at 28 and 112 days, it was below 0.7 mmol/mol creatinine demonstrating the non-inferiority of the probiotic formula at these times (Table 2). These results show that non-inferiority was met at day 28, but was not met at days 7 and 14 (Table 2). These results were confirmed in a more robust analysis using the Hodges-Lehmann estimator to calculate the 95% CI and Wilcoxon rank sum test to test differences (data not shown).
Table 2.
d-lactate concentrations (mmol/mol creatinine) of log-transformed data, mean ± SD, intention-to-treat.
| FOLLOW-UP PERIOD, DAYS | PROBIOTIC (n) | CONTROL (n) | TREATMENT EFFECT (95% CI) | P-VALUE* |
|---|---|---|---|---|
| 7 | 0.68 ± 1.27 (36) | 0.19 ± 0.91 (34) | 0.50 (0.05 to 0.96) | 0.206 |
| 14 | 0.40 ± 0.84 (36) | −0.04 ± 0.98 (35) | 0.45 (0.00 to 0.90) | 0.142 |
| 28 | 0.36 ± 1.07 (36) | 0.40 ± 0.68 (35) | −0.03 (−0.48 to 0.41) | 0.001 |
| 112 | 0.38 ± 0.9 (31) | 0.25 ± 0.85 (29) | −0.12 (−0.33 to 0.57) | 0.007 |
ANCOVA.
Abbreviations: SD, standard deviation; CI, confidence interval.
l- and total lactate concentrations and d/l-lactate ratio
l-Lactate and total lactate concentrations were not significantly different between the two groups at any time during the study (Table 3). d/l-Lactate ratios were generally higher in the probiotic group compared with those in the control group at all visits during the first 4 months but were not significantly different between groups at any time (Table 3).
Table 3.
Differences from baseline in log-transformed l, d + l and d/l-lactate concentrations (mmol/mol creatinine).
| FOLLOW-UP PERIOD, DAYS | PROBIOTIC | CONTROL | P-VALUE* | |||
|---|---|---|---|---|---|---|
| n | MEDIA (IQR) | n | MEDIA (IQR) | |||
| l-lactate | 7 | 36 | −0.05 (0.77) | 34 | −0.23 (0.57) | 0.19 5 |
| 14 | 36 | −0.05 (0.66) | 35 | −0.22 (0.76) | 0.16 0 | |
| 28 | 36 | −0.10 (0.69) | 34 | 0 (0.62) | 0.829 | |
| 112 | 31 | −0.66 (0.57) | 29 | −0.52 (0.70) | 0.362 | |
| d + l-lactate | 7 | 36 | −0.02 (0.83) | 34 | −0.21 (0.56) | 0.168 |
| 14 | 36 | −0.07 (0.65) | 35 | −0.24 (0.74) | 0.131 | |
| 28 | 36 | −0.12 (0.74) | 34 | 0 (0.63) | 0.847 | |
| 112 | 31 | −0.66 (0.51) | 29 | −0.48 (0.68) | 0.257 | |
| d/l-lactate | 7 | 36 | 0.60 (0.82) | 34 | 0.51 (1.88) | 0.410 |
| 14 | 36 | 0.52 (1.31) | 35 | 0.23 (1.35) | 0.357 | |
| 28 | 36 | 0.60 (2.05) | 34 | 0.30 (1.71) | 0.445 | |
| 112 | 31 | 1.00 (1.79) | 29 | 0.84 (1.52) | ||
For comparison between groups.
Abbreviation: IQR, interquartile range.
Blood acid excess and pH
On day 14, neither blood acid excess nor pH showed significant differences between groups (mean ± SD, 0.88 ± 1.27 mmol/L vs. 0.74 ± 1.28 mmol/L for blood acid excess and 7.39 ± 0.02 vs. 7.38 ± 0.02 for pH in the probiotic and control groups, respectively, P > 0.05 for both).
Stool bacterial counts
On day 14, the detectability of Bifidobacterium, Lactobacillus, and L. reuteri in the test group was significantly higher than that in the control group (P-value ranged from 0.005 to 0.032). Stool bacterial measurements at 14 days indicate that more than 80% of infants in the test group had detectable levels of bifidobacteria compared with ca. 50% of those in the control group (Table 4). Similarly, lactobacilli were detected in about 60% infants in the test group compared with ca. 30% in the control group (Table 4). There was no statistical difference between the two groups with respect to the detectability of C. difficile, Enterobacteriaceae, and total bacteria levels.
Table 4.
Stool bacterial counts (colony forming units/gram) on day 14, intention-to-treat.
| BACTERIA QUANTIFIED | PROBIOTIC | CONTROL | ||||
|---|---|---|---|---|---|---|
| n | % DETECTIBLE | MEDIAN (IQR) | n | % DETECTIBLE | MEDIAN (IQR) | |
| Bifidobacteria | 29 | 80.6 | 4.0 × 109 (6.9 × 109) |
18 | 51.4 | 1.1 × 1010 (1.6 × 1010) |
| Clostridium difficile | 16 | 44.42 | 2.0 × 109 (3.2 × 109) |
8 | 22.9 | 1.4 × 109 (1.7 × 109) |
| Enterobacteriaciae | 35 | 97.2 | 1.4 × 109 (2.1 × 109) |
35 | 100 | 1.9 × 109 (2.5 × 109) |
| Lactobacillus reuteri | 36 | 100 | 6.8 × 106 (2.9 × 107) |
28 | 80.0 | 7.6 × 105 (3.5 × 106) |
| Lactobacilli | 21 | 58.3 | 4.6 × 107 (8.3 × 107) |
11 | 31.4 | 3.5 × 107 (4.2 × 108) |
| Total bacteria | 36 | 100 | 1.3 × 1010 (1.4 × 1010) |
35 | 100 | 1.5 × 1010 (1.9 × 1010) |
Abbreviation: IQR, inter quartile range.
At month 4, similarly, the detectability of Bifidobacterium, Lactobacillus, and L. reuteri in the test group was significantly higher than that in the control group (P-value ranged from 0.006 to 0.024). There was no statistical difference between the two groups with respect to the detectability of Enterobacteriaceae and total bacteria levels.
Growth
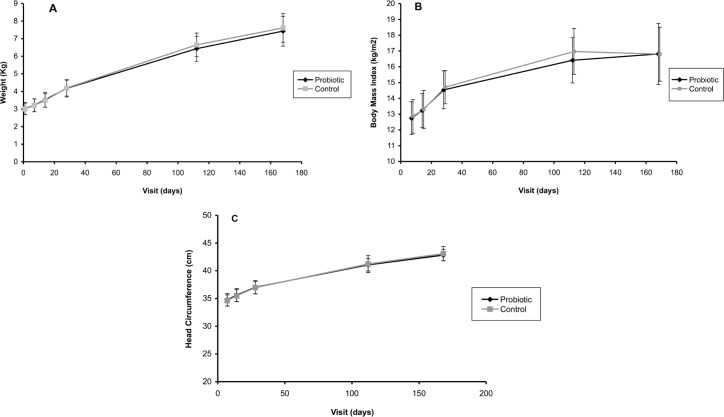
Weight, BMI, and head circumference measurements were not significantly different between the two groups at any time (Fig. 2). The lower bound of the 95% CI around the difference in mean daily weight gain between the probiotic and control groups on days 28 and 168 was above the –3 g/day margin in all infants, indicating the non-inferiority of the probiotic formula in terms of daily weight gain. For the study population as a whole (probiotic and control groups), weight gain compared with WHO median curves and using the non-inferiority margin of –3 g/day was normal (95% CI: [3.58–11.13]).
Figure 2.
Growth measurements during the six-month study period (ITT population). Mean growth measurements and SDs (indicated with the error bars) are shown. Significant differences are marked by *(P < 0.05). Panel A, weight; panel B, body mass index (BMI); panel C, head circumference.
Sleeping and crying patterns
During the first month of life, there were no statistically significant differences between the test and control groups with respect to sleep duration and number of wake-ups at night. At four months, there were no statistically significant differences between the test and control groups with respect to crying duration and number of wake-ups at night. Night-time sleep was slightly longer (20 minutes more) in the test group versus control group but did not reach statistical significance (P = 0.093).
Gastrointestinal tolerance and stool characteristics
There was a significantly lower median number of spitting in the test group compared to that in the control group at day 28 (2 vs. 3, P = 0.048) and month 4 (1.67 vs. 2.33, P = 0.047). There were fewer vomiting episodes in the test group versus control group (3 vs. 17%); however, statistical significance was not achieved (P = 0.105) likely as a result of low numbers (one vs. five episodes). There was no difference between the groups with respect to flatulence frequency.
There was no statistical difference between the groups in stool frequency during the first month of life. The test group had significantly lower frequency of hard stools and higher percentage of soft stools versus control group at day 28 (4.4 vs. 13.0% for hard stools, respectively, P = 0.001; 71.1 vs. 57.3% for soft stools, respectively, P = 0.018).
AEs
The occurrence of serious and non-serious AEs was comparable between the two groups. Non-serious AEs were reported in 20% of infants in the probiotics group and 23% of infants in the control group. Most of these (five in the probiotics group and six in the control group) were respiratory system disorders in both groups.
In all, 5% of infants in each group had a serious AE during the study (Table 5).
Table 5.
Serious adverse events (SAE) occurring in infants during the trial, n (%), intention-to-treat.
| SAE* BY SYSTEM ORGAN CLASS | PROBIOTIC (N = 44) | CONTROL (N = 44) |
|---|---|---|
| Body as a whole-general disorders | 0 | 1 (2) |
| Gastro-intestinal system disorders | 1 (2) | 1 (2) |
| Respiratory system disorders | 1 (2) | 0 |
According to the International Classification of Diseases.
Discussion
The results for urinary d-lactate concentrations indicate that a starter formula with L. reuteri (test) compared with the same formula without L. reuteri (control) in regard to increase in urinary d-lactate levels meets the non-inferiority margin at 28 days and 4 months of age. An increase in the urinary d-lactate excretion initially observed at earlier time points of 7 and 14 days for the L. reuteri group versus control group appears to be transient, with the concentrations coming down to the levels of the control group at 28 days and remaining similar to the control group at month 4. The increases at the earlier times were not associated with any sign of d-lactate acidosis as confirmed by the values for blood pH and acid excess. Also, both groups were comparable with respect to secondary endpoints related to lactate.
Compared with urinary d-lactic acid production in healthy infants reported previously,10,13 the concentrations observed in both formula groups in the current study appeared to be within normal ranges even though they increased transiently in the probiotic group. In the study aimed at establishing normal urinary d-lactate concentrations in healthy infants and children, Haschke and colleagues reported a median urinary d-lactate concentration of 6.4 mmol/mol creatinine, and a concentration of 33.9 mmol/mol creatinine at the 95th percentile in infants zero to six months old.10 The figures were higher among a different population (in the same study) of 1 month–2.5 years olds, where the median was 39.9 mmol/mol creatinine and the 95th percentile 40.8 mmol/mol creatinine. The maximum concentration for the 95th percentile observed in our study was 40.8 mmol/mol creatinine, which occurred in the probiotics group on day 7. Although our study was designed based on this study and the empirical choice of detecting a two-fold increase in urinary d-lactate concentration, the clinical relevance for the non-inferiority margin remains to be established.
In a more recent study of d-lactate concentrations in healthy six-month-old infants fed a formula containing Lactobacillus johnsonii, baseline urinary d-lactate ranged from 0.7 to 99.4 mmol/mol creatinine in the L. johnsonii group and 0.6 to 218.4 mmol/mol creatinine in the control group.13 After four weeks of study formula intake, d-lactate concentrations ranged from 5.6 to 77.6 mmol/mol creatinine in those fed the L. johnsonii formula and 1.8 to 127.6 mmol/mol creatinine in those fed the control formula. This indicates variability in d-lactate concentration that is unlikely to be attributed to Lactobacillus intake.
Concerns have been raised regarding the use of d-lactate-producing lactobacilli as probiotics in infants.9 Based on current available literature no case of probiotic-induced acidosis has been reported in healthy infants. On the other hand, cases of d-lactic acidosis have been reported in infants and adults with short bowel syndrome or small intestinal bypass.2,14,15 In these patients, d-lactic acidosis is caused by Lactobacillus overgrowth in the small intestine, which results from malabsorption of food and an acidic environment that creates a somewhat selective environment that favors Lactobacillus overgrowth.
Ku et al reported a case of probiotics-induced d-lactic acidosis in a young child and reviewed the literature for similar cases in the pediatrics population.16 In all of the d-lactic acidosis cases identified, infants had underlying short bowel syndrome. Uribarri and colleagues also reviewed the literature of d-lactic acidosis in the general population and found that in almost all cases identified, it occurred in patients with either short bowel syndrome or a resected small intestine.2
Although our results are consistent with the ranges of urinary d-lactate concentrations reported earlier in healthy infants,10,13 they also confirm that our study population (probiotic and control) is far below the pathological ranges described elsewhere in adults.2 To our knowledge, metabolic acidosis has been primarily assessed in adults, and mostly based on measurements of d-lactate concentrations in blood. Our results further generate data to support the establishment of safe reference values of d-lactate using urinary measurements.
In the study by Bongaerts and colleagues where d-lactic acidosis was linked to Lactobacillus overgrowth in children with short small bowel patients, stool lactobacilli counts were 1010–1012 CFUs/g stool.14 By contrast in our study, Lactobacillus counts during the period of exclusive feeding with L. reuteri-containing formula did not exceed 5 × 107 CFU/g stool. Furthermore, neither blood pH nor other AEs that would be considered similar to symptoms of d-lactic acidosis were reported in our study. Overall, infants in both formula groups grew normally and tolerated the study formulae well. All infants were healthy and did not show any clinical issues at any time, confirming the safety of the starter formula supplemented with L. reuteri. These results are also consistent with those reported by Connolly et al. who evaluated the safety of L. reuteri at similar daily doses in infants.9
Well-designed randomized controlled trials to address concerns regarding probiotic-induced d-lactic acidosis in healthy infants are scarce. Our study provides important contribution to address this deficiency. This study is the first randomized controlled trial whose primary objective was to evaluate the effect of L. reuteri intake in infants on d-lactic acid concentrations.
It is worth noting that the presence of L. reuteri, lactobacilli, and bifidobacteria was significantly higher in the probiotic group. These findings indicate a favorable impact of the supplementation with L. reuteri on the gut microbiota composition with increased presence of beneficial microbial populations such as bifidobacteria and lactobacilli.
In addition, the infants receiving L. reuteri had a significantly lower number of spitting compared to the control group infants at day 28 and month 4. This is in agreement with the study of Indrio et al who found that the administration of L. reuteri in infants with regurgitation reduced significantly its frequency by accelerating gastric emptying.17
In conclusion, our study confirms that supplementing starter formula with L. reuteri is safe, is well tolerated, and supports normal infant growth.
Acknowledgment
The authors thank Makda Fisseha for medical writing services on behalf of Nestlé. The authors are also grateful to Enea Rezzonico for microbiology support and analysis, Dominik Grathwhol for statistical analysis and Sophie Pecquet for medical publication management.
Footnotes
Author Contributions
Conceived and designed the experiments: KP, PS. Analyzed the data: LAT, PS. Contributed to the writing of the manuscript: KP, EF, DE, LAT, PS. Agree with manuscript results and conclusions: KP, EF, DE, LAT, PS. Jointly developed the structure and arguments for the paper: KP, LAT, PS. Made critical revisions and approved final version: LAT, PS. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Joseph Zhou, Editor in Chief
FUNDING: This study was funded by Nestec Ltd, Vevey, Switzerland. The funder had no influence over the content of this paper.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
TRIAL REGISTRATION: ClinicalTrials.gov NCT01119170.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
REFERENCES
- 1.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uribarri J, Oh MS, Carroll HJ. d-lactic acidosis A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine (Baltimore) 1998;77:73–82. doi: 10.1097/00005792-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Mack DR. d(–)-lactic acid-producing probiotics, d(–)-lactic acidosis and infants. Can J Gastroenterol. 2004;18:671–675. doi: 10.1155/2004/342583. [DOI] [PubMed] [Google Scholar]
- 4.Phypers B, Pierce T. Lactate physiology in health and disease. Cont Educ Anaesth Crit Care Pain. 2006;6:128. [Google Scholar]
- 5.Flick MJ, Konieczny SF. Identification of putative mammalian d-lactate dehydrogenase enzymes. Biochem Biophys Res Commun. 2002;295:910–916. doi: 10.1016/s0006-291x(02)00768-4. [DOI] [PubMed] [Google Scholar]
- 6.Ewaschuk JB, Naylor JM, Zello GA. d-lactate in human and ruminant metabolism. J Nutr. 2005;135:1619–1625. doi: 10.1093/jn/135.7.1619. [DOI] [PubMed] [Google Scholar]
- 7.Oh MS, Uribarri J, Alveranga D, Lazar I, Bazilinski N, Carroll HJ. Metabolic utilization and renal handling of d-lactate in men. Metabolism. 1985;34:621–625. doi: 10.1016/0026-0495(85)90088-5. [DOI] [PubMed] [Google Scholar]
- 8.Connolly E, Lönnerdal B. d(–)-lactic acid-producing bacteria: safe to use in infant formulas. Nutrafoods. 2004;3:37–49. [Google Scholar]
- 9.Connolly E, Abrahamsson T, Bjorksten B. Safety of d(–)-lactic acid producing bacteria in the human infant. J Pediatr Gastroenterol Nutr. 2005;41:489–492. doi: 10.1097/01.mpg.0000176179.81638.45. [DOI] [PubMed] [Google Scholar]
- 10.Haschke-Becher E, Baumgartner M, Bachmann C. Assay of d-lactate in urine of infants and children with reference values taking into account data below detection limit. Clin Chim Acta. 2000;298:99–109. doi: 10.1016/s0009-8981(00)00272-2. [DOI] [PubMed] [Google Scholar]
- 11.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods. 2008;5(3):235–237. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haschke-Becher E, Brunser O, Cruchet S, Gotteland M, Haschke F, Bachmann C. Urinary d-lactate excretion in infants receiving Lactobacillus johnsonii with formula. Ann Nutr Metab. 2008;53:240–244. doi: 10.1159/000185642. [DOI] [PubMed] [Google Scholar]
- 14.Bongaerts G, Bakkeren J, Severijnen R, et al. Lactobacilli and acidosis in children with short small bowel. J Pediatr Gastroenterol Nutr. 2000;30:288–293. doi: 10.1097/00005176-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Bongaerts G, Tolboom J, Naber T, Bakkeren J, Severijnen R, Willems H. d-lactic acidemia and aciduria in pediatric and adult patients with short bowel syndrome. Clin Chem. 1995;41:107–110. [PubMed] [Google Scholar]
- 16.Ku W, Lau D, Huen K. Probiotics provoked d-lactic acidosis in short bowel syndrome: case report and literature review. Hong Kong J Paediatr. 2006;11:246–254. [Google Scholar]
- 17.Indrio F, Riezzo G, Raimondi F, et al. Lactobacillus reuteri accelerates gastric emptying and improves regurgitation in infants. Eur J Clin Invest. 2011;41:417–422. doi: 10.1111/j.1365-2362.2010.02425.x. [DOI] [PubMed] [Google Scholar]