Abstract
Background
Calcium is an essential mineral often taken as a daily, long-term nutritional supplement. Data suggests that once-daily dosing is important with regard to long-term compliance of both drugs and nutritional supplements.
Objective
This study was undertaken to compare the bioavailability of a single serving of two calcium supplements in healthy, premenopausal women.
Design
A two-period, crossover bioavailability study of a single serving of calcium citrate tablets (two tablets=500 mg calcium) versus a single serving of calcium carbonate powder (one packet of powder=1,000 mg calcium) was performed in healthy women aged between 25 and 45. All subjects were on a calcium-restricted diet 7 days prior to testing and fasted for 12 h before being evaluated at 0, 1, 2, and 4 h after oral administration of the test agents. Blood measurements for total and ionized calcium and parathyroid hormone were performed and adverse events were monitored.
Results
Twenty-three women were evaluable with a mean age of 33.2±8.71. Results showed that administration of a single serving of a calcium carbonate powder resulted in greater absorption in total and ionized calcium versus a single serving of calcium citrate tablets at 4 h (4.25±0.21 vs. 4.16±0.16, p=0.001). There were minimal side effects and no reported serious adverse events.
Conclusions
This study shows that a single serving of a calcium carbonate powder is more bioavailable than a single serving of calcium citrate tablets. This may be beneficial for long-term compliance.
Keywords: calcium, calcium carbonate, calcium citrate, absorption
Calcium is an essential mineral that has a wide variety of biologic functions. Approximately 75% of dietary calcium is obtained from milk and dairy products and the recommended daily amount for adults is about 1 g/day. However, there is tremendous variability in the bioavailability of calcium in humans related to factors including dietary patterns, health and disease state(s), and digestion and absorption, and in large segment of the population, calcium intake may fall below the actual recommended intake (1). Calcium is frequently obtained by the consumption of calcium-rich foods such as dairy products and fortified foods but calcium dietary supplements are an important means by which optimal calcium intake can be reached in those who cannot meet their need by ingesting conventional foods. Moreover, calcium supplementation should be considered in individuals with osteopenia or osteoporosis, perimenopausal and postmenopausal women, mothers who breastfeed, vegans, amenorrheic women, residents of long-term care facilities, and individuals who are lactose intolerant, receiving chronic corticosteroid, or are under immunosuppressant therapy (1).
Calcium supplements may come in a variety of forms and delivery systems. The most common forms include calcium carbonate, calcium citrate, and calcium gluconate. Oral delivery forms vary and include tablets, capsules, soft-chews, powders, and liquids. While the dosage and effectiveness of a product depends on the therapeutic coverage of the agent, it has been demonstrated that patients prefer to take medications and/or nutritional agents once-daily as opposed to multiple doses throughout the day (2, 3). One recent study demonstrated that subjects taking a once-daily dose of a prescription medication had up to 44% more adherent days compared with patients receiving twice-daily dosing who adhered to dosing about 13–26% of the time (2). The authors also suggest that compliance may be even lower in patients taking nutritional agents (2). Other data has shown that reducing dose frequency also provides better quality of life, patient satisfaction, and reduced cost (4).
Based on these factors, the aim of our study was to test the bioavailability of a single serving of two commercially available calcium supplements. Our hypothesis was that oral administration of a single serving of calcium carbonate powder would have superior bioavailability to a single serving of calcium citrate tablets in healthy women and, if true, may provide a better alternative with regard to absorption should patients forget to take their second or third daily serving of a calcium supplement.
Materials and methods
Subjects
We studied 24 women aged between 25 and 45 in an institutional review board (IRB) approved study (Novum IRB, Pittsburg, PA). Consecutive subjects were screened for eligibility following written informed consent. Subjects that were included met the following criteria: subject was able to come to the clinic the night before each of the study visits by 9 pm and agreed to fast through the night, subject was a premenopausal female of any ethnic origin, was in good health as determined by medical history and physical examination, had a body mass index (BMI) between 18 and 30 kg/m2, and agreed to adhere to a low calcium diet for the entire study duration (about 15–21 days) as measured by a daily food log. Excluded from the study were subjects younger than 25 or older than 45, had reached menopause, were currently taking any calcium supplement or multivitamin (washout of 7 days was allowed), had a known medical history of calcium or vitamin D malabsorption, any current or recent diagnosis thought to affect bone or mineral metabolism or parameters related to calcium bioavailability such as hypo- or hyperparathyroidism, hypercalcemia, liver disease, history of renal calculi, or diabetes, taking any medication(s) (e.g. corticosteroids, proton pump inhibitors) within 30 days of the study, had participated in a clinical study involving administration of an investigational drug which in the judgment of the investigator may affect absorption or elimination of calcium within the past 30 days, donated blood, plasma, or platelets in the past 30 days, had any clinically significant illness within 30 days of the start of the study, were pregnant or were planning pregnancy or any subjects who in the opinion of the investigator should not participate in the study.
Study design
The study was a two-period crossover bioavailability and pharmacodynamic study of two commercially available calcium dietary supplements: Study Agent: 1-Cal-EZ®, a single-dose consists of (one packet; approximately 1 teaspoon) 2.5 g of calcium carbonate (1,000 g elemental calcium) and 1,000 IU vitamin D3 cholecalciferol – the powder contains no binders, fillers, or lactose (United Nutrition, Fairfax, VA) – and Study Agent 2: Citrical®, a single-dose consists of two tablets containing 500 mg elemental calcium citrate, 400 IU vitamin D (cholecalciferol), and 5 mg sodium. Inert ingredients in the tablets include tablet binders; polyethylene glycol, croscarmellose sodium, hydroxypropyl methylcellulose, magnesium silicate, titanium dioxide (color), propylene glycol dicaprylate/dicaprate, oligofructose-enriched inulin and magnesium stearate (Bayer Healthcare, Morristown, NJ). Both agents were commercially purchased from a local drugstore. Healthy women who signed the voluntary informed consent form at visit 0 agreed to consume a low calcium diet for 7 days prior to visit 1. Subjects were instructed to avoid all dairy, cheeses, and yogurts and to comply with a low calcium diet as kept in a daily logbook. On the night before visit 1, the subjects arrived at the clinic by 9 pm and were only allowed to have water throughout the night. On visit 1, the subjects were evaluated at time 0–visit 1 (pre-test agent) followed by administration of the study agent (tablet or powder) with a light, calcium-restricted breakfast. The breakfast consisted of the following: white bread toast not enriched with calcium, grape jelly, decaffeinated coffee with sweetener (if desired), and water as desired. No dairy products or calcium enriched food products were allowed for consumption. Subjects were then evaluated at subsequent times (hours 1, 2, and 4) during which blood and urine were taken in order to perform the assays described below. Subjects then left the clinic and were instructed to continue on for an additional 7 days of adherence to the low calcium diet. On the night before visit 2, subjects completed the same schedule (i.e. they arrived at the clinic by 9 pm and fasted through the night). On visit 2, subjects were then given the test agent (tablet or powder) with the same calcium-restricted breakfast and were evaluated at the same time points for blood and urine assays. When consuming the powdered test agent in either period, the test agent was sprinkled on the toast.
Assays
Serum and urine calcium was measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Eurofins Laboratory, Breda, Norway). Intact parathyroid hormone (iPTH) was measured using Siemens Chemiluminescence Immunoassay (Siemens Medical Solutions USA, Inc., Malvern, PA). Serum ionized calcium was calculated based on methods previously described (5).
Statistical analysis
All statistical tests were defined prospectively within the IRB-approved protocol. The primary outcome measures were the area under the curve (AUC) at 1 and 4 h for serum total and ionized calcium; the difference between the responses to each study agent was measured by t-test. Secondary outcomes included calculation of differences between the time to maximum serum concentration (Tmax) and magnitude of elevation (Cmax). AUC was calculated by the trapezoidal method. Cmax and Tmax were analyzed by taking the observed values for concentration and time and fitting the means of the timed serum increments for each source. Secondary outcome measures also included analysis of serum iPTH as measured by Tmin and magnitude of difference. Urine calcium/creatinine ratio was measured by AUC calculated by the trapezoidal method.
Results
Demographics
Twenty-four women were enrolled in the study and 23 completed both study periods and were considered evaluable. The mean age of the study subjects was 33.2±8.71 years.
Response curves
Serum calcium
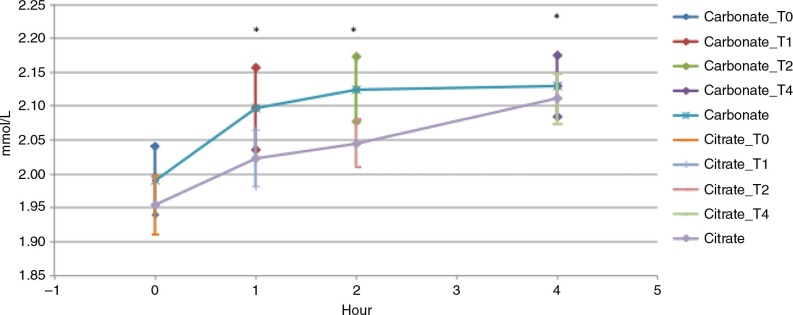
Figure 1 demonstrates the change in total serum calcium concentration from baseline to 4 h following oral administration of calcium carbonate (powder) and calcium citrate (tablet) with a light, calcium restricted meal. The response curve following calcium carbonate ingestion showed a significantly greater total AUC compared to the total AUC following calcium citrate tablet administration (p=0.001). This was also demonstrated after calcium carbonate administration versus calcium citrate administration at all time points (1, 2, and 4 h, [p=0.0056, p=0.0001, p=0.001]), AUC 1 (at 1 h), AUC 2 (at 2 h), and AUC 3 (at 4 h) (Fig. 1 and Table 1). The magnitude of elevation of serum calcium absorption (Cmax) was significantly larger following calcium carbonate powder administration versus calcium citrate tablet administration (p=0.01) (Table 1). The time to maximum concentration (Tmax) of calcium carbonate powder absorption was also significantly smaller versus calcium citrate tablet absorption p=0.04 (Table 1).
Fig. 1.
Serum total calcium, calcium carbonate powder versus calcium citrate tablets. Serum total calcium area under the curve from baseline to 4 h. T-test between carbonate vs. citrate, *p<0.05.
Table 1.
Serum calcium pharmacokinetic parameters
| Calcium carbonate powder | Calcium citrate tablets | ||
|---|---|---|---|
| Parameter | N=24 | N=23 | p |
| Total serum calcium | |||
| AUC 1 (mg/dL * h) | 2.04±0.13 | 1.99±0.09 | 0.0056 |
| AUC 2 | 2.11±0.12 | 2.03±0.08 | 0.0001 |
| AUC 3 | 4.25±0.21 | 4.16±0.16 | 0.001 |
| Total AUC | 8.41±0.44 | 8.18±0.08 | 0.001 |
| Cmax | 2.18±0.13 | 2.13±0.08 | 0.01 |
| Tmax | 2.63±1.24 | 3.23±1.32 | 0.04 |
| Ionized calcium | |||
| AUC 1 | 0.88±0.05 | 0.860±0.04 | 0.02 |
| AUC 2 | 0.91±0.05 | 0.88±0.04 | 0.0004 |
| AUC 3 | 1.82±0.09 | 1.77±0.07 | 0.005 |
| Total AUC | 3.62±0.18 | 3.52±0.15 | 0.001 |
| Cmax | 0.94±0.06 | 0.91±0.04 | 0.0031 |
| Tmax | 2.21±1.14 | 2.65±1.53 | 0.13 |
Serum ionized calcium
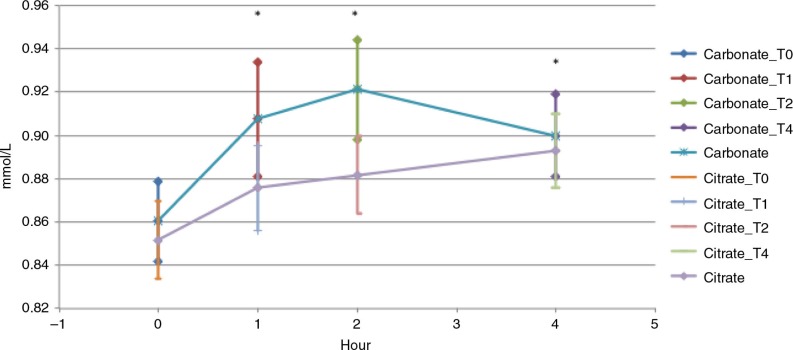
Figure 2 demonstrates the change in total serum ionized calcium concentration from baseline to 4 h following oral administration of calcium carbonate and calcium citrate. The total AUC of serum ionized calcium was also significantly greater after calcium carbonate administration versus calcium citrate administration p=0.001 (Table 1).
Fig. 2.
Serum ionized calcium, carbonate powder vs. calcium citrate tablets. Serum ionized calcium area under the curve from baseline to 4 h. T-test between carbonate vs. citrate, *p<0.05.
Serum iPTH
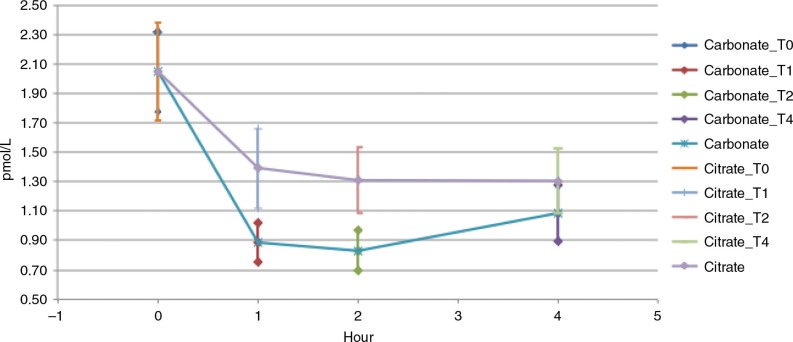
Figure 3 demonstrates the response curve of serum iPTH from baseline to 4 h following calcium carbonate administration versus calcium citrate administration. The response curve after calcium carbonate powder administration showed a significantly greater decline (cMin) compared with that of the calcium citrate tablet (p=0.0007).
Fig. 3.
Serum iPTH, calcium carbonate powder vs. calcium citrate tablets. Serum iPTH cMin from baseline to 4 h.
Urinary calcium
Cumulative increment in urinary calcium excretion was not different following calcium carbonate powder versus calcium citrate tablet administration (data not shown).
Adverse events
There were no serious adverse events in the study. There were two reports of headache in two different subjects, one in the calcium powder administration time and one in the calcium tablet administration period. There was one report of fatigue in a third subject in the calcium powder administration period. There were no gastrointestinal side effects reported in the study period.
Discussion
This study demonstrates that the bioavailability of a single serving of calcium carbonate powder was greater than that of a single serving of calcium citrate tablets in healthy, premenopausal women who took the study agents with a light, low-calcium meal. In this study, change in total AUC in serum total calcium was higher after administration of the calcium carbonate powder versus calcium citrate tablets demonstrating that more calcium was absorbed after the single serving of calcium carbonate powder versus the single serving of calcium citrate tablets. The AUC for calcium carbonate powder was also significantly higher at 1, 2, and 4 h versus calcium citrate tablet ingestion. The bioavailability of the single serving of calcium carbonate powder was also demonstrated by serum decline in PTH, changes that are expected based on known pharmacokinetic analyses of calcium bioavailability and known suppression of parathyroid function with concurrent calcium absorption. There was no significant difference between the two groups in PTH at baseline.
The magnitude of elevation after the ingestion of a single serving of a calcium carbonate powder was significantly larger than that after the ingestion of a single serving of calcium citrate tablets, demonstrating that more calcium was absorbed. The time to maximum absorption (Tmax) of calcium carbonate powder was also significantly different than Tmax of calcium citrate tablets at 2.63±1.24 h versus 3.23±1.32 h, p=0.0036, demonstrating that a single serving of calcium carbonate powder was absorbed approximately 40 minutes more rapidly than a single serving of calcium citrate tablets. We were unable to detect a difference in urine calcium excretion with either administration possibly due to the effect of normal calcium homeostasis regulation within the body.
In this study, we controlled for dietary effects on calcium absorption and excretion by limiting the intake of dietary calcium in subjects for at least 7 days prior to the test periods. It has also been demonstrated that most intestinal adaption based on dietary availability of calcium occurs within 1 week (6, 7). We also controlled for test measurements by having the subjects arrive to the study site the night before and remain fasted throughout the night with water only.
Compared to other studies, we have demonstrated that a single serving of calcium carbonate powder was better absorbed than a single serving of calcium citrate tablets. Previous studies testing calcium carbonate and calcium citrate tablets head-to-head have shown increased absorption of calcium citrate in healthy subjects and in those after gastric bypass (8, 9). However, to our knowledge there have been no studies testing the two calcium anion forms as powders head-to-head. Overall absorption of agents is a critical step in basic pharmacology, and it has been shown that the absorbability of some agents in tablet form differs when taken in other oral forms such as liquids or powders (10).
There are a number of limitations to this study. We were unable to measure intestinal calcium absorption because it is not possible to use radiolabeled isotopes for already commercially prepared dietary agents (11). A second limitation is the differences in dose of the study agents as the amount of calcium carbonate powder in the single serving is 1 g versus the amount of calcium citrate in the single serving is two tablets at 500 mg. While this limitation is in regard to the equivalence of the dose at baseline, the intent of our study was to test the bioavailability of a single serving in order to determine how the study agent was absorbed at a single time point following a single serving administration. It has also been shown that differences in calcium bioavailability are evident in items such as fortified orange juice that claim to have an equivalent dosage of calcium, and this relates to the concept that labeling versus equivalence should be noted by both clinicians and consumers (12). As aforementioned, numerous systematic reviews have examined the effect of daily medication dosing frequency on adherence in patients with a variety of chronic conditions and have demonstrated that patients are more compliant with regimens that require once-daily dosing compared to twice or three times a day dosing, especially when agents are taken for a long (chronic) period of time (2). This is particularly relevant to nutritional supplements like calcium as the use of a calcium nutritional supplement is often indicated for long-term daily use. Despite the fact that the recommended dosage for many calcium supplements is two or three times per day, people may forget to take a second or third dose of a nutritional or dietary supplement. To that end, it is critical to take an agent that is well absorbed pointing to greater effectiveness in the long-term. Moreover, if people do take the additional recommended serving(s) it can be argued that the calcium carbonate powder would be continually better absorbed with multiple servings throughout the day. A third limitation is that we performed our study in healthy premenopausal women and it can be argued that our study could have been performed in postmenopausal women. However, based on general recommendations for equivalence studies, we chose to perform this study in premenopausal women because considerable differences in absorption in postmenopausal women have been noted (13, 14). Our fourth limitation was that this analysis lacked an un-supplemented control group. Finally, our study showed that there were minimal side effects reported with either study agent.
Conclusions
We found that a single serving of calcium carbonate powder was more bioavailable and more rapidly absorbed than a single serving of calcium citrate tablets in healthy, premenopausal women. Calcium carbonate powder may be an advantageous delivery form in subjects who dislike or are unable to take tablets. Moreover, if subjects forget to take their second serving of calcium, this study suggests that absorption of a single serving of calcium carbonate powder is greater than a single serving of two calcium citrate tablets.
Conflict of interest and funding
This study was funded by Besins Healthcare, Herndon, VA. Peter Bua is employed by Besins Healthcare, Herndon, VA.
References
- 1.Brunton LL, Chabner BA, Knollmann BC, editors. 12th ed. New York: McGraw-Hill Medical Books; 2011. Goodman & Gilman's the pharmacological basis of therapeutics. [Google Scholar]
- 2.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manage Care. 2009;15:e22–33. [PubMed] [Google Scholar]
- 3.Coleman CI, Limone B, Sobieraj DM, Lee S, Roberts MS, Kaur R, et al. Dosing frequency and medication adherence in chronic disease. J Manage Care Pharm. 2012;18:527–39. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter A, Anton SE, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25:2307–35. doi: 10.1016/s0149-2918(03)80222-9. [DOI] [PubMed] [Google Scholar]
- 5.Price RI, Kent GN, Rosman KJ, Gutteridge DH, Reeve J, Allen JP, et al. Kinetics of intestinal calcium absorption in humans measured using stable isotopes and high-precision thermal ionization mass spectrometry. Biomed Environ Mass Spectrom. 1990;19:353–9. doi: 10.1002/bms.1200190605. [DOI] [PubMed] [Google Scholar]
- 6.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98:13324–9. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centeno V, de Barboza GD, Marchionatti A, Rodríguez V, Tolosa de Talamoni N. Molecular mechanisms triggered by low-calcium diets. Nutr Res Rev. 2009;22:163–74. doi: 10.1017/S0954422409990126. [DOI] [PubMed] [Google Scholar]
- 8.Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med. 1990;323:878–83. doi: 10.1056/NEJM199009273231305. [DOI] [PubMed] [Google Scholar]
- 9.Tondapu P, Provost D, Adams-Huet B, Sims T, Chang C, Sakhaee K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes Surg. 2009;19:1256–61. doi: 10.1007/s11695-009-9850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song YM, Sheu WH, Lee WJ. Acute biochemical variations induced by calcium citrate and calcium carbonate in Type 2 diabetic patients: impaired calcium absorption in Type 2 diabetic patients with prolonged gastric emptying time. J Diabet Complications. 2001;15:97–102. doi: 10.1016/s1056-8727(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 11.Abe H, Otsuka M. Effects of lubricant-mixing time on prolongation of dissolution time and its prediction by measuring near infrared spectra from tablets. Drug Dev Ind Pharm. 2012;38:412–19. doi: 10.3109/03639045.2011.608679. [DOI] [PubMed] [Google Scholar]
- 12.Heaney RP, Rafferty K, Dowell MS, Bierman J. Calcium fortification systems differ in bioavailability. J Am Diet Assoc. 2005;105:807–9. doi: 10.1016/j.jada.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989;4:469–75. doi: 10.1002/jbmr.5650040404. [DOI] [PubMed] [Google Scholar]
- 14.FDA. Draft guidance for industry. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm [cited 15 October 2013]