Abstract
We have demonstrated that diets containing fish oil and pectin (FO/P) reduce colon tumor incidence relative to control (corn oil and cellulose [CO/C]) in part by inducing apoptosis of DNA-damaged colon cells. Relative to FO/P, CO/C promotes colonocyte expression of the antiapoptotic modulator, Bcl-2, and Bcl-2 promoter methylation is altered in colon cancer. To determine if FO/P, compared with CO/C, limits Bcl-2 expression by enhancing promoter methylation in colon tumors, we examined Bcl-2 promoter methylation, mRNA levels, colonocyte apoptosis and colon tumor incidence in azoxymethane (AOM)-injected rats. Rats were provided diets containing FO/P or CO/C, and were terminated 16 and 34 weeks after AOM injection. DNA isolated from paraformaldehyde-fixed colon tumors and uninvolved tissue was bisulfite modified and amplified by quantitative reverese transcriptase-polymerase chain reaction to assess DNA methylation in Bcl-2 cytosine–guanosine islands. FO/P increased Bcl-2 promoter methylation (P = 0.009) in tumor tissues and colonocyte apoptosis (P = 0.020) relative to CO/C. An inverse correlation between Bcl-2 DNA methylation and Bcl-2 mRNA levels was observed in the tumors. We conclude that dietary FO/P promotes apoptosis in part by enhancing Bcl-2 promoter methylation. These Bcl-2 promoter methylation responses, measured in vivo, contribute to our understanding of the mechanisms involved in chemoprevention of colon cancer by diets containing FO/P.
Keywords: Bcl-2, DNA methylation, epigenetics, fish oil, pectin
Introduction
Epigenetic regulation of gene transcription occurs through mechanisms that include DNA methylation and histone modification.1,2 DNA methylation occurs primarily at cytosine–guanosine dinucleotides (CpG) by the addition of a methyl group on the 5′ carbon position of cytosine. CpG islands are CpG-rich regions in or near the promoter region of genes, that when unmethylated allow the transcription of genes.1 Aberrant methylation of promoter CpG islands has been accepted as a crucial contributor to cancer development3,4 because the extent of promoter methylation in tumors is associated with disease progression, recurrence and survival rates in colon cancer.5,6 A hallmark of tumorigenesis is an inhibition of apoptosis, and genes involved in apoptosis can be transcriptionally regulated through DNA methylation.7 Importantly, aberrant DNA methylation is potentially reversible by natural agents in the diet, such as soy isoflavones, apple polyphenols, green tea polyphenols and biologically active compounds present in vegetables,8–11 which may lead to induction of apoptosis in the colon.
We have previously demonstrated that the combination of fish oil and pectin (FO/P), compared with a diet containing corn oil and cellulose (CO/C) inhibits colon tumor formation.12 Fish oil is high in n-3 polyunsaturated fatty acids (PUFA), i.e. eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), whereas corn oil is high in the n-6 fatty acid, linoleic acid (LA, 18:2n-6). Pectin is a highly fermentable fiber that yields butyrate upon microbial fermentation, whereas cellulose is poorly fermented. Fish oil did not influence proliferation, but did significantly enhance colonocyte differentiation and apoptosis.12 Pectin reduced the numbers of cells per crypt column, but this was not the result of reduced proliferation. Similar to fish oil, pectin’s major effect on cell kinetics was to increase apoptosis, and the increase in apoptosis was greatest when fish oil was combined with pectin.12 Since that study, we have demonstrated that FO/P contribute to the regulation of apoptosis through a variety of mechanisms, including changes in membrane-associated signaling cascades,13,14 and calcium trafficking.15,16
Another of the mechanisms by which FO/P enhances apoptosis is suppression of Bcl-2 levels, an antiapoptotic mediator.17,18 Bcl-2 obstructs the intrinsic apoptotic pathway by preventing the release of cytochrome c from the mitochondria, thereby suppressing the downstream caspase activation.19 In leukemia cells, Bcl-2 is hypomethylated leading to a high Bcl-2 expression.20 These observations are relevant because high levels of Bcl-2 are observed in the metastatic state of various types of cancer including colon cancer.21 Therefore, we hypothesized that the suppression of Bcl-2 expression in response to a FO/P diet may result from a previously unidentified modulation of the Bcl-2 promoter region. To test this, we determined the methylation status of specific CpG dinucleotides of the Bcl-2 promoter region and the transcription level of Bcl-2. This study provides evidence that FO/P modulates apoptosis in part by epigenetic regulation of Bcl-2 expression in rat AOM-induced colon carcinomas.
Materials and methods
Animals and study design
The animal use protocol was approved by the Institutional Animal Care and Use Committee of Texas A&M University and conformed to National Institutes of Health (NIH) guidelines. Ninety-six male Sprague-Dawley rats (Harlan Teklad, Madison, WI, USA) were housed individually in a temperature and humidity controlled animal facility with a 12 h light/dark cycle. Five-week-old rats were allowed to acclimate to their new environments for one week prior to starting the experimental diets. The rats consumed the experimental diets for one week prior to being injected with the colon-specific carcinogen azoxymethane (AOM, Sigma, St Louis, MO, USA, 15 mg/kg body weight, subcutaneous) twice, one week apart. Twenty-two rats (CO/C n = 12, FO/P n = 10) were terminated at 16 weeks and 74 rats (CO/C, n = 41; FO/P, n = 33) were used for final tumor analysis at 34 weeks after the second AOM injection. Unfasted rats were killed by CO2 overdose and cervical dislocation.
Diets
Rats were provided a diet containing either FO/P or CO/C as previously described.22 The FO/P diet contained higher amounts of n-3 PUFA (EPA and DHA) and pectin, a highly fermentable fiber. The CO/C diet had higher amounts of n-6 PUFA (LA) and cellulose, a poorly fermented fiber. The fish oil diet included 3.5 g corn oil/100 g diet to meet essential fatty acid requirements. All diets contained oils at 15% by weight and 30% of calories, an amount that corresponds to current human dietary recommendations. The amount of fiber in the diet was 6% by weight, which is equivalent to 30 g/d for humans, also within the recommended range for humans. The antioxidant levels in the FO/P and CO/C diets were 1.5 g/kg α-tocopherol, 1 g/kg γ-tocopherol and 0.025% tertiary butylhydroquinone. Fresh diets were provided daily to prevent lipid oxidation.
Colon cancer incidence
Colons from rats terminated 34 weeks after the second AOM injection were used to determine tumor incidence. Tumors were counted and tumor tissues were fixed in 4% PFA for four hours and embedded in paraffin blocks for histological examination. Tumor sections (4 μm) were stained with hematoxylin and eosin, and tumors were classified as adenomas or adenocarcinomas,12 and adenocarcinomas were counted for colon cancer incidence.
In situ apoptosis
Apoptosis was measured by TdT-mediated UTP-biotin nick end labeling (TUNEL assay) of fragmented pieces of DNA using 4 μm sections of PFA-fixed, paraffin-embedded tissue. Apoptotic cells with condensed chromatin, apoptotic bodies and intense brown staining were counted in 25 crypt columns for each animal. The apoptotic index was calculated as 100 times the mean number of apoptotic cells per crypt column divided by the mean number of cells per crypt column.12
DNA extraction and preparation
Genomic DNA was extracted from 10 μm PFA-fixed colon carcinomas and non-involved tissue sections using a QIAamp DNA FFPE tissue kit (Qiagen, Valencia, CA, USA). In addition, to prepare a universally methylated reference sample, rat liver genomic DNA was treated with CpG methyltransferase (M.SssI, New England Biolabs, Ipswich, MA, USA), which methylates cytosines in CpG dinucleotides with S-adenosyl methionine (SAM) as a methyl donor. Briefly, genomic DNA of rat liver was treated with M.SssI (0.05 units/μL final concentration) and SAM overnight, followed by another overnight incubation with an additional M.SssI (0.09 units/μL final concentration) and SAM.23 The M.SssI-treated DNA was purified using a DNA Clean and Concentrator kit (Zymo Research, Irvine, CA, USA).
Sodium bisulfite modification and DNA recovery
Two microgram of genomic DNA or M.SssI treated DNA was sodium bisulfite modified using a Zymo EZ DNA Methylation Kit (Zymo Research, Irvine, CA, USA) as per the manufacturer’s instructions. Sodium bisulfite converts unmethylated cytosines to uracils, but does not affect the methylated cytosines.
Quantative polymerase chain reaction detection of Bcl-2 promoter methylation status
After sodium bisulfite conversion, genomic and M.SssI treated DNA were amplified by fluorescence-based Quantative polymerase chain reaction (qPCR) using an ABI 7900 unit. Serial dilutions of M.SssI treated DNA were included on each plate to generate a standard curve. PCR amplification was performed with a final reaction mixture of 25 μL consisting of 600 nmol/L of each primer, 200 nmol/L probe, 200 μmol/L dNTPs, 3.5 mmol/L MgCl2, 10 × TaqMan buffer without Uracil DNA glycosylase (AMPerase), 10 × stabilizer and water. Bisulfite-converted DNA was amplified at 95°C for 10 min, followed by 50 cycles at 95°C for 15 s and 60°C for one minute.24 Primers and probes, designed specifically for bisulfite-converted DNA, were used for Bcl-2 and Col2a1, a reference gene to normalize for input DNA. Bcl-2 primers and probe were designed to contain seven CpG dinucleotides in a CpG island located in the first exon of Bcl-2 (Figure 1). Primers and the probe were designed using Primer Express Software 3.0 (Applied Biosystem, Foster City, CA, USA) in a region of the Col2a1 gene that lacks any CpG dinucleotides to allow for equal amplification, regardless of methylation levels. The percentage of fully methylated molecules at a specific locus was calculated by dividing the Bcl-2:Col2a1 ratio of a sample by the Bcl-2:Col2a1 ratio of M.SssI-treated DNA and multiplying by 100. The primer and probe sequences are listed in Table 1.
Figure 1.
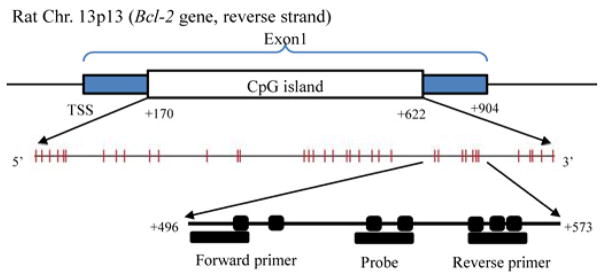
Genomic structure of rat Bcl-2 and the location of primers and probe for DNA methylation. The | and ■ represent cytosine–guanosine (CpG) dinucleotides. (A color version of this figure is available in the online journal)
Table 1.
Primer and probe sequence of promoter methylation
| Gene | GenBank number | Amplicon start, end | Forward primer 5′ to 3′ | Reverse primer 5′ to 3′ | Probe sequence 5′ to 3′ |
|---|---|---|---|---|---|
| Bcl-2 | NM_016993 | 496–573 | GGATATTTTTGTAAAGTCGCGACG | ACCTATAATCCACCTAACCCTCCG | 6FAM-CTAAAAATAACTTCTCTCGTCGCT-MGB* |
| Col2a1 | NM_012929 | 13,169–13,244 | GGTATTTTTGTTGTTTTGAAGAG-TAGTTG | CCTTCTCTCCTCCTTAAACTCCAA | 6FAM-ACCTCTAATCCCTCCCAAA-MGB |
MGB refers to a Minor Groove Binder non-fluorescent quencher in the 3′ terminus of the probe (MGBNFQ)
RNA extraction and transcription level measurement using quantitative reverse transcription-PCR
Total RNA was extracted from colon carcinomas using Totally RNA (Ambion, Grand Island, NY, USA), followed by treatment with DNase. cDNA was synthesized from 2 μg of total RNA using oligo dT primer and SuperScript II reverse transcriptase (Invitrogen, Grand Island, NY, USA). Quantitative reverse transcription-PCR was performed using an ABI 7900 and TaqMan gene expression assays (Applied Biosystems) to quantify mRNA expression levels of Bcl-2. The expression level of Bcl-2 (Rn99999125_m1) was normalized to Eukaryotic 18S rRNA expression (Hs99999901_s1).17 Accuracy of the assay was validated by establishing linearity of response for each assay. Negative controls consisted of reverse transcription reaction components without RT enzyme.
Statistical analyses
Data were analyzed using independent samples t-test to determine the effect of diet (FO/P versus CO/C) on apoptosis and Bcl-2 methylation. Colon tumor incidence was analyzed by Chi square analysis and reported as the percentage of rats bearing tumors. The relationship between DNA methylation and gene expression was determined by regression analysis. The values are reported as means ± standard error of the mean. Differences between treatments were regarded as significant when P values were < 0.05.
Results
Food intake and body weight gain
There were no differences in food intake between diet groups at either time point (data not shown). Initial body weight was similar between the CO/C (67.94 ± 1.34 g) and FO/P (66.58 ± 1.51 g) rats, and because weight gains were not affected by diet, final body weight was similar between the CO/C (520.67 ± 7.51 g) and FO/P (526.40 ± 11.74 g) rats.
FO/P diet promotes apoptosis and reduces colon tumor incidence
Rats receiving the FO/P diet (51.5%, 17 of 33) had a significantly lower colon tumor incidence compared with those receiving the control CO/C diet (75.6%, 31 of 41) (P = 0.016, Figure 2a). In addition, rats consuming the FO/P diet had a higher (P = 0.028) number of apoptotic cells per crypt column (0.85 ± 0.17) compared with those consuming the CO/C diet (0.47 ± 0.08). This resulted in an elevated apoptotic index (percentage of apoptotic cells in crypt column) in the non-tumor (uninvolved) regions of the colon in FO/P rats at 16 and 34 weeks after AOM injection, compared with CO/C (P = 0.020, Figure 2b).
Figure 2.
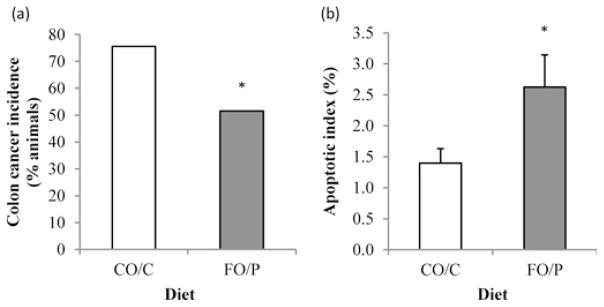
(a) Colon tumor incidence (n = 74), (b) the effect of diet on mean of apoptotic index in rats terminated at 16 and 34 weeks after azoxymethane injections (n = 40). The apoptotic index was calculated as 100 times the mean number of apoptotic cells per crypt column divided by the mean number of cells per crypt column. The data are means ± standard error of the mean. *Different from CO/C, P < 0.05. FO/P, fish oil/pectin diet and CO/C, corn oil/cellulose diet
FO/P enhances Bcl-2 promoter methylation in tumors at 34 weeks after AOM injections
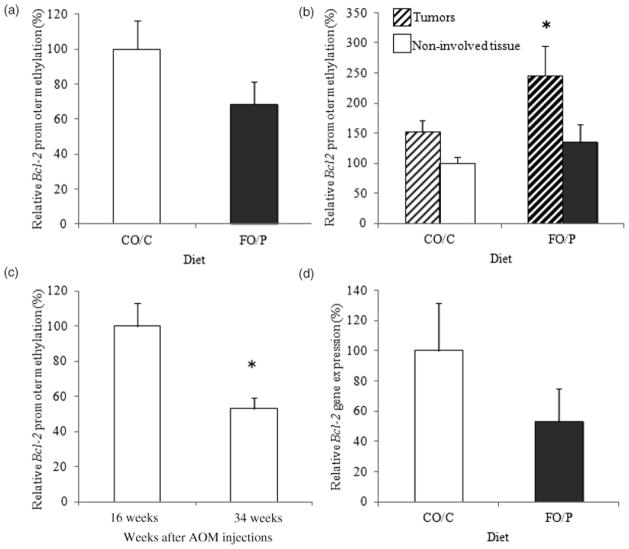
At the 16 weeks time point, Bcl-2 promoter methylation tended to decline with the FO/P diet, but this effect was not significant (CO/C 0.94 ± 0.16, FO/P 0.65 ± 0.12, P = 0.083, Figure 3a). In contrast, at the 34 weeks time point, FO/P enhanced Bcl-2 promoter methylation, which was significant (P = 0.009) in carcinomas (CO/C 0.57 ± 0.06, FO/P 0.91 ± 0.19) but not in uninvolved colonic mucosa (CO/C 0.37 ± 0.04, FO/P 0.50 ± 0.11) (P = 0.20, Figure 3b). Bcl-2 promoter methylation was lower in uninvolved tissues at 34 weeks (0.42 ± 0.05) compared with those of 16 weeks (0.79 ± 0.10) (P = 0.001, Figure 3c), which likely reflects the continued progression of tumorigenesis.
Figure 3.
(a) Relative Bcl-2 promoter methylation 16 weeks after azoxymethane (AOM) injections (6 rats/group). (b) Relative Bcl-2 promoter methylation 34 weeks after AOM injections (14–25 rats/group). (c) Relative Bcl-2 promoter methylation of uninvolved tissues 16 and 34 weeks after AOM injections (n = 12 at 16 weeks, n = 36 at 34 weeks). Bcl-2 promoter methylation at each time point includes values for both the FO/P and CO/C groups. (d) Relative Bcl-2 gene expression in carcinomas (3–4 rats/group). The data are means ± SEM. *Different from CO/C, P < 0.05. FO/P, fish oil pectin diet and CO/C, corn oil/cellulose diet
Bcl-2 gene expression is negatively regulated by promoter methylation
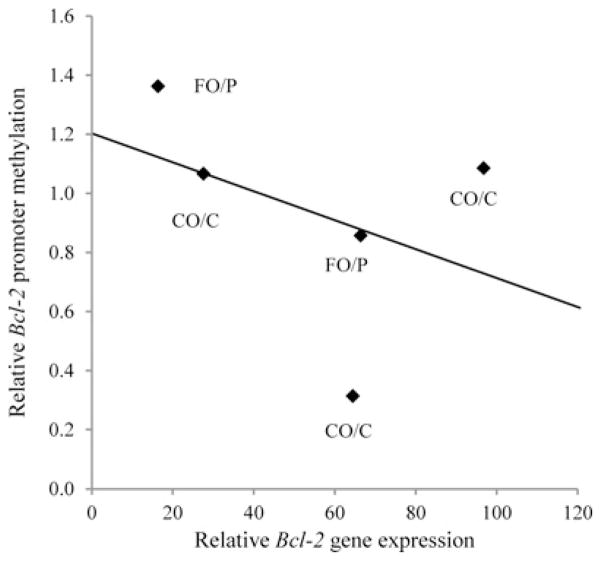
Our hypothesis was that FO/P would increase Bcl-2 promoter methylation, leading to lower gene transcription. In carcinomas collected at 34 weeks, Bcl-2 promoter methylation was elevated in rats receiving the FO/P diet, which was associated with numerically lower Bcl-2 transcript levels in carcinomas compared with CO/C (P = 0.13, Figure 3d). When the extent of Bcl-2 promoter methylation was compared with Bcl-2 expression in carcinomas of all rats, the anticipated negative correlation (slope=−0.005, R2 = 0.16) was observed, even though it was not significant (P = 0.25) (Figure 4).
Figure 4.
Negative correlation of Bcl-2 promoter methylation and gene expression in carcinomas (slope=−0.005, R2 = 0.16, P = 0.25)
Discussion
DNA methylation is a well-established epigenetic mechanism that leads to changes in gene expression without DNA sequence alterations. Aberrant hypermethylation of promoters is a recognized mechanism by which the expression of tumor suppressor genes is inhibited during colon cancer development.3,4 In contrast to hypermethylation of tumor suppressor genes, a region in the first exon of Bcl-2 oncogene is completely demethylated allowing high-levels of Bcl-2 expression in B-cell chronic lymphocytic leukemia.20 Aberrant promoter methylation has been proposed as a prognostic marker of cancers, and the extent of methylation in a tumor is associated with disease progression, recurrence and survival rates in various types of cancer.25–27 For instance, colon cancer patients with promoter hypermethylation exhibit shorter cancer-specific survival compared with those without promoter hypermethylation.6
The combination of FO/P has been extensively studied to identify its chemoprotective mechanisms in colon carcinogenesis, including suppression of PPARδ and prostaglandin E2 concentrations,28 and especially its ability to synergistically induce apoptosis.15,16,29 Apoptosis can be induced by changes in the regulation of histone acetylation, another epigenetic means of regulating gene transcription.30,31 In addition, we have recently demonstrated that FO/P modulates non-coding microRNA in AOM-induced colon cancer, another epigenetic mechanism whereby FO/P regulates gene transcription.32,33
In this study, we investigated the ability of FO/P to modulate the methylation based epigenetic regulation of the Bcl-2 gene in the AOM-induced colon cancer model. Methylation of the Bcl-2 promoter was targeted because we previously demonstrated that the combination of FO/P, compared with CO/C, suppressed the level of Bcl-2 expression, which occurred in parallel with the induction of colonocyte apoptosis.17 Therefore, the goal of this paper was to determine if a diet enriched in FO/P modulates the level of Bcl-2 gene expression by enhancing promoter methylation in vivo.
As we previously reported,34 the incidence of colon tumors in rats consuming a FO/P diet was lower than in those consuming a CO/C diet. Part of the protection against tumor formation may be attributable to the enhanced apoptotic index in the rats consuming FO/P compared with those from rats consuming CO/C (Figure 2).
Our results indicated that AOM-induced colon carcinomas exhibited a higher relative methylation of the Bcl-2 promoter in rats consuming a chemoprotective FO/P diet, compared with CO/C. Interestingly, this effect was not observed in the uninvolved tissue sections. Although no other studies have investigated the effect of diet on methylation of Bcl-2, several reports have demonstrated dietary effects on DNA methylation in other genes within cancer cells or animal models of cancer. For example, Nandakumar et al.10 demonstrated that green tea polyphenols restored the expression of the tumor suppressor genes Cip1/p21 and p16INK4a in human skin cancer cells by suppressing DNA methylation. Fang et al.8 reported that genistein, a soy isoflavone, reversed hypermethylation and reactivated RARβ, p16INK4a and MGMT in human esophageal squamous cancer cells. Recently, the methylation state of the tumor suppressor C/EBPδ in leukemia cells was found to be regulated by eicosapentaenoic acid.35 Using the AOM-treated ApcMin/+ mouse model, Volate et al.36 determined that consumption of low concentrations of green tea led to demethylation of the RXRα promoter region, which restored RXRα expression in colon mucosa. The current study is the first to demonstrate Bcl-2 promoter methylation regulation by diet in vivo.
Babidge et al.37 demonstrated that specific CpG dinucleotides in the Bcl-2 gene were methylated in 20% of human colorectal tumors, but that four CpG dinucleotides in the promoter region were not methylated in any colorectal tumors. In the current study, Bcl-2 promoter methylation declined from the levels observed at the intermediate stage of tumorigenesis (16 weeks) to those found in the non-involved tissues at 34 weeks after AOM injections. By modulating the transcriptional regulation of Bcl-2 at the tumor stage, levels of apoptosis were suppressed as is recognized to occur in colon cancer.17,18,38 However, the FO/P diet was able to enhance Bcl-2 promoter methylation in carcinomas, which should enhance apoptosis. Indeed, Koelink et al.39 demonstrated that decreased tumor apoptosis was associated with a significantly higher probability of disease recurrence in colon tumors. Since cancer cells are resistant to the induction of apoptosis, reversing Bcl-2 promoter hypomethylation by a FO/P diet could offer a therapeutic approach.
The levels of Bcl-2 transcripts were numerically lower in tumors from rats consuming a diet containing FO/P compared with CO/C. Even though the difference between diet groups was not significant, the pattern of response was similar to previous data,17 and reflects the elevated methylation state in carcinomas from the FO/P rats in this study. An extensive comparison could not be made due to the relatively small number of samples for which both DNA and RNA was available. However, other researchers have demonstrated that diets rich in fish oil prevented the expression of Bcl-2 in the colon cancer cells,38,40 as well as other cell types.41 Moreover, this result is comparable to those of Zhu et al.42, who reported that the Bcl-2 gene promoter is hypomethylated in colorectal cancer tissues, and that Bcl-2 methylation was inversely correlated with Bcl-2 protein expression in those tissues. Similar results were also demonstrated by Carvalho et al.43 who found that Bcl-2 mRNA expression was inversely correlated with promoter methylation in advanced stage prostate carcinomas.
In summary, we have shown that dietary FO/P promotes apoptosis in part by enhancing Bcl-2 promoter methylation in colon tumors relative to a CO/C diet. This finding contributes to our understanding of the mechanisms whereby diets containing FO/P are chemoprotective against colon cancer. In order to fully appreciate how a diet containing FO/P modulates DNA methylation during colon carcinogenesis, the effects of FO/P on DNA methyltransferases (the enzymes responsible for DNA methylation) should be investigated.
Acknowledgments
This study was supported by NSBRI NASA NCC 9-58 (JRL, NDT), NASA NAG-9-1523 (JRL, NDT) and NIH (CA59034 [RSC], CA129444 [RSC], CA057030 [RJC] and CA61750 [JRL]).
Footnotes
Author contributions: All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; YC, NDT and LAD conducted the experiments; YC, NDT and RJC performed statistical analyses; and YC, NDT, RSC and JRL materially participated in data interpretation and manuscript preparation.
References
- 1.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 2.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 3.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–93. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 4.Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, Murtaugh MA, Wolff RK, Slattery ML. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–45. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–6. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlin AM, Palmqvist R, Henriksson ML, Jacobsson M, Eklof V, Rutegard J, Oberg A, Van Guelpen BR. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res. 2010;16:1845–55. doi: 10.1158/1078-0432.CCR-09-2594. [DOI] [PubMed] [Google Scholar]
- 7.Gopisetty G, Ramachandran K, Singal R. DNA methylation and apoptosis. Mol Immunol. 2006;43:1729–40. doi: 10.1016/j.molimm.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–41. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 9.Fini L, Selgrad M, Fogliano V, Graziani G, Romano M, Hotchkiss E, Daoud YA, De Vol EB, Boland CR, Ricciardiello L. Annurca apple polyphenols have potent demethylating activity and can reactivate silenced tumor suppressor genes in colorectal cancer cells. J Nutr. 2007;137:2622–8. doi: 10.1093/jn/137.12.2622. [DOI] [PubMed] [Google Scholar]
- 10.Nandakumar V, Vaid M, Katiyar SK. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32:537–44. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Breda SG, van Delft JH, Engels LG, Kleinjans JC, Mathers JC. Methylation status of CpG islands in the promoter region of genes differentially expressed in colonic mucosa from adenoma patients and controls in response to altered vegetable intake. Br J Nutr. 2009;101:1295–9. doi: 10.1017/s0007114508083529. [DOI] [PubMed] [Google Scholar]
- 12.Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–30. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 13.Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–2. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 14.Fan YY, Zhang J, Barhoumi R, Burghardt RC, Turner ND, Lupton JR, Chapkin RS. Antagonism of CD95 signaling blocks butyrate induction of apoptosis in young adult mouse colonic cells. Am J Physiol. 1999;277:C310–9. doi: 10.1152/ajpcell.1999.277.2.C310. [DOI] [PubMed] [Google Scholar]
- 15.Kolar S, Barhoumi R, Jones CK, Wesley J, Lupton JR, Fan YY, Chapkin RS. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca(2+) loading and apoptosis in colonocytes. Cancer. 2011;117:5294–303. doi: 10.1002/cncr.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolar SS, Barhoumi R, Callaway ES, Fan YY, Wang N, Lupton JR, Chapkin RS. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca(2+) accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G935–43. doi: 10.1152/ajpgi.00312.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turk HF, Kolar SS, Fan YY, Cozby CA, Lupton JR, Chapkin RS. Linoleic acid and butyrate synergize to increase Bcl-2 levels in colonocytes. Int J Cancer. 2011;128:63–71. doi: 10.1002/ijc.25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong MY, Chapkin RS, Davidson LA, Turner ND, Morris JS, Carroll RJ, Lupton JR. Fish oil enhances targeted apoptosis during colon tumor initiation in part by downregulating Bcl-2. Nutr Cancer. 2003;46:44–51. doi: 10.1207/S15327914NC4601_06. [DOI] [PubMed] [Google Scholar]
- 19.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 20.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–8. [PubMed] [Google Scholar]
- 21.Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–49. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Pickering JS, Lupton JR, Chapkin RS. Dietary fat, fiber, and carcinogen alter fecal diacylglycerol composition and mass. Cancer Res. 1995;55:2293–8. [PubMed] [Google Scholar]
- 23.Campan M, Weisenberger DJ, Trinh B, Laird PW. MethyLight. Methods Mol Biol. 2009;507:325–37. doi: 10.1007/978-1-59745-522-0_23. [DOI] [PubMed] [Google Scholar]
- 24.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D, Danenberg PV, Laird PW. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:e32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Zhao J, Chen XF, Li W, Liu R, Lei Z, Liu X, Peng X, Xu K, Chen J, Liu H, Zhou QH, Zhang HT. CpG island methylator phenotype involving tumor suppressor genes located on chromosome 3p in non-small cell lung cancer. Lung Cancer. 2008;62:15–22. doi: 10.1016/j.lungcan.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Prosper F, Heiniger A, Torres A. Lack of CpG island methylator phenotype defines a clinical subtype of T-cell acute lymphoblastic leukemia associated with good prognosis. J Clin Oncol. 2005;23:7043–9. doi: 10.1200/JCO.2005.01.4944. [DOI] [PubMed] [Google Scholar]
- 27.Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L, Peters JH, DeMeester SR, DeMeester TR, Skinner KA, Laird PW. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61:3410–8. [PubMed] [Google Scholar]
- 28.Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, Ford JR, Braby LA, Chapkin RS, Turner ND, Lupton JR. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–6. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–8. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 30.Cho Y, Kim H, Turner ND, Mann JC, Wei J, Taddeo SS, Davidson LA, Wang N, Vannucci M, Carroll RJ, Chapkin RS, Lupton JR. A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J Nutr. 2011;141:1029–35. doi: 10.3945/jn.110.134973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crim KC, Sanders LM, Hong MY, Taddeo SS, Turner ND, Chapkin RS, Lupton JR. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis. 2008;29:1415–20. doi: 10.1093/carcin/bgn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077–84. doi: 10.1093/carcin/bgp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah MS, Schwartz SL, Zhao C, Davidson LA, Zhou B, Lupton JR, Ivanov I, Chapkin RS. Integrated microRNA and mRNA expression profiling in a rat colon carcinogenesis model: effect of a chemo-protective diet. Physiol Genomics. 2011;43:640–54. doi: 10.1152/physiolgenomics.00213.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr. 1998;128:491–7. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 35.Ceccarelli V, Racanicchi S, Martelli MP, Nocentini G, Fettucciari K, Riccardi C, Marconi P, Di Nardo P, Grignani F, Binaglia L, Vecchini A. Eicosapentaenoic acid demethylates a single CpG that mediates expression of tumor suppressor CCAAT/enhancer-binding protein delta in U937 leukemia cells. J Biol Chem. 2011;286:27092–102. doi: 10.1074/jbc.M111.253609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volate SR, Muga SJ, Issa AY, Nitcheva D, Smith T, Wargovich MJ. Epigenetic modulation of the retinoid X receptor alpha by green tea in the azoxymethane-Apc Min/+ mouse model of intestinal cancer. Mol Carcinog. 2009;48:920–33. doi: 10.1002/mc.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babidge WJ, Butler LM, Burton MA, Cowled PA. Methylation of CpG sites in exon 2 of the bcl-2 gene occurs in colorectal carcinoma. Anticancer Res. 2001;21:2809–14. [PubMed] [Google Scholar]
- 38.Chen ZY, Istfan NW. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2000;63:301–8. doi: 10.1054/plef.2000.0218. [DOI] [PubMed] [Google Scholar]
- 39.Koelink PJ, Sier CF, Hommes DW, Lamers CB, Verspaget HW. Clinical significance of stromal apoptosis in colorectal cancer. Br J Cancer. 2009;101:765–73. doi: 10.1038/sj.bjc.6605220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habermann N, Schon A, Lund EK, Glei M. Fish fatty acids alter markers of apoptosis in colorectal adenoma and adenocarcinoma cell lines but fish consumption has no impact on apoptosis-induction ex vivo. Apoptosis. 2010;15:621–30. doi: 10.1007/s10495-010-0459-y. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh-Choudhury T, Mandal CC, Woodruff K, St Clair P, Fernandes G, Choudhury GG, Ghosh-Choudhury N. Fish oil targets PTEN to regulate NFkappaB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res Treat. 2009;118:213–28. doi: 10.1007/s10549-008-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Q, Jin Z, Yuan Y, Lu Q, Ge D, Zong M. Impact of MTHFR gene C677T polymorphism on Bcl-2 gene methylation and protein expression in colorectal cancer. Scand J Gastroenterol. 2011;46:436–45. doi: 10.3109/00365521.2010.537682. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho JR, Filipe L, Costa VL, Ribeiro FR, Martins AT, Teixeira MR, Jeronimo C, Henrique R. Detailed analysis of expression and promoter methylation status of apoptosis-related genes in prostate cancer. Apoptosis. 2010;15:956–65. doi: 10.1007/s10495-010-0508-6. [DOI] [PubMed] [Google Scholar]