Abstract
Cellular differentiation, by definition, is epigenetic. Genome-wide profiling of pluripotent cells and differentiated cells suggests global chromatin remodeling during differentiation, resulting in progressive transition from a relatively open chromatin configuration to a more compact state. Genetic studies in mouse models demonstrate major roles for a variety of histone modifiers and chromatin remodelers in key developmental transitions, such as the segregation of embryonic and extraembryonic lineages in blastocyst stage embryos, the formation of the three germ layers during gastrulation, and differentiation of adult stem cells. Furthermore, rather than merely stabilizing the gene expression changes driven by developmental transcription factors, evidence is emerging that chromatin regulators have multifaceted roles in cell fate decisions.
Introduction
Virtually all cells of an organism share the same genome but exhibit different phenotypes and carry out diverse functions. Individual cell types, characterized by distinct gene expression patterns, are generated during development and then stably maintained. The chromatin state – the packaging of DNA with histone and nonhistone proteins – has profound effects on gene expression and is believed to contribute to the establishment and maintenance of cell identities. Indeed, developmental transitions are accompanied by dynamic changes in chromatin states.
The assembly and compaction of chromatin are regulated by multiple mechanisms, including DNA modifications (e.g. cytosine methylation and cytosine hydroxymethylation), post-translational modifications (PTMs) of histones (e.g. phosphorylation, acetylation, methylation and ubiquitylation), incorporation of histone variants (e.g. H2A.Z and H3.3), ATP-dependent chromatin remodeling, and non-coding RNA (ncRNA)-mediated pathways. In recent years, significant progress has been made in understanding the roles of histone modifications and chromatin remodeling in cellular differentiation, which will be the focus of this review. For perspectives of other chromatin regulators (DNA methylation and hydroxymethylation, histone variants and ncRNAs) in pluripotency, differentiation and development, we refer readers to other recent reviews1–4.
PTMs of histones may directly affect chromatin compaction and assembly or serve as binding sites for effector proteins, including other chromatin-modifying or chromatin-remodeling complexes, and ultimately influence transcription initiation and/or elongation. Most, if not all, histone PTMs are reversible. Many enzymes involved in their addition and removal have been identified. These include histone acetyltransferases (HATs, also known as lysine acetyltransferases (KATs)) and histone deacetylases (HDACs, also known as lysine deacetylases (KDACs)), lysine methyltransferases (KMTs) and lysine demethylases (KDMs), and ubiquitylation enzymes (E1, E2 and E3 enzymes) and deubiquitylases (DUBs). These enzymes often exist in multisubunit complexes and modify specific residues on the N-terminal tails or within the globular domains of core histones (H2A, H2B, H3, and H4). For example, in the two repressive Polycomb group (PcG) protein complexes, Polycomb repressive complex 1 (PRC1) contains RING1A or RING1B, which catalyze monoubiquitylation of H2A at lysine 119 (H2AK119ub1), and PRC2 contains EZH2, which catalyzes trimethylation of H3 at lysine 27 (H3K27me3). Additionally, some Trithorax group (TrxG) protein complexes contain the MLL family of methyltransferases that catalyze H3K4me3. Beyond PTMs of histones, chromatin compaction is also affected by ATP-dependent chromatin remodeling complexes that utilize energy from ATP hydrolysis to exchange histones and reposition or evict nucleosomes. Approximately 30 genes encoding the ATPase subunits have been identified in mammals. Based on the sequence and structure of these ATPases, chromatin-remodeling complexes are divided into four main families: SWI/SNF, ISWI, CHD and INO80 complexes5. Many histone modifiers and chromatin remodelers have been implicated in stem cell pluripotency, cellular differentiation and development.
In this Review, we focus on studies using mammalian systems. We will first describe chromatin states in stem cells and their alterations during differentiation, highlighting findings from recent genome-wide profiling studies. This information provides important clues to the functions of chromatin regulators and to the overall organization of chromatin in pluripotent versus differentiated cells. We will then review recent discoveries from genetic studies in mouse models to highlight the importance of various chromatin modifiers and remodelers in key developmental transitions. Finally, we will discuss emerging evidence of new roles for chromatin regulators in cell fate decisions.
Epigenetic landscape in ES cells
Stem cells usually exist in small numbers in developing embryos and somatic tissues, which makes it difficult to study the molecular mechanisms governing stem cell self-renewal and differentiation in vivo. Embryonic stem (ES) cells, derived from the inner cell mass (ICM) of blastocysts, can be maintained and expanded indefinitely in culture while retaining their differentiation potential. Thus, ES cells are widely used as an experimental system for investigating the epigenetic regulation of stem cells.
Open chromatin of ES cells
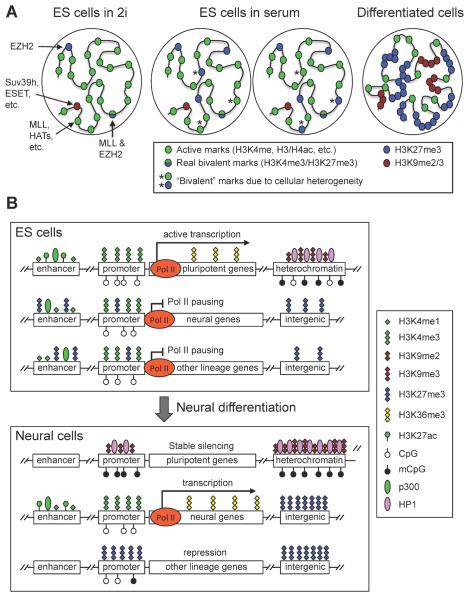
A unique network of transcription factors, including the core pluripotency factors OCT4, SOX2 and NANOG, is involved in the establishment and maintenance of ES cell pluripotency. ES cells also possess distinctive chromatin features. Electron microscopy indicates that undifferentiated human ES cells have less heterochromatin than do differentiated cells6. Staining of H3K9me3 and heterochromatin protein 1 (HP1), as well as fluorescence in situ hybridization (FISH) analysis of the major satellite DNA repeats, also suggest that constitutive heterochromatin is less condensed in undifferentiated ES cells. Consistently, major architectual chromatin proteins, such as HP1 and linker histones, are hyperdynamic and bind loosely to chromatin in these cells7. Genome-wide maps of epigenetic modifications from both mouse and human ES cells also revealed widespread active chromatin domains, characterized by enrichment of histone acetylation and H3K4me3 and hypomethylation of DNA8–11. The hyperactivity of the ES cell genome leads to widespread expression of coding and noncoding elements12. Collectively, these findings indicate that ES cells have a globally `open' and dynamic chromatin state (Fig. 1A).
Figure 1. Chromatin states in pluripotent and differentiated cells.
(A) ES cells have a globally `open' chromatin state, characterized by the enrichment of active histone marks such as histone acetylation and H3K4 methylation, whereas differentiated cells have a more compact chromatin state, characterized by expanded domains of repressive histone marks such as H3K27me3, H3K9me2 and H3K9me3. ES cells cultured in 2i medium are highly similar to the naïve pluripotent cells in ICM of blastocysts, and ES cells cultured in serum are more heterogeneous. Some H3K4me3 and H3K27me3 `bivalent' marks may reflect the cellular heterogeneity, especially when ES cells are cultured in serum. (B) Major chromatin features in different genomic regions. In ES cells, the enhancers of both pluripotency-associated genes and developmental genes are enriched with H3K4me1 and p300. The presence of H3K27ac makes enhancers of pluripotency-associated genes active, whereas the lack of H3K27ac and enrichment of H3K27me3 keep enhancers of developmental lineage-commitment genes in a `poised' state. The promoters of pluripotency-associated genes and lineage-commitment genes are also believed to be active and `poised', respectively. Transcriptional elongation is prevented at lineage-commitment genes due to promoter-proximal RNA polymerase II (Pol II) pausing. Upon differentiation toward a specific lineage (e.g. neural lineage), lineage-specific genes acquire active marks at enhancer and promoter regions, and Pol II pausing is released to allow productive elongation. Genes of other lineages lose enhancer marks and gain H3K27me3 at promoters, resulting in repression. Pluripotency-associated genes gain H3K9 methylation and DNA methylation and become stably silenced. During differentiation, heterochromatin regions — characterized by H3K9me2 and H3K9me3, HP1 binding and DNA methylation — are expanded and become more condensed. H3K27me3 in intergenic regions and repressed genes also expands to large domains.
Despite a highly active transcriptome, repression of lineage-specific genes is essential for maintaining ES cell pluripotency. A subset of developmental genes seem to be enriched with `bivalent domains', containing both repressive H3K27me3 and activating H3K4me3 marks, in ES cells8, 13–16. Recent evidence suggests that the two marks do not co-exist on the same H3 tail but can occur on the opposite H3 tails in the same nucleosome17. Bivalent domains have also been identified in pluripotent ICM and epiblast cells of early mouse embryos, multipotent hematopoietic stem cells (HSCs), and zebrafish blastomeres18–22. After differentiation of ES cells, most bivalent genes lose one of the marks and become monovalent14. These findings led to the notion that bivalent domains keep key developmental genes in a silent but `poised' state in pluripotent cells. This hypothesis, however, has been a topic of debate. Bivalency does not seem to be a universal feature of pluripotent and multipotent cells. For example, analysis of developing Xenopus and Drosophila embryos and mouse hair follicle stem cells (HF-SCs) identified few bivalent domains23–25. In addition, bivalency is not unique to pluripotent cells, as bivalent domains are also present, albeit in smaller numbers, in differentiated cells such as T lymphocytes, mouse embryonic fibroblasts, and neurons14, 26, 27. Furthermore, the number of bivalent domains in ES cells could have been overestimated due to heterogeneity of histone marks in populations of cultured ES cells. ES cells grown in standard medium (which contains serum factors) include subpopulations of differentiating cells and exhibit heterogeneity in morphology and expression of pluripotency factors28, 29. Hong et al. analyzed fractionated human ES cell subpopulations and found that some lineage-specific genes that are marked by bivalent domains according to bulk assays on unfractionated cells are actually monovalent (either H3K4me3 or H3K27me3) in distinct cell populations30. Mouse ES cells can be maintained in a naïve state in the absence of serum using a defined medium, known as 2i medium, containing inhibitors of mitogen-activated protein kinase kinase (MEK) and glycogen synthase kinase 3 (GSK3)31. Recently, Marks et al. showed that mouse ES cells grown in 2i medium, compared to those grown in serum-containing medium, exhibit highly similar H3K4me3 profiles, but substantially reduced prevalence of H3K27me3 at promoters, many fewer bivalent domains, and lower, rather than higher, expression of lineage-specific genes32. Thus, a large proportion of bivalent domains in ES cells cultured in serum are due to acquisition of H3K27me3 at promoters (Fig. 1A), further calling into question the significance of `bivalency' in naïve pluripotent cells.
Chromatin dynamics during differentiation
ES cell differentiation is accompanied by global chromatin remodeling, resulting in progressive transition from the open chromatin configuration described above to a more compact and repressive state. Microscopically, heterochromatin foci become more condensed and abundant in differentiated cells, which correlates with significantly less dynamic exchange of chromatin proteins6, 7. Genome-wide analysis of H3K9me2 identified large organized chromatin K9-modifications (LOCKs), which generally occur in gene-poor facultative heterochromatin. These domains show significant increases in both genome coverage (4% versus 31%) and average size (43 kb versus 93 kb) as undifferentiated mouse ES cells progress through differentiation in vitro33. H3K27me3 also progresses from focal distributions in ES cells to expanded domains over silent genes and intergenic regions in differentiated cells34, 35 (Fig. 1A). Notwithstanding the evidence and prevailing view of global chromatin remodeling, there are reports suggesting a greater role for local chromatin changes during cellular differentiation. For example, Lienert et al. showed that, during neuronal differentiation of ES cells, H3K9me2 exhibits no global increase, but instead discrete local changes, particularly in genic regions36.
During differentiation, ES cells silence pluripotency genes and gain the phenotype of distinct differentiated cells by activating lineage-specific genes and repressing lineage-inappropriate genes. Recently, multiple groups performed genome-wide transcriptional and epigenetic profiling of cells derived from directed differentiation of ES cells representing various lineages and defined differentiation stages37–44. From these studies, a global picture of epigenetic and gene expression alterations during differentiation is beginning to emerge (Fig. 1B). For example, active genes generally contain H3K4me3 at their promoters, and H3K4me1, H3K4me2 and H3K27ac at their enhancers. Repressed loci are enriched with either H3K27me3 or DNA methylation, which appear to repress distinct loci. Xie et al. reported that promoters of developmental regulators that are active in early developmental stages tend to be CG rich and mainly employ H3K27me3 upon silencing in nonexpressing lineages; by contrast, somatic-tissue-specific promoters, which are active later in development, are largely CG poor, and often show high levels of DNA methylation upon subsequent repression44. At putative distal regulatory elements, Gifford et al. found lineage-specific transitions from high DNA methylation to H3K4me1 or H3K27me3. These alterations occur at many sites that do not seem to change gene expression during early stages of differentiation, raising the possibility that these changes are epigenetic priming events that facilitate gene expression at later stages43. Another interesting finding from the genome-wide studies is that some genes with similar expression profiles during differentiation show considerable variations in chromatin states. For example, during cardiac differentiation, genes associated with metabolic function share a similar chromatin pattern, whereas those involved in contractile function and sarcomere structure have a distinct pattern, even though these two groups of genes have similar temporal and spatial expression profiles39, 40. These findings imply that epigenetic regulation ensures coordinated expression of functionally related genes during differentiation. In summary, information about the chromatin state in ES cells and chromatin dynamics during ES cell differentiation could shed light on the functions of chromatin regulators in stem cell pluripotency and cellular differentiation.
Chromatin states in adult stem cells
Many adult tissues harbor multipotent stem cells, which have the ability for life-long self-renewal and the ability to differentiate into a number of tissue-specific cell types. Adult stem cells are critical for tissue homeostasis and regeneration. For example, HSCs give rise to all the blood cell types and are responsible for the constant renewal of blood, and neural stem cells (NSCs) produce the three primary cell types in the central nervous system — neurons, astrocytes and oligodendrocytes — and are the source of adult neurogenesis.
While the scarcity of stem cells in most tissues remains a major challenge in studying adult stem cells, several groups were able to isolate sufficient quantities of adult stem cells from tissues to conduct transcriptional and epigenetic profiling studies. Results from the limited number of studies presently available support the notion that the chromatin states of adult stem cells are intermediate between those of pluripotent cells and terminally differentiated cells. For example, while the chromatin of adult stem cells is globally less `open' compared with that of ES cells, a common set of stemness genes, including regulators of chromatin, transcription, cell cycle and survival, is marked by H3K4me3 and is active in both HF-SCs and ES cells25. In HSCs, H3K4me3 is more prevalent compared with differentiated progeny, and enhancers of differentiation genes are marked by monomethylation of H3K4, H3K9, and H3K27, which is likely involved in the maintenance of activation potential required for differentiation21.
In the skin, HF-SCs drive synchronized cycles of hair follicle growth (anagen), destruction (catagen) and rest (telogen). Lien et al. profiled global mRNA and histone methylation marks in quiescent (telogen) and activated (anagen) HF-SCs and their committed, transit-amplifying cell (TAC) progeny. During the transition from a quiescent state to an active, proliferative state, HF-SCs show induction of cell cycle regulators without global alterations in mRNA and histone modification patterns. However, transitioning from HF-SCs to TACs involves substantial changes in transcriptional and chromatin profiles, including PcG-mediated repression of HF-SC genes and derepression of PcG-silenced TAC regulators25. Likewise, comparisons of histone modification maps and gene expression profiles of human CD133+ HSCs and CD36+ erythrocyte precursor cells revealed that epigenetic changes correlate with changes in gene expression during erythrocyte differentiation. Specifically, gene expression positively correlates with H3K4me3, H3K4me1, H3K9me1, H3K36me3, and H4K20me1 and negatively correlates with H3K9me3 and H3K27me321. Mesenchymal stem cells (MSCs) are present in several tissues, including bone marrow, umbilical cord, and adipose tissue, and can be expanded in culture and induced to differentiate into various lineages (such as osteoblasts, chondrocytes and adipocytes). Recent reports indicate that, when MSCs are induced to differentiate, histone modifications exhibit dynamic changes, whereas promoter DNA methylation shows only modest changes that do not correlate significantly with changes in gene expression45–47. Comparisons of the DNA methylation maps of stem cells, progenitor cells and terminally differentiated cells of the blood and skin lineages also suggest that in vivo differentiation of HSCs and HF-SCs is associated with relatively small changes in DNA methylation48. Previous studies revealed that during multi-step differentiation of mouse ES cells, most DNA methylation changes occur during the initial step of differentiation10, 27. Therefore, it is likely that promoter DNA methylation patterns have been largely established by the adult stem cell stage and that histone modifications play important roles in subsequent differentiation.
Genetic studies in mouse models
Development from a zygote to an organism is a complex process that involves multiple key cell fate decisions. During mammalian development, the zygote and cells of early cleavage stage embryos are totipotent, as they are capable of giving rise to all embryonic and extraembryonic tissues. The first cell lineage specification event occurs before implantation and results in the segregation of trophoblast (outer layer) cells and ICM cells at the blastocyst stage. Following implantation, trophoblasts develop into placental tissues, and the pluripotent ICM develops into the epiblast, which differentiates to form the three germ layers – ectoderm, mesoderm and endoderm – during gastrulation. The germ layers, which are multipotent, will give rise to specific tissues and organs in the developing embryo. Genetic stuides in mouse models demonstrate major roles for a variety of chromatin modifiers and remodelers in key developmental transitions.
Preimplantation development and ES cell identity
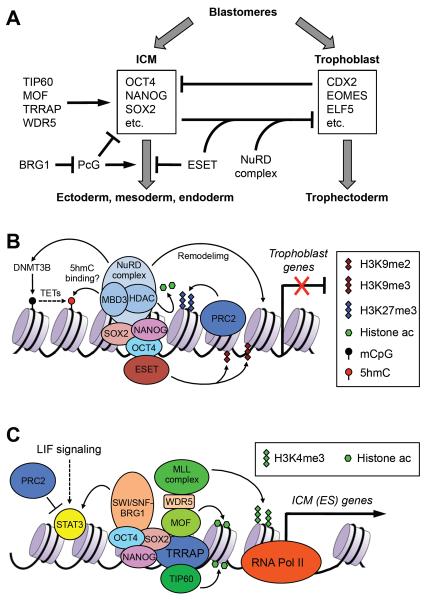
Proper segregation of the ICM and trophoblast lineages at the blastocyst stage requires the transcription factors OCT4 (which determines commitment to the embryonic lineage) and CDX2 (which specifies the trophoblast lineage). Reciprocal inhibition between the OCT4 and CDX2 transcription networks reinforces ICM-specific and trophoblast-specific expression patterns49–51 (Fig. 2A). ESET (also known as SETDB1 and KMT1E), a histone methyltransferase that represses gene expression by catalyzing the formation of H3K9me2 and H3K9me3, appears to function as a co-repressor for OCT4 in this context. In mouse embryos, zygotic Eset expression begins at the blastocyst stage, specifically in ICM cells. Null mutation of Eset results in preimplantation lethality and prevents proper development of the ICM and establishment of ES cell lines52. Depletion of Eset in ES cells, by shRNA-mediated knockdown or genetic ablation, induces differentiation, particularly towards the trophoblast lineage53–56. The phenotypes of Eset-deficient embryos and ES cells are similar to those of Oct4 mutants49. Molecular analyses revealed that ESET and OCT4 physically interact54, 55. OCT4 seems to recruit ESET for repression of developmental regulators in ICM cells, especially those involved in trophoblast differentiation such as Cdx253–56 (Fig. 2AB).
Figure 2. Chromatin regulators involved in the segregation of embryonic and extraembryonic lineages during preimplantation development.
(A) The transcription factors specifying the inner cell mass (ICM) and trophoblast cells reciprocally antagonize each other. Chromatin regulators maintain the identity of ICM cells by repressing the trophectoderm transcriptional program, preventing differentiation toward the three germ layers, promoting the expression of pluripotency factors, and functioning as co-regulators or effectors of pluripotency factors. Chromatin regulators that promote trophoblast differentiation are less well understood. (B) In ICM cells, ESET functions as an OCT4 co-repressor to repress trophoblast genes by depositing H3K9me2 and H3K9me3. The NuRD complex is also involved in the repression of trophoblast genes, possibly by multiple mechanisms. The NuRD complex has chromatin-remodeling activity that alters DNA–histone interactions. HDAC1 and HDAC2, which are components of NuRD, deacetylate histones, and deacetylation of H3K27 has been shown to facilitate PRC2 binding and H3K27 methylation. NuRD may also promote DNA methylation by inducing DNMT3B expression. Recent evidence suggests that the MBD3 subunit can bind 5-hydroxymethylcytosine (5hmC). (C) In ICM cells, pluripotency factors, such as OCT4, NANOG and SOX2, recruit histone-modifying enzymes (e.g. TIP60, MOF and MLL complexes) and chromatin-remodeling complexes (e.g. SWI/SNF-BRG1) to ICM-specifying genes (including Oct4, Nanog and Sox2 themselves) and their targets to create `open' chromatin states. One of the effects of the SWI/SNF-BRG1 complex is to maintain chromatin accessibility at STAT3-binding targets by opposing PcG-mediated repression, thus enhancing LIF signaling.
The nucleosome remodeling and deacetylation (NuRD) corepressor complex also plays a role in maintaining the barrier between the embryonic and trophectodermal cell fates. Deletion of methyl-CpG-binding domain protein 3 (Mbd3), which encodes a core component of the NuRD complex, results in peri-implantation lethality, with the ICM failing to develop to a mature epiblast57. Mbd3-deficient ES cells are viable and can self-renew, but they show inappropriate expression of trophectoderm-specific genes such as Elf5 and Eomes. Although Mbd3 deficiency alone is not sufficient to induce trophectoderm differentiation, Mbd3-deficient ES cells can be converted to trophoblast cells when cultured in ES medium without leukemia inhibitory factor (LIF) or in trophoblast stem cell medium58–60. These results suggest that the NuRD complex contributes to repression of trophectoderm determinant genes so that ES cells are not responsive to trophectoderm-inducing signals such as FGF4 (Fig. 2A). The NuRD complex has also been shown to suppress the expression of pluripotency genes in ES cells and promote lineage commitment61. The seemingly contrasting effects of the NuRD complex suggest a possible role in maintaining the balance between self-renewal and differentiation. These effects are likely mediated by complex and interconnected mechanisms, as the NuRD complex, in addition to its chromatin-remodeling and histone deacetylase activities, has been functionally linked to H3K27me3, DNA methylation, and DNA hydroxymethylation60, 62, 63 (Fig. 2B).
Components of several other enzyme complexes involved in histone modifications and ATP-dependent chromatin remodeling are essential for ICM survival and ES cell self-renewal. TIP60 and MOF, two members of the MYST family of HATs, as well as TRRAP, a common component of several HAT complexes, are required for preimplantation development. Although mouse embryos deficient for Tip60 or Mof survive to the blastocyst stage, they die shortly afterwards and blastocysts fail to hatch and survive in cuture64–66. Trrap-null embryos exhibit even more severe phenotypes, as 50% of blastocysts from Trrap+/− intercrosses degenerate inside the zona pellucida, and Trrap−/− blastocyst embryos exhibit severe growth retardation of the trophoblast layer and an absence of the ICM67. Conditional deletion of Mof or Trrap in ES cells leads to loss of self-renewal capability associated with alterations in histone acetylation and chromatin structure68, 69, consistent with a previous RNAi screen that identified Trrap and Tip60 as regulators of ES cell identity70. ES cells deficient for Trrap or Mof show dramatic downregulation of pluripotency genes and upregulation of specific differentiation markers of the three germ layers68, 69. MOF, which catalyzes H4K16ac, directly binds to pluripotency-associated genes, including Nanog, Oct4 and Sox2, and specifically regulates the NANOG transcriptional network68. WD repeat domain 5 (WDR5), a commonly shared component of the MOF and MLL complexes, also regulates ES cell self-renewal71, although its role in mammalian development remains to be determined. Via WDR5, MOF may target MLL complexes and H3K4 methylation to pluripotency-associated genes, highlighting the cooperation of various chromatin regulators in maintaining pluripotency68 (Fig. 2AC).
BRG1, the ATPase subunit of the SWI/SNF-BRG1 chromatin-remodeling complex, is present through preimplantation development72. Maternal Brg1 is required for zygotic genome activation at the two-cell stage73, and zygotic Brg1 is essential for the survival and proliferation of ICM and trophoblast cells74. BAF47 (also known as SNF5 and INI1) and BAF155 (also known as SRG3), two other components of the SWI/SNF complex, are also required for peri-implantation development75, 76. Depletion of Brg1 in ES cells results in loss of self-renewal and induces differentiation77, 78. Genome-wide analysis revealed that BRG1 colocalizes extensively with pluripotency factors in ES cells, suggesting that the SWI/SNF-BRG1 compex is an important component of the pluripotency network77, 79. The cytokine LIF can support self-renewal of murine ES cells by activating signal transducer and activator of transcription 3 (STAT3). A recent study indicates that the SWI/SNF-BRG1 complex maintains chromatin accessibility at STAT3-binding targets by opposing PcG-mediated repression80 (Fig. 2C).
In summary, the chromatin modifiers and remodelers described above regulate preimplantation development and maintain the identity of ICM and ES cells by suppressing the trophectoderm transcriptional program, preventing differentiation toward the three germ layers, promoting the expression of pluripotency factors, and functioning as co-regulators or effectors of pluripotency factors (Fig. 2A).
Postimplantation development and ES cell differentiation
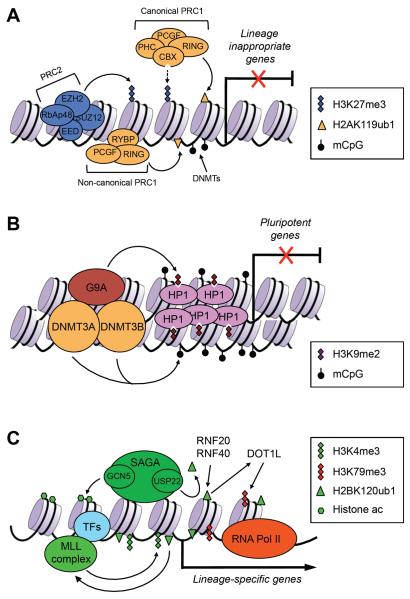
An important post-implantation developmental event is gastrulation, through which the three germ layers are formed, resulting in the establishment of the basic body plan. A large number of chromatin regulators have been implicated in this process. Among the most extensively studied are PcG proteins. Embryos lacking EZH2, the histone methyltransferase catalytic subunit of the PRC2 complex, initiate but fail to complete gastrulation and die soon after implantation81. Deletion of the PRC2 core component EED or SUZ12 results in similar phenotypes82, 83. RING1B, the histone ubiquitylation catalytic subunit of PRC1, and some other PRC1 components (e.g. RYBP and L3MBTL2) are also essential for gastrulation and early embryogenesis84–86. Consistent with the developmental phenotypes, ES cells deficient for PRC1 and/or PRC2 functions are capable of self-renewal, but exhibit inappropriate derepression of lineage-specific genes and differentiation defects86–91. PcG proteins are thus key components of a network that represses developmental genes during differentiation (Fig. 3A).
Figure 3. Chromatin regulators involved in gene regulation during postimplantation development and cellular differentiation.
(A) PcG proteins play important roles in repressing developmental genes. The PRC2 complex deposits the repressive H3K27me3 marks, which create binding sites for the canonical, CBX-containing PRC1 complex. The noncanonical, RYBP-containing PRC1 complex binds to chromation via H3K27me3-independent mechanisms. RING1A and RING1B, components of the PRC1 complexes, mediate the repressive H2AK119ub1 modification. DNA methylation, mediated by DNA methyltransferases (DNMTs), is also important in repressing lineage-commitment genes, especially those with low CpG-content promoters. (B) G9A is critical for silencing pluripotency-associated genes in postimplantation embryos and differentiated cells. G9A mediates H3K9me2, which induces heterochromatinization by recruiting HP1. G9A also recruits DNMT3A and DNMT3B, which initiate de novo DNA methylation. (C) In differentiating cells, lineage-specific transcription factors (TFs) recruit chromatin-modifying complexes, such as the MLL complex and the SAGA complex, to lineage-specific genes to create `open' chromatin states. The MLL complex deposits the active H3K4me3 marks. The SAGA complex has at least two enzymatic activities: histone acetylation by GCN5 and H2BK120 deubiquitylation by USP22. H2BK120ub1, mediated by RNF20 and RNF40, is preferentially enriched in the coding regions of differentiation-related genes, but not pluripotency-associated genes. H2BK120ub1 has been shown to promote MLL-mediated H3K4 methylation and DOT1L-mediated H3K79 methylation.
Pluripotency genes are rapidly repressed upon differentiation and remain stably silenced in differentiated cells. G9A (also known as EHMT2), a histone methyltransferase that catalyzes mainly H3K9me2 in euchromatin, appears to be a key component of the machinery that silences pluripotency genes. Embryos lacking G9A display prolonged expression of Oct4 and Nanog, severe growth retardation and early lethality92, 93. G9A-deficient ES cells show normal self-renewal, but fail to stably silence Oct4 and exhibit differentiation defects92, 94. Inactivation of Oct4 following embryo implantation is a multi-step process that involves direct inhibition of transcription, followed by local heterochromatinization and de novo DNA methylation. G9A is not required for the initial Oct4 repression upon differentiation, but G9A-mediated H3K9 methylation is necessary for subsequent heterochromatinization and de novo DNA methylation at the Oct4 locus94. A recent study suggests that some signaling pathways influence differentiation by altering G9A expression93. While H3K9 methylation and heterochromatinization may contribute to de novo DNA methylation, G9A can also promote DNA methylation independently of its histone methyltransferase activity by recruting the de novo DNA methyltransferases DNMT3A and DNMT3B95–97. DNA methylation profiling revealed that pluripotency genes and germline-specific genes are major tagets of differentiation-coupled de novo DNA methylation10, 27, and genetic evidence indicates that DNMT3A and DNMT3B are required for methylation of the Oct4 and Nanog promoters in differentiating ES cells and postimplantation embryos98. Taken together, these findings suggest that histone methylation and DNA methylation function cooperatively to ensure complete and stable silencing of pluripotency genes (Fig. 3B).
During differentiation, proper activation of lineage-specific genes is equally important as inactivation of lineage-inappropriate genes and pluripotency genes. Multiple epigenetic factors associated with gene activation have been implicated in gene expression during cellular differentiation and embryogenesis (Fig. 3C). For example, embryos lacking GCN5 — a HAT that is part of the SAGA complex and serves as a coactivator for multiple transcription factors — show loss of mesodermal tissues due to apoptosis and early embryonic lethality99, 100, and Gcn5-null ES cells form smaller embryoid bodies than wild-type ES cells101. Interestingly, loss of GCN5 HAT activity is only partly responsible for these phenotypes, as embryos homozygous for GCN5 catalytic site mutations survive until mid-gestation, when they exhibit severe neural tube closure defects102. Subsequent studies revealed that deletion of GCN5 affects the activity of a second enzyme in the SAGA complex, USP22, which deubquitylates histone H2B and non-histone proteins such as TRF1 and FBP1103, 104. The more severe phenotype of mice bearing Gcn5 null mutations compared with Gcn5 catalytic mutations probably reflects the combined loss of GCN5 and USP22 activities.
Several recent studies revealed that monoubiquitylation of H2B on lysine 120 (H2BK120ub1), a mark that is associated with highly transcribed genes, significantly increases upon differentiation of stem cells105–107. H2BK120ub1 is preferentially enriched in the coding regions of differentiation-related genes, but not in pluripotency genes107. Inhibition of H2BK120ub1, either by depletion of the RNF20–RNF40 E3 ligase complex or by ectopic expression of an H2B-K120R mutant, attenuates the upregulation of lineage-specific genes and impairs cellular differentiation105–107. H2BK120ub1 promotes H3K4 and H3K79 methylation, two modifications also associated with gene activation108 (Fig. 3C).
Epigenetic modifiers in adult stem cell functions
Conditional knockout models, which circumvent the embryonic and postnatal lethality that often occur in mice with germline gene deletions, indicate that many chromatin regulators involved in cell fate decisions during embryogenesis also play important roles in adult stem cell functions. For example, recent studies suggest that BRG1 plays key roles in the proliferation and differentiation of HF-SCs, as well as hair regeneration and epidermal repair109. However, some chromatin modifiers seem to be critical in adult stem cells, but not during embryonic development110, 111. For instance, mice lacking TET1, a 5-methylcytosine (5mC) dioxygenase that converts 5mC to 5-hydroxymethylcytosine (5hmC), are viable and fertile112, but exhibit reduced self-renewal of NSCs in adult brain and impaired hippocampal neurogenesis111.
Multifaceted roles of chromatin regulators
Although the classic view that transcription factors are major `drivers' of differentiation and chromatin modifiers are primarily responsible for stabilizing the differentiated states was important in the early stages of understanding the general roles of these two groups of proteins, this model has proven too simplistic to explain the complexity of the interactions between transcription factors and chromatin regulators. Recent evidence suggests that chromatin regulators are involved in priming transcriptional responses before cell fate decisions, modulating gene expression during cellular differentiation, and transmitting epigenetic marks through cell divisions to maintain the identity of differentiated cells.
Epigenetic pre-patterning for lineage specification
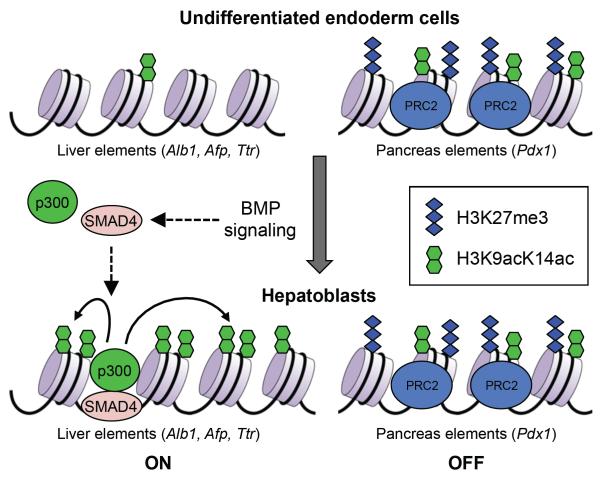
Transcription factors preferentially bind to `open' chromatin. Thus, epigenetic mechanisms may set the stage for lineage-specific transcription factors by creating and maintaining a permissive chromatin environment. Indeed, an emerging theme from recent studies is that epigenetic pre-patterning occurs before cell fate decisions. In one study, Szutorisz et al. differentiatied mouse ES cells toward the B-cell lineage and investigated the epigenetic regulation of gene expression. They found that a cis-acting element in the immunoglobulin lambda-like polypeptide 1 (Igll1; also known as λ5)–pre-B lymphocyte gene 1 (VpreB1) locus is marked by histone H3ac and H3K4me2 at a discrete site in undifferentiated ES cells. The marked region expands during differentiation and becomes a localized center for transcription factors and RNA polymerase II recruitment before full activation of the Igll1 and VpreB1 genes at the pre-B cell stage113. Similar epigenetic pre-patterning has been demonstrated in the fate choice of liver and pancreas in the embryonic endoderm. Xu et al. showed that the liver and pancreas regulatory elements have distinct chromatin patterns in undifferentiated endoderm cells. When the cells differentiate into hepatoblasts, H3K9ac and H3K14ac promote expression of hepatic genes, whereas H3K27me3 appears to repress the expression of pancreatic genes114 (Fig. 4). The concept of transcriptional priming by chromatin changes is reinforced by recent studies of higher-order chromatin structure during induced `dedifferentiation'. Circular chromosome conformation capture with high-throughput sequencing (4C-seq) reveals that, during somatic cell reprogramming into induced pluripotent cells (iPSCs), the establishment of long-range interchromosomal interactions with the Oct4 and Nanog loci precedes trancriptional activation of these genes115, 116. Recent genome-wide mapping studies suggest that epigenetic pre-patterning may be a widespread phenomenon in cell fate decisions. For instance, enhancers are usually `pre-patterned' by H3K4me1 and H3K4me2 marks and the histone variants H3.3 and H2A.Z before their target genes are activated. There is evidence that the presence of H3K4me1 and H3K4me2 marks facilitate binding of `pioneer factors'117. Pioneer factor binding, albeit not sufficient for gene activation, opens up chromatin and imparts competence for transcription (Box 1). Epigenetic pre-patterning may be important for the spatio-temporal regulation of gene expression during development.
Figure 4. Epigenetic pre-patterning for lineage specification.
In multipotent endoderm cells, regulatory elements of liver-specific genes and pancreas-specific genes are `pre-patterned' with distinct chromatin marks; both the active H3K9acK14ac marks and the repressive H3K27me3 mark are enriched in the regulatory elements of pancreas-expressed genes (such as Pdx1), but they are low or undetectable in the regulatory elements of liver-expressed genes (such as Alb1, Afp and Ttr). In response to BMP signaling, SMAD4 recruits p300 to the regulatory elements of liver-expressed genes to stimulate histone acetylation and induce hepatic specification. Meanwhile, the PRC2 complex maintains the level of H3K27me3 in the regulatory elements of pancreas-expressed genes to prevent pancreas specification.
Chromatin modifiers as co-regulators of transcription
Many histone-modifying enzymes are components of co-regulator complexes, which function cooperatively with transcription factors to modulate gene expression. In most cases, the core co-regulator complexes have no DNA-binding capability, and DNA-binding transcription factors, DNA methylation, and non-coding RNAs have all been implicated in the recruitment of histone-modifying complexes. The heterogeneity in subunit composition of co-regulator complexes may also confer target selectivity and functional specificity. This idea is best supported by results from recent studies of the highly heterogeneous PRC1 complexes (Box 2). Canonical, chromobox homolog protein (CBX)-containing PRC1 complexes require the presence of H3K27me3 for their genomic localization, whereas non-canonical, RING1 and YY1-binding protein (RYBP)-containing PRC1 complexes lack CBX proteins and exhibit H3K27me3-independent recruitment and H2A ubiquitylation118, 119. PRC1 complexes are also recruited to CpG islands by the H3K36-specific demethylase KDM2B120, 121. Canonical PRC1 complexes containing different CBX proteins appear to have nonoverlapping functions as well. CBX7, the predominant CBX protein in ES cells, is required for pluripotency, whereas CBX2, CBX4 and CBX8, which become upregulated upon differentiation, function in lineage commitment122, 123.
Co-regulators are often referred to as co-activators or co-repressors. For instance, HAT-containing complexes (e.g. SAGA) usually function as co-activators whereas HDAC-containing compexes (e.g. Sin3) generally serve as co-repressors. A surprising finding from recent genome-wide mapping studies is that some classic `co-repressors' are associated with not only repressed genes but also with actively transcribed loci. For example, chromatin immunoprecipitation followed by sequencing (ChIP-seq) analysis of multiple HATs and HDACs in human T cells revealed that all the HDACs examined are highly enriched in active genes and only a minor fraction of them are associated with silent genes124. The yeast Rpd3S HDAC has also been shown to be recruited to transcribed chromatin to prevent cryptic initiation of transcription within the coding region125. The precise control of gene expression levels is critical for cell fate determination, and co-repressors may play important roles in this fine-tuning of gene expression.
Inheritance of chromatin modifications
Cellular identities, once established, are remarkably stable. Although cellular identities can be experimentally reprogrammed by cell fusion or forced expression of pluripotency-associated or lineage-specific factors, cellular reprogramming is a slow and inefficient process. Chromatin modifications, such as DNA methylation and H3K9me3, present major barriers for somatic cell reprogramming into iPSCs, highlighting the importance of chromatin modifications in cellular memory126. Indeed, the efficiency of iPSC derivation can be increased by modulating chromatin regulators such as DNMTs, KMTs and chromatin remodelers127–132. For example, a recent study showed that, strikingly, depletion of MBD3 results in reprogramming efficiency of up to 100% within seven days132. Interestingly, compared to primary cells, cells grown as adherent cultures in the presence of serum tend to form pronounced macroscale H3K9me3 domains, which may hinder reprogramming35.
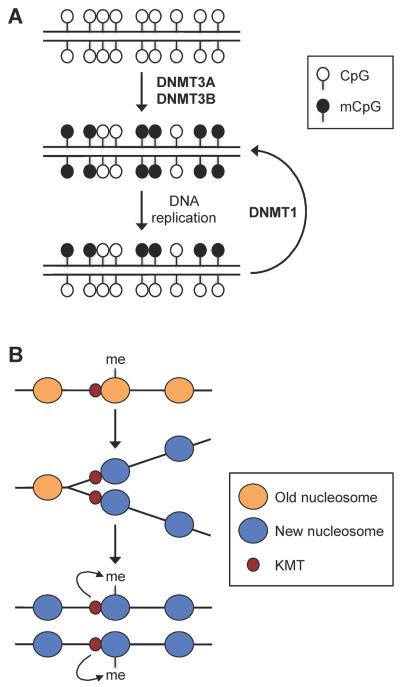
A fundamental question to our understanding of long-term maintenance of cellular identity is how chromatin modifications are passed to daughter cells through cell divisions. It is widely accepted that symmetric CpG methylation is faithfully maintained during DNA replication by a mechanism that involves semi-conservative segregation and templated copying (Fig. 5A). However, the mechanisms by which histone modifications are mitotically inherited are poorly understood. Several models have been proposed to explain the inheritance of histone methylation marks, which show fairly slow turnover and thus have the potential to be mitotically heritable133. Recent evidence suggests that, at least in some cases, histone-modifying enzymes, rather than the histone marks, persist through DNA replication. Petruk et al. showed that, in Drosophila melanogaster embryos, H3K4me3 and H3K27me3 are replaced by nonmethylated H3 following DNA replication whereas the H3K4 methyltransferase Trithorax and the H3K27 methyltransferase Enhancer-of-Zeste remain associated with newly replicated DNA134. In vitro experiments also revealed continuous association of PRC1 complex with replicating DNA135. These results support a model that histone methyltransferase complexes associated with nascent DNA re-establish histone methylation marks on newly assembled nucleosomes (Fig. 5B). It will be important to determine the generality and significance of this model in epigenetic inheritance.
Figure 5. Inheritance of DNA methylation and histone methylation marks through DNA replication.
(A) Semi-conservative maintenance of symmetric CpG methylation. During early embryogenesis, DNA methylation patterns are established by the de novo DNA methyltransferases DNMT3A and DNMT3B. After each round of DNA replication, the maintenance methyltransferase DNMT1 `copies' the CpG methylation patterns from the parental strand onto the daughter strand. (B) Role of lysine methyltransferases (KMTs) in maintaining histone methylation. During DNA replication, methylated histones are replaced by unmodified histones, but KMTs remain associated with newly replicated DNA at specific loci. Following DNA replication, the enzymes methylate the newly incorporated histones to re-establish the methylation patterns.
Conclusions
Recent technological advances have led to comprehensive epigenomic maps in pluripotent and differentiated cells. The results support the notion that differentiation is accompanied by dynamic changes in chromatin states, implying important functions for chromatin regulators in cell fate decisions. Although a global picture of the chromatin states in pluripotent cells and their changes during differentiation is emerging, it is far from complete. Several prevalent histone modifications have been the focus of most published studies, and the majority of histone modifications have not been explored136. Moreover, various histone marks function collaboratively and coordinately in biological processes. An important area of future research is to determine the `meanings' of different combinations of histone modifications.
Most of the published genome-wide chromatin modification studies have compared undifferentiated ES cells with in vitro differentiated cells. Although ES cells can recapitulate many aspects of early embryogenesis, their epigenome is not identical to that of ICM cells and varies in different culture conditions. Additionally, ES cells from different species may represent different developmental stages. There is evidence that human `ES cells' are actually similar to mouse epiblast-derived stem cells (EpiSCs) rather than mouse ES cells137. Furthermore, differentiation of ES cells, in most cases, produces heterogenous cell populations. In the future, we expect that highly sensitive technologies, including single-cell assays, will be developed so that small numbers of stem cells or other types of cells isolated from animals and humans can be directly profiled.
Loss-of-function and gain-of-function studies are powerful approaches for investigating the roles of individual genes in biological processes. Genetic studies in mice and other model organisms have clearly demonstrated the importance of chromatin regulators in major developmental transitions. However, the developmental functions of many other chromatin regulators remain to be explored. It is worth noting that phenotypes of mutant animals may not be entirely attributable to chromatin defects. Most `histone' modifiers likely also modify nonhistone proteins, and loss-of-function and gain-of-function models could facilitate the identification of these nonhistone substrates. A bottleneck is that for many modifications, `pan' antibodies that recognize diverse substates marked by the modification are not readily available, and it is difficult to identify some PTMs by mass spectrometry. Another major challenge is to determine the biological functions of modifications on nonhistone proteins. These modifications may be `read' by protein domains that recognize such marks in histones, and they may be subject to regulatory crosstalk, where different modifications regulate one another, as is observed in histones. PTM crosstalk can even occur between histone and nonhistone proteins, perhaps foreshadowing the discovery of chromatin signaling cascades138. Genome-wide studies comparing wild-type and mutant cells will no doubt continue to provide new clues to the full range of histone modifier functions.
Consistent with their fundamental role in differentiation, many chromatin modifiers and remodelers have been implicated in various human diseases, including cancer139. In the coming years, we expect to see intense research on the mechanisms by which malfunctions of chromatin regulators contribute to these diseases. Some chromatin alterations are potentially reversible, which raises the exciting possibility of correcting chromatin states as a therapeutic strategy.
Box 1 Chromatin modifications and pioneer factor binding.
Pioneer factors are a special class of transcription factors that are capable of accessing their DNA target sites in compact chromatin and presumably bind to the genome prior to the binding of other factors. Multiple proteins have been shown to possess the properties of pioneer factors. These include the FOXA factors, GATA factors, PU.1 and FOXD3117. Recent studies suggest that the reprogramming factors OCT4, SOX2 and KLF4 also act as pioneer factors. Soufi et al. showed that the vast majority of reprogramming-factor-binding events early in somatic cell reprogramming occur within closed chromatin130. Pioneer factor binding is thought to impart competence for future gene expression by opening up the local chromatin and facilitating subsequent recruitment of additional transcription factors and other regulatory proteins117.
A defining feature of pioneer factors is their ability to access condensed chromatin without the aid of other factors, including chromatin modifiers and remodelers. However, pioneer factor binding can be positively or negatively affected by special chromatin features. FOXA1 binding in breast cancer cells is facilitated by the absence of DNA methylation, nucleosome depletion, and the presence of H3K4me1 and H3K4me2 marks117. Likewise, epigenetic pre-patterning of the liver regulatory elements in undifferentiated endoderm cells correlates with FOXA, GATA4 and GATA6 binding114. In human somatic cells, megabase-scale chromatin domains enriched with H3K9me3 prevent binding of OCT4, SOX2, KLF4, and MYC and impede reprogramming of these cells to pluripotency130. Chromatin modifications and pioneer factors likely function synergistically to impart competency for transcription.
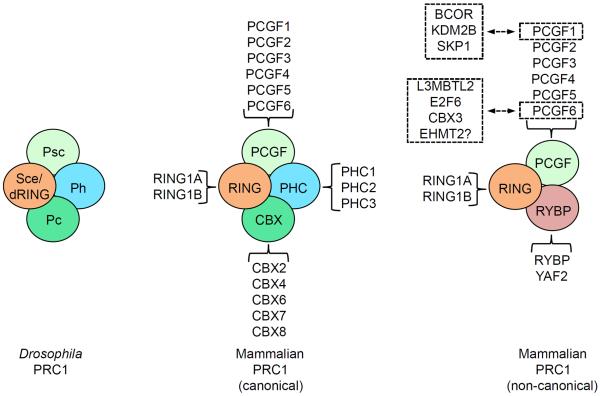
Box 2 Heterogeneous compositions of mammalian PRC1 complexes.
In Drosophila melanogaster, the core PRC1 complex contains Polycomb (Pc), a chromodomain-containing protein that binds to H3K27me3, Sex combs extra (Sce; also known as dRING), an E3 ligase that catalyzes H2A monoubiquitylation, Posterior sex combs (Psc), a large protein that is capable of inducing chromatin compaction, and Polyhomeotic (Ph). Each core subunit has two or more homologs in mammals, known as Chromobox homolog proteins (CBX2, CBX4, CBX6, CBX7 and CBX8), Ring finger proteins (RING1A and RING1B), Polycomb group ring finger proteins (PCGF1 (or Nervous system polycomb-1, NSPC1), PCGF2 (or MEL18), PCGF3, PCGF4 (or B lymphoma Mo-MLV insertion region 1 homolog, BMI1), PCGF5 and PCGF6 (or MEL18 and BMI1-like ring finger, MBLR)) and Polyhomeotic homolog proteins (PHC1, PHC2 and PHC3), respectively. Combinatorial association of these different homologs give rise to multiple canonical mammalian PRC1 complexes with distinct properties and functions. Moreover, recent studies have identified non-canonical PRC1 complexes, which contain RING1 and YYI-binding protein (RYBP) or a related protein YY1-associated factor 2 (YAF2) instead of CBX proteins118, 119. Non-canonical PRC1 complexes have also been shown to associate with other proteins via individual subunits. Via PCGF1, non-canonical PRC1 complexes interact with BCL6 corepressor (BCOR), Lysine demethylase 2B (KDM2B), and S-phase kinase-associated protein 1 (SKP1) to form the BCOR corepressor complex120, 121. Via PCGF6, components of non-canonical PRC1 complexes interact with Lethal(3) malignant brain tumor-like 2 (L3MBTL2), E2F transcription factor 6 (E2F6), Chromobox homolog 3 (CBX3, also known as heterochromatin protein 1γ, HP1γ), and perhaps the H3K9me2-specific methyltransferase G9A to form the Polycomb repressive complex 1-like 4 (PRC1L4) complex140.
Online summary
Embryonic stem (ES) cells have a globally `open' and dynamic chromatin state.
H3K4me3 and H3K27me3 bivalency observed in ES cells cultured in the presence of serum could largely reflect cellular heterogeneity. Bivalency is not a universal and unique feature of pluripotent and multipotent cells.
ES cell differentiation is accompanied by global chromatin remodeling, resulting in progressive transition from a relatively open chromatin configuration to a more compact and repressive state.
Dynamic changes in histone modifications also occur during adult stem cell differentiation.
Genetic studies in knockout mice suggest major roles of a variety of chromatin modifiers and remodelers in key developmental transitions, such as the segregation of embryonic (inner cell mass) and extraembryonic (trophoblast) lineages at the blastocyst stage and the formation of the three germ layers (ectoderm, mesoderm and endoderm) during gastrulation.
Epigenetic `pre-patterning' in pluripotent and multipotent cells may be important in lineage specification.
Chromatin modifiers often exist in multisubunit co-regulator complexes. The heterogeneous compositions of these complexes contribute to target selectivity and functional specificity.
Some classic `co-repressors' are associated with actively transcribed loci, which may prevent cryptic initiation of transcription and fine-tune transcription.
Recent evidence suggests that, at least in some cases, histone methylation enzymes, rather than histone methylation marks, persist through DNA replication, which provides a possible mechanism for inheritance of histone modifications.
Acknowledgements
Work in the T.C. laboratory is supported by The Cancer Prevention and Research Institute of Texas (CPRIT). T.C. is a CPRIT Scholar in Cancer Research. S.Y.R.D. support includes two grants from NIH, GM067718 and GM096472.
Glossary definitions
- Cytosine methylation
The addition of a methyl group to the 5th carbon in cytosines, which occurs predominantly in the context of CpG dinucleotides (the `p' refers to the phosphodiester bond linking a cytosine (C) and a guanine (G)). Cytosine methylation, often referred to as DNA methylation, is a major form of DNA modification. Promoter methylation correlates with gene silencing.
- Cytosine hydroxymethylation
A form of DNA modification that is generated by oxidation of 5-methylcytosine, a reaction mediated by the ten-eleven translocation (TET) family of hydroxylases. 5-hydroxymethylcytosine (5hmC) is an intermediate in DNA demethylation and may also serve as a stable epigenetic mark.
- CpG island
A genomic region that contains a high content of CpG dinucleotides. CpG islands are found in many mammalian promoters.
- Non-coding RNAs (ncRNAs)
Functional RNA molecules that are not translated into proteins. These include small ncRNAs (e.g. microRNAs, siRNAs) and long ncRNAs (lncRNAs, e.g. Xist, HOTAIR). ncRNAs regulate gene expression at various levels, such as transcription, splicing, mRNA stability, and translation.
- Polycomb group (PcG) proteins
A family of chromatin regulatory proteins that are typically involved in repressing gene expression, partly through trimethylation of H3K27 and monoubiquitylation of H2AK119.
- Trithorax group (TrxG) proteins
A family of chromatin regulatory proteins that typically activate gene expression through trimethylation of H3K4 and/or ATP-dependent chromatin remodeling.
- Totipotent
The ability of a cell to give rise to differentiated cells of all tissues, embryonic and extraembryonic, in an organism (e.g. zygote).
- Pluripotent
The ability of a cell to differentiate into all three germ layers (endoderm, mesoderm and ectoderm) and give rise to all fetal or adult cell types (e.g. cells of the inner cell mass (ICM) of blastocyst stage embryos).
- Multipotent
The ability of a cell to differentiate into multiple but limited cell types (e.g. cells of the embryonic germ layers and adult stem cells).
- Blastocyst
An early stage embryo that has undergone the first cell lineage specification, resulting in two primary cell types, the inner cell mass and the trophoblasts.
- Inner cell mass (ICM)
A group of cells inside a mammalian blastocyst that give rise to the embryo.
- Trophoblast
The outer layer of the mammalian blastocyst that will eventually develop to form part of the placenta.
- Trophoblast stem cell
A multipotent cell capable of producing all trophoblast cell types in culture and in vivo.
- Gastrulation
A phase of early embryonic development, during which the three germ layers are formed.
- Ectoderm
The outermost layer of the three embryonic germ layers that gives rise to epidermis (skin, hair, eyes, etc.) and the nervous system.
- Mesoderm
The middle of the three embryonic germ layers that gives rise to muscle, cartilage, bone, blood, connective tissue, etc.
- Endoderm
The innermost layer of the three embryonic germ layers that gives rise to the epithelia of the digestive and respiratory systems, liver, pancreas, etc.
- Euchromatin
A form of chromatin that is relatively decondensed and transcriptionally active.
- Heterochromatin
Highly condensed chromatin that is transcriptionally inactive.
- Constitutive heterochromatin
Structural regions of chromosomes, such as centromeres and telomeres, that are devoid of genes.
- Facultative heterochromatin
Tightly packed chromatin regions in which genes are silenced in a given cell type.
- Chromatin dynamics
Changes in chromatin structure, composition and positioning.
- Zygote
Fertilized egg before cleavage occurs (1-cell stage embryo).
- Implantation
An early developmental stage at which the embryo adheres to the wall of the uterus.
- Zona pellucida
A thick glycoprotein membrane surrounding the plasma membrane of an oocyte.
- Leukemia inhibitory factor (LIF)
An interleukin 6 class cytokine that is often added in mouse embryonic stem (ES) cell cultures to inhibit differentiation.
- Epiblast-derived stem cells (EpiSCs)
Pluripotent stem cells derived from the late epiblast layer of postimplantation embryos.
- Major satellite DNA
Tandemly repeating DNA sequences that are present primarily in the pericentromeric regions of the mouse genome.
- Hatch
When a blactocyst bursts out of the protective zona pellucida.
- Nucleosome remodeling and deacetylation (NuRD) corepressor complex
A multisubunit complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Its components include the CHD family of ATPases Mi-2α/Mi-2β, HDAC1/HDAC2, MTA1/MTA2/MTA3, MBD2/MBD3, and RbAp46/RbAp48.
- SAGA complex
A multisubunit complex, named after the yeast Spt-Ada-Gcn5 acetyltransferase complex, that is conserved in eukaryotic organisms. It has histone acetyltransferase activity, mediated by the GCN5 subunit, and histone deubiquitylase activity, mediated by the USP22 subunit. It also contains subunits important for interactions with transcriptional activators and general transcription machinery. The SAGA complex functions as a co-activator.
- Dedifferentiation
Conversion of a differentiated cell to a pluripotent or multipotent cell.
- Pioneer factors
A special group of trancription factors that can directly bind condensed chromatin to initiate regulatory events in chromatin. Pioneer factor binding, albeit not sufficient for gene activation, is thought to establish competence for gene expression by loosening chromatin and facilitating binding of other transcription factors and regulatory proteins.
Biographies
Dr. Taiping Chen is an Associate Professor in the Department of Molecular Carcinogenesis at The University of Texas MD Anderson Cancer Center (MDACC). He is also a member of the Center for Cancer Epigenetics at MDACC. Dr. Chen received his Ph.D. from McGill University in 2000 and held a Research Investigator position at Novartis Institutes for Biomedical Research, Cambridge, Massachusetts, USA, from 2004 to 2011. His research focuses on the functions of epigenetic modifiers in various biological processes, including mammalian development, epigenetic reprogramming in germ cells and early embryos, and tumorigenesis.
Dr. Sharon Y.R. Dent is Professor and Chair of the Department of Molecular Carcinogenesis at The University of Texas MD Anderson Cancer Center (MDACC) and Director of the Center for Cancer Epigenetics. Dr. Dent's research is directed at defining the functions of histone modifying enzymes both in normal biological processes and in disease states, using genetic approaches in yeast, in mice, and in human cells. Dr. Dent is now working to determine how misregulation of these activities contributes to human disease, including highly aggressive cancers and neurological disorders.
Footnotes
Competing interests statement The authors declare no competing financial interests.
References
- 1.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nature Rev. Genet. 2013;14:204–20. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 2.Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nature Rev. Mol. Cell Biol. 2013;14:341–56. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skene PJ, Henikoff S. Histone variants in pluripotency and disease. Development. 2013;140:2513–24. doi: 10.1242/dev.091439. [DOI] [PubMed] [Google Scholar]
- 4.Castel SE, Martienssen RA. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nature Rev. Genet. 2013;14:100–12. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–84. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SH, Kook MC, Kim EY, Park S, Lim JH. Ultrastructure of human embryonic stem cells and spontaneous and retinoic acid-induced differentiating cells. Ultrastruct. Pathol. 2004;28:229–38. doi: 10.1080/01913120490515595. [DOI] [PubMed] [Google Scholar]
- 7.Meshorer E, et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 2006;10:105–16. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuara V, et al. Chromatin signatures of pluripotent cell lines. Nature Cell Biol. 2006;8:532–8. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]; This study, as well as Bernstein et al. (below), described the identification of bivalent domains (containing both repressive H3K27me3 and activating H3K4me3 marks) in lineage-specific genes in mouse ES cells cultured in normal serum-containing medium.
- 9.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meissner A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–70. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lister R, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efroni S, et al. Global transcription in pluripotent embryonic stem cells. Cell Stem Cell. 2008;2:437–47. doi: 10.1016/j.stem.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]; This study, as well as Azuara et al. (above), described the identification of bivalent domains (containing both repressive H3K27me3 and activating H3K4me3 marks) in lineage-specific genes in mouse ES cells cultured in normal serum-containing medium.
- 14.Mikkelsen TS, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao XD, et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2007;1:286–98. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Pan G, et al. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Voigt P, et al. Asymmetrically modified nucleosomes. Cell. 2012;151:181–93. doi: 10.1016/j.cell.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided evidence that H3K4me3 and H3K27me3 marks do not co-exist on the same histone H3 tail, but can occur on opposite H3 tails in the same nucleosome.
- 18.Alder O, et al. Ring1B and Suv39h1 delineate distinct chromatin states at bivalent genes during early mouse lineage commitment. Development. 2010;137:2483–92. doi: 10.1242/dev.048363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahl JA, Reiner AH, Klungland A, Wakayama T, Collas P. Histone H3 lysine 27 methylation asymmetry on developmentally-regulated promoters distinguish the first two lineages in mouse preimplantation embryos. PloS One. 2010;5:e9150. doi: 10.1371/journal.pone.0009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugg-Gunn PJ, Cox BJ, Ralston A, Rossant J. Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc. Natl. Acad. Sci. USA. 2010;107:10783–90. doi: 10.1073/pnas.0914507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui K, et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vastenhouw NL, et al. Chromatin signature of embryonic pluripotency is established during genome activation. Nature. 2010;464:922–6. doi: 10.1038/nature08866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akkers RC, et al. A hierarchy of H3K4me3 and H3K27me3 acquisition in spatial gene regulation in Xenopus embryos. Dev. Cell. 2009;17:425–34. doi: 10.1016/j.devcel.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuettengruber B, et al. Functional anatomy of polycomb and trithorax chromatin landscapes in Drosophila embryos. PLoS Biol. 2009;7:e13. doi: 10.1371/journal.pbio.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien WH, et al. Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell. 2011;9:219–32. doi: 10.1016/j.stem.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roh TY, Cuddapah S, Cui K, Zhao K. The genomic landscape of histone modifications in human T cells. Proc. Natl. Acad. Sci. USA. 2006;103:15782–7. doi: 10.1073/pnas.0607617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohn F, et al. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol. Cell. 2008;30:755–66. doi: 10.1016/j.molcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K, Lopes SM, Tang F, Surani MA. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. 2008;3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–18. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- 30.Hong SH, et al. Cell fate potential of human pluripotent stem cells is encoded by histone modifications. Cell Stem Cell. 2011;9:24–36. doi: 10.1016/j.stem.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–92. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Marks H, et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper compared transcriptome and epigenome profiles of mouse ES cells grown in serum and 2i medium and showed that 2i-treated (naïve) ES cells exhibit lower expression of lineage-specific genes, reduced H3K27me3 at promoters, and fewer bivalent domains, suggesting that a large proportion of bivalent domains in ES cells cultured in serum are due to acquisition of H3K27me3 at promoters.
- 33.Wen B, Wu H, Shinkai Y, Irizarry RA, Feinberg AP. Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nature Genet. 2009;41:246–50. doi: 10.1038/ng.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pauler FM, et al. H3K27me3 forms BLOCs over silent genes and intergenic regions and specifies a histone banding pattern on a mouse autosomal chromosome. Genome Res. 2009;19:221–33. doi: 10.1101/gr.080861.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell. 2013;152:642–54. doi: 10.1016/j.cell.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study mapped multiple histone modifications in a diverse collection of human tissues, blood lineages, and stem cells, and the results supported the notion that developmental specification is accompanied by progressive chromatin restriction. The paper also showed that cells cultured in serum exhibit prominent features of constitutive heterochromatin, including the formation of macroscale H3K9me3 domains.
- 36.Lienert F, et al. Genomic prevalence of heterochromatic H3K9me2 and transcription do not discriminate pluripotent from terminally differentiated cells. PLoS Genet. 2011;7:e1002090. doi: 10.1371/journal.pgen.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creyghton MP, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA. 2010;107:21931–6. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rada-Iglesias A, et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–83. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wamstad JA, et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell. 2012;151:206–20. doi: 10.1016/j.cell.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paige SL, et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. 2012;151:221–32. doi: 10.1016/j.cell.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rada-Iglesias A, et al. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell. 2012;11:633–48. doi: 10.1016/j.stem.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie R, et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224–37. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gifford CA, et al. Transcriptional and Epigenetic Dynamics during Specification of Human Embryonic Stem Cells. Cell. 2013;153:1149–63. doi: 10.1016/j.cell.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie W, et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell. 2013;153:1134–48. doi: 10.1016/j.cell.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikkelsen TS, et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–69. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen AL, Jacobsen BM, Reiner AH, Andersen IS, Collas P. Promoter DNA methylation patterns of differentiated cells are largely programmed at the progenitor stage. Mol. Biol. Cell. 2010;21:2066–77. doi: 10.1091/mbc.E10-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herlofsen SR, et al. Genome-wide map of quantified epigenetic changes during in vitro chondrogenic differentiation of primary human mesenchymal stem cells. BMC Genomics. 2013;14:105. doi: 10.1186/1471-2164-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bock C, et al. DNA methylation dynamics during in vivo differentiation of blood and skin stem cells. Mol. Cell. 2012;47:633–47. doi: 10.1016/j.molcel.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 50.Niwa H, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–29. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 51.Strumpf D, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 52.Dodge JE, Kang YK, Beppu H, Lei H, Li E. Histone H3-K9 methyltransferase ESET is essential for early development. Mol. Cell. Biol. 2004;24:2478–86. doi: 10.1128/MCB.24.6.2478-2486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bilodeau S, Kagey MH, Frampton GM, Rahl PB, Young RA. SetDB1 contributes to repression of genes encoding developmental regulators and maintenance of ES cell state. Genes Dev. 2009;23:2484–9. doi: 10.1101/gad.1837309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan P, et al. Eset partners with Oct4 to restrict extraembryonic trophoblast lineage potential in embryonic stem cells. Genes Dev. 2009;23:2507–20. doi: 10.1101/gad.1831909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeap LS, Hayashi K, Surani MA. ERG-associated protein with SET domain (ESET)-Oct4 interaction regulates pluripotency and represses the trophectoderm lineage. Epigenet. Chromatin. 2009;2:12. doi: 10.1186/1756-8935-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lohmann F, et al. KMT1E mediated H3K9 methylation is required for the maintenance of embryonic stem cells by repressing trophectoderm differentiation. Stem Cells. 2010;28:201–12. doi: 10.1002/stem.278. [DOI] [PubMed] [Google Scholar]
- 57.Kaji K, Nichols J, Hendrich B. Mbd3, a component of the NuRD co-repressor complex, is required for development of pluripotent cells. Development. 2007;134:1123–32. doi: 10.1242/dev.02802. [DOI] [PubMed] [Google Scholar]
- 58.Kaji K, et al. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nature Cell Biol. 2006;8:285–92. doi: 10.1038/ncb1372. [DOI] [PubMed] [Google Scholar]
- 59.Zhu D, Fang J, Li Y, Zhang J. Mbd3, a component of NuRD/Mi-2 complex, helps maintain pluripotency of mouse embryonic stem cells by repressing trophectoderm differentiation. PloS One. 2009;4:e7684. doi: 10.1371/journal.pone.0007684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Latos PA, et al. NuRD-dependent DNA methylation prevents ES cells from accessing a trophectoderm fate. Biol. Open. 2012;1:341–52. doi: 10.1242/bio.2012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds N, et al. NuRD suppresses pluripotency gene expression to promote transcriptional heterogeneity and lineage commitment. Cell Stem Cell. 2012;10:583–94. doi: 10.1016/j.stem.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reynolds N, et al. NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression. EMBO J. 2012;31:593–605. doi: 10.1038/emboj.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yildirim O, et al. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell. 2011;147:1498–510. doi: 10.1016/j.cell.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta A, et al. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol. Cell. Biol. 2008;28:397–409. doi: 10.1128/MCB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas T, Dixon MP, Kueh AJ, Voss AK. Mof (MYST1 or KAT8) is essential for progression of embryonic development past the blastocyst stage and required for normal chromatin architecture. Mol. Cell. Biol. 2008;28:5093–105. doi: 10.1128/MCB.02202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Y, et al. Homozygous disruption of the Tip60 gene causes early embryonic lethality. Dev. Dyn. 2009;238:2912–21. doi: 10.1002/dvdy.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herceg Z, et al. Disruption of Trrap causes early embryonic lethality and defects in cell cycle progression. Nature Genet. 2001;29:206–11. doi: 10.1038/ng725. [DOI] [PubMed] [Google Scholar]
- 68.Li X, et al. The histone acetyltransferase MOF is a key regulator of the embryonic stem cell core transcriptional network. Cell Stem Cell. 2012;11:163–78. doi: 10.1016/j.stem.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawan C, et al. Histone acetyltransferase cofactor Trrap maintains self-renewal and restricts differentiation of embryonic stem cells. Stem Cells. 2013;31:979–91. doi: 10.1002/stem.1341. [DOI] [PubMed] [Google Scholar]
- 70.Fazzio TG, Huff JT, Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–74. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; In an RNAi screen of ~1000 loci encoding chromatin proteins, this study identified ~70 potential ES cell regulators, including multiple components of the Tip60-p400 complex that are required for the maintenance of ES cell identity.
- 71.Ang YS, et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–97. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeGouy E, Thompson EM, Muchardt C, Renard JP. Differential preimplantation regulation of two mouse homologues of the yeast SWI2 protein. Dev. Dyn. 1998;212:38–48. doi: 10.1002/(SICI)1097-0177(199805)212:1<38::AID-AJA4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Bultman SJ, et al. Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev. 2006;20:1744–54. doi: 10.1101/gad.1435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bultman S, et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell. 2000;6:1287–95. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 75.Klochendler-Yeivin A, et al. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 2000;1:500–6. doi: 10.1093/embo-reports/kvd129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JK, et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol. Cell. Biol. 2001;21:7787–95. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kidder BL, Palmer S, Knott JG. SWI/SNF-Brg1 regulates self-renewal and occupies core pluripotency-related genes in embryonic stem cells. Stem Cells. 2009;27:317–28. doi: 10.1634/stemcells.2008-0710. [DOI] [PubMed] [Google Scholar]
- 78.Ho L, et al. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc. Natl. Acad. Sci. USA. 2009;106:5181–6. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho L, et al. An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc. Natl. Acad. Sci. USA. 2009;106:5187–91. doi: 10.1073/pnas.0812888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho L, et al. esBAF facilitates pluripotency by conditioning the genome for LIF/STAT3 signalling and by regulating polycomb function. Nature Cell Biol. 2011;13:903–13. doi: 10.1038/ncb2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Carroll D, et al. The polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell. Biol. 2001;21:4330–6. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faust C, Schumacher A, Holdener B, Magnuson T. The eed mutation disrupts anterior mesoderm production in mice. Development. 1995;121:273–85. doi: 10.1242/dev.121.2.273. [DOI] [PubMed] [Google Scholar]
- 83.Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004;23:4061–71. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Voncken JW, et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc. Natl. Acad. Sci. USA. 2003;100:2468–73. doi: 10.1073/pnas.0434312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pirity MK, Locker J, Schreiber-Agus N. Rybp/DEDAF is required for early postimplantation and for central nervous system development. Mol. Cell. Biol. 2005;25:7193–202. doi: 10.1128/MCB.25.16.7193-7202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin J, et al. The polycomb group protein L3mbtl2 assembles an atypical PRC1-family complex that is essential in pluripotent stem cells and early development. Cell Stem Cell. 2012;11:319–32. doi: 10.1016/j.stem.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyer LA, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 88.Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol. Cell. Biol. 2007;27:3769–79. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen X, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leeb M, et al. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 2010;24:265–76. doi: 10.1101/gad.544410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hisada K, et al. RYBP represses endogenous retroviruses and preimplantation- and germ line-specific genes in mouse embryonic stem cells. Mol. Cell. Biol. 2012;32:1139–49. doi: 10.1128/MCB.06441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tachibana M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–91. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamamizu K, et al. Protein kinase A determines timing of early differentiation through epigenetic regulation with G9a. Cell Stem Cell. 2012;10:759–70. doi: 10.1016/j.stem.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 94.Feldman N, et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nature Cell Biol. 2006;8:188–94. doi: 10.1038/ncb1353. [DOI] [PubMed] [Google Scholar]
- 95.Epsztejn-Litman S, et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nature Struct. Mol. Biol. 2008;15:1176–83. doi: 10.1038/nsmb.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tachibana M, Matsumura Y, Fukuda M, Kimura H, Shinkai Y. G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. EMBO J. 2008;27:2681–90. doi: 10.1038/emboj.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong KB, et al. DNA methylation in ES cells requires the lysine methyltransferase G9a but not its catalytic activity. EMBO J. 2008;27:2691–701. doi: 10.1038/emboj.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li JY, et al. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol. Cell. Biol. 2007;27:8748–59. doi: 10.1128/MCB.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu W, et al. Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nature Genet. 2000;26:229–32. doi: 10.1038/79973. [DOI] [PubMed] [Google Scholar]
- 100.Yamauchi T, et al. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc. Natl. Acad. Sci. USA. 2000;97:11303–6. doi: 10.1073/pnas.97.21.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin W, et al. Developmental potential of Gcn5(−/−) embryonic stem cells in vivo and in vitro. Dev. Dyn. 2007;236:1547–57. doi: 10.1002/dvdy.21160. [DOI] [PubMed] [Google Scholar]
- 102.Bu P, Evrard YA, Lozano G, Dent SY. Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol. Cell. Biol. 2007;27:3405–16. doi: 10.1128/MCB.00066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atanassov BS, et al. Gcn5 and SAGA regulate shelterin protein turnover and telomere maintenance. Mol. Cell. 2009;35:352–64. doi: 10.1016/j.molcel.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Atanassov BS, Dent SY. USP22 regulates cell proliferation by deubiquitinating the transcriptional regulator FBP1. EMBO Rep. 2011;12:924–30. doi: 10.1038/embor.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fuchs G, et al. RNF20 and USP44 regulate stem cell differentiation by modulating H2B monoubiquitylation. Mol. Cell. 2012;46:662–73. doi: 10.1016/j.molcel.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karpiuk O, et al. The histone H2B monoubiquitination regulatory pathway is required for differentiation of multipotent stem cells. Mol. Cell. 2012;46:705–13. doi: 10.1016/j.molcel.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 107.Chen S, Li J, Wang DL, Sun FL. Histone H2B lysine 120 monoubiquitination is required for embryonic stem cell differentiation. Cell Res. 2012;22:1402–5. doi: 10.1038/cr.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wright DE, Wang CY, Kao CF. Histone ubiquitylation and chromatin dynamics. Front. Biosci. 2012;17:1051–78. doi: 10.2741/3973. [DOI] [PubMed] [Google Scholar]
- 109.Xiong Y, et al. Brg1 governs a positive feedback circuit in the hair follicle for tissue regeneration and repair. Dev. Cell. 2013;25:169–81. doi: 10.1016/j.devcel.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 110.Wu Z, et al. Dnmt3a regulates both proliferation and differentiation of mouse neural stem cells. J. Neurosci. Res. 2012;90:1883–91. doi: 10.1002/jnr.23077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang RR, et al. Tet1 Regulates Adult Hippocampal Neurogenesis and Cognition. Cell Stem Cell. 2013 doi: 10.1016/j.stem.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dawlaty MM, et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9:166–75. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szutorisz H, et al. Formation of an active tissue-specific chromatin domain initiated by epigenetic marking at the embryonic stem cell stage. Mol. Cell. Biol. 2005;25:1804–20. doi: 10.1128/MCB.25.5.1804-1820.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu CR, et al. Chromatin “prepattern” and histone modifiers in a fate choice for liver and pancreas. Science. 2011;332:963–6. doi: 10.1126/science.1202845. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that the liver and pancreas regulatory elements have distinct epigenetic patterns in multipotent embryonic endoderm cells, suggesting that epigenetic “pre-patterning” may be important in cell fate decisions.
- 115.Apostolou E, et al. Genome-wide chromatin interactions of the Nanog locus in pluripotency, differentiation, and reprogramming. Cell Stem Cell. 2013;12:699–712. doi: 10.1016/j.stem.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei Z, et al. Klf4 organizes long-range chromosomal interactions with the oct4 locus in reprogramming and pluripotency. Cell Stem Cell. 2013;13:36–47. doi: 10.1016/j.stem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 117.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–41. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tavares L, et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell. 2012;148:664–78. doi: 10.1016/j.cell.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that PRC1 complexes target to closely overlapping sites in wild-type and PRC2-deficient mouse ES cells and that H3K27me3-independent recruitment of PRC1 activity is mediated by RYBP-containing (non-canonical) PRC1 complexes.
- 119.Gao Z, et al. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell. 2012;45:344–56. doi: 10.1016/j.molcel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified six major groups of PRC1 complexes in mammalian cells and showed that CBX-cotaining (canonical) and RYBP-containing (non-canonical) PRC1 complexes exhibit distinct genome localization patterns and functions.
- 120.Wu X, Johansen JV, Helin K. Fbxl10/Kdm2b recruits polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol. Cell. 2013;49:1134–46. doi: 10.1016/j.molcel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 121.He J, et al. Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nature Cell Biol. 2013;15:373–84. doi: 10.1038/ncb2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Loghlen A, et al. MicroRNA regulation of Cbx7 mediates a switch of Polycomb orthologs during ESC differentiation. Cell Stem Cell. 2012;10:33–46. doi: 10.1016/j.stem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morey L, et al. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell. 2012;10:47–62. doi: 10.1016/j.stem.2011.12.006. [DOI] [PubMed] [Google Scholar]; These two papers (O'Loghlen et al. and Morey et al.) showed that different CBX proteins have distinct functions in ES cells: CBX7 is required for pluripotency, and CBX2, CBX4 and CBX8 are involved in differentiation.
- 124.Wang Z, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–31. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this work, genome-wide mapping revealed that the majority of HDACs are enriched in active genes and that “poised” genes are subject to dynamic cycles of acetylation and deacetylation by transient HAT/HDAC binding.
- 125.Li B, et al. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science. 2007;316:1050–4. doi: 10.1126/science.1139004. [DOI] [PubMed] [Google Scholar]
- 126.Papp B, Plath K. Epigenetics of reprogramming to induced pluripotency. Cell. 2013;152:1324–43. doi: 10.1016/j.cell.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singhal N, et al. Chromatin-Remodeling Components of the BAF Complex Facilitate Reprogramming. Cell. 2010;141:943–55. doi: 10.1016/j.cell.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 129.Onder TT, et al. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors' initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study mapped the genome-wide binding sites of OCT4, SOX2, KLF4 and c-Myc (reprogramming factors) at an early stage of somatic cell reprogramming and found extensive binding of these factors to chromatin, including at enhancers of genes that promote reprogramming, suggesting that these factors may function as pioneer factors.
- 131.Chen J, et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nature Genet. 2013;45:34–42. doi: 10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- 132.Rais Y, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]; This work showed that trancription factor-induced reprogramming of MBD3-depleted cells to pluripotency leads to nearly 100% reprogramming efficiency within days, highlighting the importance of chromatin regulators in maintaining cellular identity.
- 133.Corpet A, Almouzni G. Making copies of chromatin: the challenge of nucleosomal organization and epigenetic information. Trends Cell Biol. 2009;19:29–41. doi: 10.1016/j.tcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 134.Petruk S, et al. TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell. 2012;150:922–33. doi: 10.1016/j.cell.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that, in Drosophila embryos, histone methyltransferase complexes (TrxG and PcG proteins), rather than histone methylation marks (H3K4me3 and H3K27me3), persist through DNA replication, thus providing a mechanism for epigenetic inheritance.
- 135.Francis NJ, Follmer NE, Simon MD, Aghia G, Butler JD. Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell. 2009;137:110–22. doi: 10.1016/j.cell.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tan M, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–28. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 138.Johnson DG, Dent SY. Chromatin: receiver and quarterback for cellular signals. Cell. 2013;152:685–9. doi: 10.1016/j.cell.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 140.Trojer P, et al. L3MBTL2 protein acts in concert with PcG protein-mediated monoubiquitination of H2A to establish a repressive chromatin structure. Mol. Cell. 2011;42:438–50. doi: 10.1016/j.molcel.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]