Abstract
Elderly patients are inherently predisposed to dysphagia predominately because of comorbid health conditions. With the aging of the population in the United States, along with the increased prevalence of obesity and gastroesophageal reflux disease, healthcare providers will increasingly encounter older patients with either oropharyngeal or esophageal disease and complaints of dysphagia. Useful tests to evaluate dysphagia include the videofluoroscopic swallowing study and the fiberoptic endoscopic evaluation of swallowing. Swallow rehabilitation is useful to help patients compensate for swallowing difficulty and ultimately help strengthen the neuromusculature involved in swallowing.
Keywords: Esophageal dysphagia, oropharyngeal dysphagia, aging, swallow function, videofluoroscopic swallowing study, fiberoptic endoscopic evaluation of swallowing
The word dysphagia, which comes from the Greek words dys (difficulty) and phagia (to eat), refers to the sensation of food being delayed or hindered in its passage from the mouth to the stomach. Dysphagia may be classified anatomically as either oropharyngeal or esophageal. Oropharyngeal, or transfer, dysphagia is related to the initiation of the swallow (ie, the movement of a food bolus from the hypopharynx to the esophagus). Esophageal dysphagia arises in the body of the esophagus and relates to difficulty in passing food to the stomach. Dysphagia may result either from mechanical obstruction or altered motor function along the area of food passage.
The elderly are at an increased risk for development of dysphagia due to illnesses that affect the swallowing mechanism. To better serve this vulnerable group of patients, healthcare providers must inquire about the presence of dysphagia and have a working knowledge of its pathophysiology. This review addresses several commonly encountered questions about caring for elderly persons with dysphagia, including a discussion on the mechanisms crucial to normal swallowing and how they may be affected by the aging process.
How Common Is Dysphagia in the Elderly Population?
The true prevalence of dysphagia is higher in the elderly population than the general population. Although the prevalence of dysphagia in the Midwestern US population was reported to be 6% to 9%,1 its prevalence in community-dwelling persons over age 50 years is estimated to be between 15% and 22%.2-4 The prevalence of dysphagia is even higher in those residing in assisted living facilities and nursing homes, where up to 40% to 60% of residents are reported to have feeding difficulties.4,5 Irrespective of age, dysphagia is commonly associated with certain diseases such as cerebrovascular accidents, amyotrophic lateral sclerosis, Parkinson disease (PD), myasthenia gravis, and tardive dyskinesia, all of which increase in prevalence with aging. The prevalence of dysphagia is expected to increase rapidly in the near future, given the demographic trends in the United States. According to data released by the US Census Bureau, between January 2000 and July 2009, the total US population increased by 9%, the number of persons age 65 years and older increased by 13%, and the number of persons age 85 years and older increased by 32%. For the next 18 years, 10,000 more Americans will become seniors each day because of the aging Baby Boomer population. It is expected that by 2030, 1 in 5 US residents will be age 65 years or older.6 With the rapid growth of the aging population, it is not surprising that dysphagia is increasingly recognized as an important national healthcare issue that is associated with enormous cost.
What Is the Physiology of Normal Swallowing?
Swallowing represents a function that involves more than 30 nerves and muscles.7 Bolus preparation and passage from the oral cavity into the esophagus are volitional, while further passage across the aerodigestive tract to the stomach is reflexive. After initiation of a swallow, it takes less than 1 second for a bolus to reach the esophagus2 and an additional 10 to 15 seconds to complete the swallow. On average, a person performs approximately 600 swallows each day effortlessly. The swallowing centers are bilaterally represented within the central nervous system, and the degree of each hemispheric representation seems to be critical in determining recovery of the swallowing function after a dysphagic stroke.8
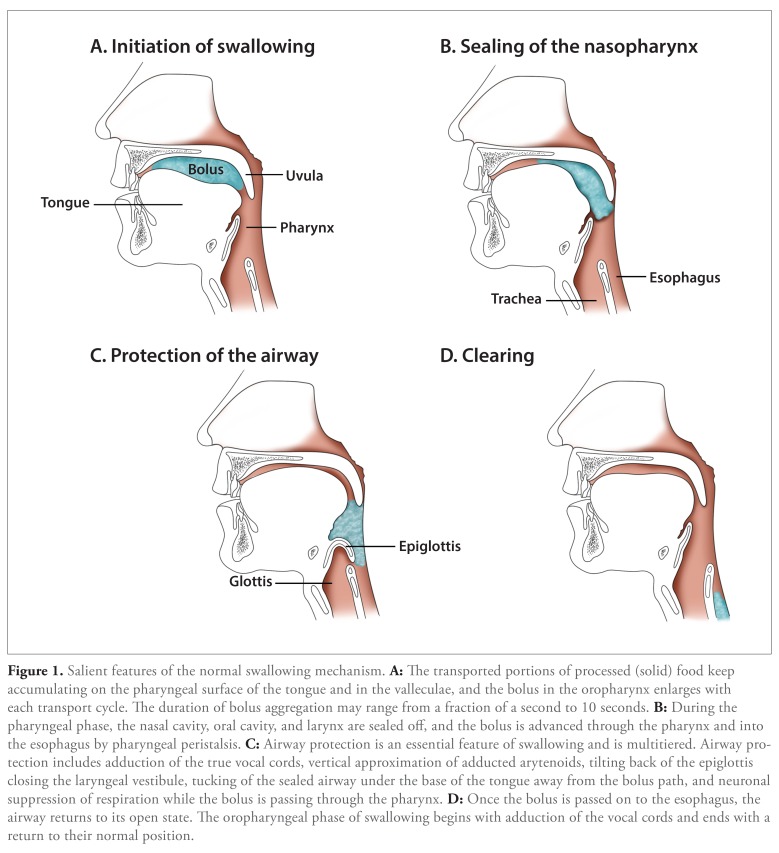
Swallowing is conventionally described as 3 anatomic phases: oral, pharyngeal, and esophageal (Figure 1). The oral phase is the volitional component of swallowing activity, which prepares and propels food into the pharynx and involves use of the cranial nerves V (trigeminal), VII (facial), and XII (hypoglossal). The pharyngeal phase of swallowing involves sealing of the airway and projection of the bolus into the esophagus. This mechanism is mediated reflexively and involves cranial nerves V (trigeminal), X (vagus), XI (accessory), and XII (hypoglossal). The esophageal phase further propels the bolus into the stomach by coordinated esophageal peristalsis and relaxation of the lower esophageal sphincter. Salient features of the normal swallowing mechanism are depicted in Figure 1.
Figure 1.
Salient features of the normal swallowing mechanism. A: The transported portions of processed (solid) food keep accumulating on the pharyngeal surface of the tongue and in the valleculae, and the bolus in the oropharynx enlarges with each transport cycle. The duration of bolus aggregation may range from a fraction of a second to 10 seconds. B: During the pharyngeal phase, the nasal cavity oral cavity and larynx are sealed off, and the bolus is advanced through the pharynx and into the esophagus by pharyngeal peristalsis. C: Airway protection is an essential feature of swallowing and is multitiered. Airway protection includes adduction of the true vocal cords, vertical approximation of adducted arytenoids, tilting back of the epiglottis closing the laryngeal vestibule, tucking of the sealed airway under the base of the tongue away from the bolus path, and neuronal suppression of respiration while the bolus is passing through the pharynx. D: Once the bolus is passed on to the esophagus, the airway returns to its open state. The oropharyngeal phase of swallowing begins with adduction of the vocal cords and ends with a return to their normal position.
What Is the Effect of Aging on the Swallowing Mechanism?
Normal aging is associated with cerebral atrophy, deterioration in nerve function, and a region-dependent decline in muscle mass,9 which may adversely affect swallowing function.10,11 Furthermore, the effects of age on the temporal evolution of isometric and swallowing pressure seems to progress with time.12-15 Thus, many asymptomatic elderly persons—84% in one report—demonstrate videofluoro-scopic changes in pharyngeal swallow compared with what is considered normal in healthy young adults.16 However, in otherwise healthy elderly persons, effects of aging on swallow remain compensated without reaching a symptomatic level. Despite radiographically documented prolongation of the oral14 and pharyngeal12 phases, the coordination among motor events measured manometrically is affected little if any14,17 and pharyngeal swallow remains remarkably robust in otherwise healthy elderly persons, as indicated by relative preservation of bolus clearance.18 Therefore, despite physiologic changes in the swallowing mechanism due to aging, dysphagia cannot be attributed to normal aging alone, and its presence suggests the need for investigation to identify potentially treatable causes.
What Are Common Causes of Dysphagia in the Elderly?
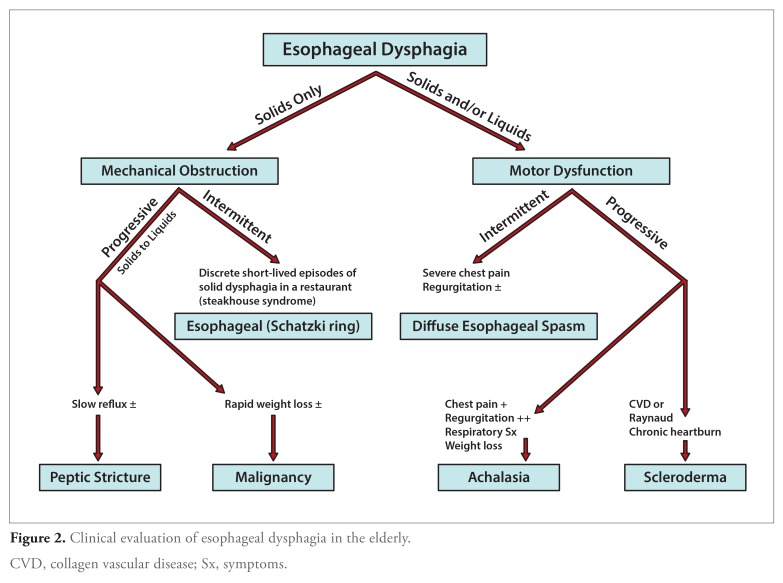
Diverse disease entities associated with either oropharyngeal (Table 1) or esophageal (Table 2) dysfunction may cause dysphagia in the elderly. Oropharyngeal dysphagia in the elderly is most commonly caused by stroke, occurring in one-third of all stroke patients.19-21 Esophageal dysphagia may result from a number of motor or mechanical causes (Table 2 and Figure 2). In some patients, no cause can be identified, and such cases have been categorized as functional dysphagia.22
Table 1.
Etiology of Oropharyngeal Dysphagia
| Structural (Mechanical) Disorders |
| — Zenker diverticulum (Killian dehiscence) |
| — Lateral pharyngeal pouch or diverticula |
| — Cricopharyngeal achalasia, bar, rings, and stenosis |
| — Proximal esophageal web (Plummer-Vinson) |
| — Oropharyngeal and laryngeal tumors |
| — Head and neck surgery |
| — Radiotherapy |
| — Extrinsic compression (cervical osteophytes, skeletal abnormalities, and thyromegaly) |
| Motor Disorders |
| Central Nervous System |
| — Stroke |
| — Head trauma |
| — Brain tumors |
| — Extrapyramidal syndromes (Parkinson disease, Huntington disease, and Wilson disease) |
| — Multiple sclerosis |
| — Cerebral palsy |
| — Dementia |
| — Metabolic encephalopathies |
| — Tardive dyskinesia (phenothiazine use) |
| Peripheral Nervous System |
| — Amyotrophic lateral sclerosis |
| — Bulbar poliomyelitis |
| — Tabes dorsalis |
| — Guillain-Barré syndrome |
| — Drugs (botulinum toxin, procainamide, and cytotoxins) |
| Myogenic |
| — Myasthenia gravis |
| — Dermatomyositis, polymyositis |
| — Mixed connective tissue disease |
| — Hyperthyroidism, hypothyroidism |
| — Cushing syndrome |
| — Amyloidosis |
| — Paraneoplastic syndromes |
| — Drugs (amiodarone, alcohol, and statins) |
Table 2.
Etiology of Esophageal Dysphagia
| Structural (Mechanical) Disorders |
| Intrinsic Encroachment |
| — Mucosal rings and webs: Schatzki, Plummer-Vinson, or multiringed esophagus (eosinophilic esophagitis) |
| — Strictures (inflammatory or fibrotic): peptic, caustic, pill, or radiation-induced |
| — Esophageal tumors: adenocarcinoma, squamous cell carcinoma, metastatic (breast or melanoma), leiomyoma, lymphoma, or granular cell tumor |
| — Systemic diseases: scleroderma (multifactorial), pemphigus/pemphigoid, lichen planus, or Crohn’s disease |
| — Miscellaneous: postsurgery (laryngeal, esophageal, or gastric cancers), acute esophageal infections, esophageal diverticulae, or foreign bodies |
| Extrinsic Compression |
| — Mediastinal masses: lung cancer, lymphoma, lymph node, or thyromegaly |
| — Vascular compression: dysphagia lusoria (aberrant right subclavian artery), dysphagia aortica (right-sided aorta), or cardio-megaly (enlarged left atrium) |
| — Miscellaneous: cervical spine osteophytes/spondylosis or fundoplication |
| Motor Disorders |
| — Primary: achalasia, diffuse esophageal spasm, hypertensive lower esophageal sphincter, ineffective esophageal motility disorder, or nutcracker esophagus |
| — Secondary: connective tissue diseases, scleroderma, CREST syndrome, diabetes, Chagas disease, or paraneoplastic syndrome |
CREST, calcinosis, Raynaud syndrome, esophageal dysmotility, sclerodactyly, telangiectasia.
Figure 2.
Clinical evaluation of esophageal dysphagia in the elderly.
CVD, collagen vascular disease; Sx, symptoms.
Despite diverse etiologies of dysphagia, mechanistically, swallowing disruption is caused by either disordered motility or encroachment from within or outside (mechanical dysphagia) the oropharyngoesophageal bolus conduit. Identifying the distinctions between motor and mechanical or oropharyngeal and esophageal dysphagia is not only important in relation to etiology but is key in management.
What Are the Important Diagnostic Clues?
The evaluation and management of dysphagia is a multidimensional task and often requires a multidisciplinary approach. Important initial steps include confirming the presence of a swallowing dysfunction; defining its anatomic level (oropharyngeal vs esophageal), its mechanism (motor vs mechanical), and underlying specific etiology; and ascertaining the integrity of oropharyngeal swallow and the degree of risk or presence of silent or overt aspiration. Subsequent assessment must determine the patients abilities and impairments and the degree to which these impairments can be improved.
A careful history remains the cornerstone of any dysphagia evaluation,23 and a thorough review of symptoms can differentiate esophageal from oropharyngeal dysphagia24 and predict the specific cause of dysphagia with an accuracy of approximately 80% confirmed by specific testing.25,26 How a patient describes his or her difficulty and its timing, associated symptoms, and other characterizations (Table 3) may specifically denote the anatomic level of swallowing dysfunction. Although symptoms of chronic heartburn, hematemesis, coffee ground emesis, and anemia in a patient with dysphagia point to the presence of complications of gastroesophageal reflux disease (GERD), such as erosive esophagitis, peptic stricture, and esophageal adenocarcinoma, neither the presence nor absence of the symptom of heartburn has significant diagnostic value. At least one-third of patients with esophageal adenocarcinoma27 and approximately one-fourth of patients with peptic stricture28 have been shown to have no heartburn prior to diagnosis. On the other hand, more than 40% of patients with achalasia have a history of heartburn, although its cause remains controversial.29
Table 3.
Anatomic Location of Swallowing Dysfunction Based on Patients’ Description
| Oropharyngeal | Esophageal | |
|---|---|---|
| What does it feel like? | — The patient cannot initiate a swallow, or food feels like it is hanging in the neck. | — After swallowing, food sticks behind the sternum or in the epigastrium or, less commonly, in the neck. |
| When does it occur? | — Within 1 second of an attempted swallow | — A few seconds after swallowing |
| Are there associated symptoms or conditions? |
|
|
| How is relief achieved after the bolus impaction? |
|
— Regurgitation or vomiting |
| Do you have a systemic illness? | — Stroke, Parkinson disease, myasthenia, multiple or amyotrophic lateral sclerosis, thyrotoxicosis, and other related conditions | — Collagen vascular diseases such as scleroderma, CREST syndrome, rheumatoid arthritis, systemic lupus erythematosus, and Sjogren syndrome |
CREST, calcinosis, Raynaud syndrome, esophageal dysmotility, sclerodactyly, telangiectasia; GERD, gastroesophageal reflux disease
Which Medications Might Affect the Swallowing Function in the Elderly?
Drugs may affect the swallowing function in 3 major ways.30 A number of commonly used medications, through pharmacologic actions on the central nervous system, neuromuscular transmission, or myotoxic effects, may inhibit smooth and striated muscle function, hampering swallow activity and bolus transit and reducing lower esophageal sphincter tone. Both mechanisms may add up to an increased incidence and severity of GERD and peptic stricture. Additionally, drug-related xerostomia may affect the ability to chew foods, initiate swallows, and form and transport bolus. Furthermore, there is an increased risk of mouth infections, and patients may have difficulty in retaining their dentures.31
Dysphagia also may result as an adverse effect or complication of medications. Chemotherapy, immunosuppression, and long-term antibiotic therapy are well known to facilitate opportunistic esophageal infections and stricture formation. Sulfa-containing drugs also have been associated with systemic allergic complications such as Stevens-Johnson syndrome32 and may involve the food pipe.
Medication-induced esophageal injury usually caused by local irritation of the esophageal mucosa and referred to as pill esophagitis33-37 may lead to swallowing difficulties. In the United States, pill esophagitis is mostly caused by tetracyclines, potassium chloride formulations, nonsteroidal anti-inflammatory drugs, alendronate, and quinidine.38,39 The most common site for pill-associated esophagitis is near the level of the aortic arch, an area characterized by compression from the arch, skeletal to smooth muscle transition, and physiologic reduction in the amplitude of the esophageal peristaltic wave.40 The risk of the development of pill esophagitis is enhanced if medications are taken in the supine position and prior to sleeping,34 as the frequency of swallowing and the ability of the saliva to dilute medications in the esophagus are diminished during sleep.41,42 Other factors include drug intake without sufficient water/fluid, polypharmacy, the size and shape of a pill,43 esophageal motility disorders or compression,44-46 or entrapment by fixed mediastinal structures and adhesions following prior chest surgery.47-49
Elderly patients are particularly at risk for development of drug-related dysphagia and pill esophagitis because they consume more medications and are more likely to have anatomic and motility abnormalities, cardiac enlargement with concomitant compression of the mid-esophagus, and decreased saliva production.50,51 Thus, a detailed review of medications and when they are taken is an important component of dysphagia evaluation.
How Important Is the Physical Examination?
A comprehensive physical examination should be part of the initial evaluation of all dysphagic patients.52,53 Information regarding the patients speech and cognitive abilities, behavioral dysfunction, strength, and range of movement of the muscles involved in speech and swallowing directly influences decisions about the patients suitability for swallowing therapy and the type of therapy chosen.54,55
Examination of the oral cavity, head, and neck may reveal poor dentition, masses, lymphadenopathy, goiter, pharyngeal pouch, signs of thyrotoxicosis (thyrotoxic myopathy),56 or signs of prior surgery, tracheostomy, and radiotherapy. Diffuse dental erosions may point to underlying GERD. A palpable supraclavicular (Virchow) lymph node may suggest dysphagia caused by an abdominal malignancy (eg, adenocarcinoma of the esophagogastric junction). One can check laryngeal ascent by placing the index and middle fingers lightly on the hyoid and laryngeal cartilages, respectively, and asking the patient to swallow. Reduced laryngeal ascent is frequently seen in neurologic dysphagia. Physical features of collagen-vascular diseases, when present, may provide further evidence of esophageal dysfunction.
A neurologic examination is mandatory for evaluation of every dysphagia case of unknown cause and should include testing of all cranial nerves, particularly the sensory (cranial nerves V, IX, and X) and motor (cranial nerves V, VII, X, XI, and XII) components involved in swallowing. The presence of tremors, cogwheeling, rigidity, and gait disturbances may indicate PD, which is highly associated with dysphagia.57,58 The combination of motor and sensory abnormalities, particularly in the setting of longer disease duration and significant motor abnormalities, may suggest multiple sclerosis.59 Proximal muscle weakness may be due to dermatomyositis or polymyositis. Myasthenia gravis is frequently associated (up to 70% of cases) with facial and pharyngeal weakness, and dysphagia is seen in approximately 30% of myasthenics.60-62
What Is the Role of Laboratory Evaluation?
In most instances, the underlying cause of dysphagia is readily evident after obtaining a patients history and performing a physical examination. Laboratory measurements may be helpful in confirming the provisional diagnoses of dysphagia or in detecting myasthenia gravis, inflammatory myopathies, or toxic myopathies (eg, thyrotoxic or myxedema myopathy). Serologic detection of acetylcholine receptor (AChR) antibodies is virtually diagnostic of myasthenia gravis. However, AChR antibodies are absent in approximately half of myasthenics without typical ocular findings. In such cases, the tensilon (edrophonium) stimulation test can be used. Alternatively, a diagnosis may be reached by using repetitive nerve stimulation or single fiber electromyography.63,64 Muscle enzyme essays that measure, for example, serum creatinine phosphokinase or the presence of antinuclear antibodies can be helpful in diagnosing inflammatory myopathies. Needle electromyography is useful in differentiating inflammatory myosites from neurogenic disorders but is not absolutely specific.65 For a definitive diagnosis of myositis and to differentiate der-matomyositis, polymyositis, and inclusion body myositis, a muscle biopsy may be needed.66 Thyroid function tests are helpful in diagnosing myxedema and thyrotoxicosis. Thyrotoxicosis is a reversible cause of dysphagia and should always be considered in the differential diagnosis, particularly in elderly patients in whom the more classical signs of thyrotoxicosis are usually absent.67
What Tests Should Be Performed?
Five major tests are currently in use for estimating and quantifying swallowing dysfunction: barium radiography, a videofluoroscopic swallowing study (VFSS; modified barium swallow), fiberoptic endoscopic evaluation of swallowing (FEES), upper endoscopy, and esophageal manometry. These tools are aimed at obtaining objective measurements of the timing,68-71 pressure,72,73 range,74-76 and strength77,78 of movements of the swallowing apparatus, bolus flow, and clearance79-82; sensation83-85; and airway protection along with the risk or presence of aspiration.63
VFSS, often referred to as modified barium swallow, remains the most commonly used initial study for evaluating oropharyngeal dysphagia.54,86 This study is highly sensitive and can guide management for most of these patients, as it provides critical information about all 4 categories of oropharyngeal swallowing dysfunction: the inability or excessive delay in initiation of pharyngeal swallowing, aspiration of ingestate, nasopharyngeal regurgitation, and residue of ingestate within the pharyngeal cavity after swallowing. First introduced in 1988, FEES has evolved as a quick, valid, safe, low-cost, and precise bedside technique to evaluate dysphagia in all age groups and in all settings, including nursing homes and long-term care facilities.87-89 Based on fiberoptic endoscopy findings in acute stroke patients, different dysphagia severity scales have been developed to help predict the outcome and intercurrent complications.90 Despite controversies regarding their respective strengths and limitations,91-93 the 2 modalities appear to be complementary rather than substitutes for each other. Whenever there is a clinical suspicion of mechanical oropharyngeal dysphagia, the VFSS should be the first study used because it could provide an anatomic road map for subsequent FEES.
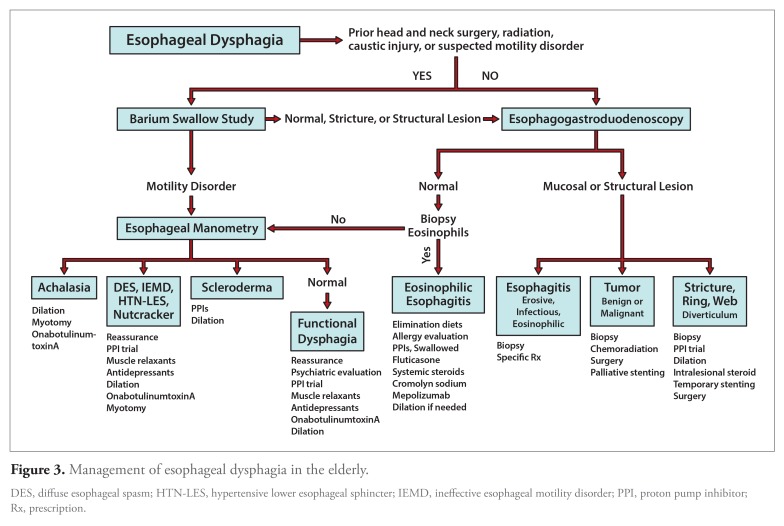
In most cases of esophageal dysphagia, diagnosis is accomplished with a careful history and endoscopy. Expert opinions are divided on whether endoscopy or barium study should be the initial evaluation in patients with suspected esophageal dysphagia (Figure 3). Endoscopy is virtually always needed in the evaluation of esophageal dysphagia, allows tissue sampling, and, in many cases, is therapeutic, obviating the need for further evaluation. Given that advantage, most US clinicians use endoscopy as the initial diagnostic study.94 In cases in which the clinical scenario suggests a proximal esophageal lesion (for example, prior neck surgery or radiation or the presence of a Zenker diverticulum), a complex stricture (caustic ingestion or radiation), or achalasia, a barium swallow may be a more sensitive and safer investigation prior to attempting an endoscopy.95 The sensitivity of a barium study for detecting structural and functional lesions can be further enhanced through a Valsalva maneuver or swallowing a solid bolus such as a marshmallow.96
Figure 3.
Management of esophageal dysphagia in the elderly.
DES, diffuse esophageal spasm; HTN-LES, hypertensive lower esophageal sphincter; IEMD, ineffective esophageal motility disorder; PPI, proton pump inhibitor; Rx, prescription.
Initial barium swallow provides a “road map” for subsequent endoscopy in the subgroup of patients with suspected complicated stricturing. In other cases, it is reasonable to proceed directly to endoscopy.97 Endoscopy remains the initial study of choice for evaluating cases of odynophagia because it allows the tissue sampling needed to differentiate esophageal neoplastic lesions from infectious, pill-induced, or peptic esophagitis.98 In an elderly patient with dysphagia and weight loss, endoscopic assessment becomes paramount because malignancy is high as a differential diagnosis.
Esophageal manometry is the gold-standard technique for evaluating esophageal motor dysfunction and is useful for establishing the diagnoses of achalasia and its variants, diffuse esophageal spasm, and ineffective esophageal motility disorder as well as identifying esophageal hypomotility associated with scleroderma and other collagen vascular diseases.99-101 Esophageal manometry is also indicated in patients with a nondiagnostic barium swallow and/or upper endoscopy, and esophageal motility abnormalities have been reported in up to 90% of such cases.102
How Should Oropharyngeal Dysphagia in Elderly Patients Be Treated?
VFSS or FEES may readily identify pharyngeal or crico-pharyngeal strictures, oropharyngeal neoplasms, posterior hypopharyngeal (Zenker) diverticula, and cervical webs. These mechanical abnormalities may require surgery, dilatation, chemotherapy, radiation therapy, or a combination of these modalities. Esophageal dilatation is usually safe and effective in 75% of cases of benign strictures or webs.103 Dilatation or cricopharyngeal myotomy (open or endoscopic with or without diverticulectomy of associated Zenker diverticulum) may be helpful in 98% of patients with upper esophageal sphincter dysfunction.104-106 Other structural abnormalities include cricopharyngeal bars, cervical osteophytes, or skeletal hyperostoses, and lateral pharyngeal diverticula also may be commonly identified during swallowing evaluation. However, these abnormalities are less convincingly linked to oropharyngeal dysphagia.
Cricopharyngeal bars are found in 5% to 19% of the patients undergoing videofluoroscopy and are controversially linked with dysphagia. They occur with equal frequency in nondysphagic persons,107 and in an overwhelming majority of dysphagic patients, they are accompanied by other esophageal pathologies that can instigate dysphagia.108 However, cricopharyngeal bars have been linked to upper esophageal sphincter dysfunction,109,110 and in some cases, cricopharyngeal myotomy can relieve the dysphagia.111 Thus, given the conflicting reports, the pathophysiologic significance of cricopharyngeal bars and the decision to treat should be guided by the particular clinical scenarios. Cervical osteophytes are also common incidental findings seen in 6% to 30% of elderly patients with dysphagia,112 but only 0.7% of patients with cervical disc disease report dysphagia.113 Surgery for cervical osteophytes is controversial,114 associated with complications,115 and should be reserved for severe cases of dysphagia that have failed conservative treatment.116 Lateral pharyngeal diverticula are found in up to 50% of asymptomatic patients.117 Despite sporadic reports of successful surgical ligation,118 the association with dysphagia is controversial.117
The resolution of pharyngeal dysfunction with return to the euthyroid state has been reported in thyrotoxicosis.56 Likewise, immunosuppressive therapy for inflammatory myopathies is associated with improved swallowing function.119,120 In contrast to the thyrotoxic and inflammatory myopathies, the management of dysphagia with neuromuscular causes poses a significant challenge in most patients. In this group, the severity of swallowing dysfunction is neither related to the severity of underlying disease nor has specific drug therapy predictably helped swallowing dysfunction.
Patients with PD frequently complain of swallowing difficulties (18.5% to 100%).121-123 Additionally, more than 50% of the patients who do not report swallowing impairments have impaired swallowing on objective evaluation,124 and the risk of silent aspiration is a great concern in these patients.125,126 The pathophysiology of dysphagia in this group of patients is not yet clear but appears to involve a nondopaminergic pathway124,127 which may explain its relative unresponsiveness to anti—parkinsonian drug therapy.128
The diagnosis of myasthenia gravis always warrants specific therapy, search for thymoma, and every precaution for preventing myasthenic crisis. However, like in PD, improvement in swallowing dysfunction is less satisfactory than other manifestations of the disease. A recent report indicates that the FEES-Tensilon Test may be a suitable tool in the diagnosis and therapy of myasthenia gravis with pharyngeal muscle weakness.129 However, these results need to be confirmed through large studies.
What Is the Value of Swallow Rehabilitation in the Elderly?
Most elderly patients with neurogenic dysphagia will require swallowing therapy. During swallow training, 2 parallel courses are often tried, which are having the patient 1.) eat some foods orally while preventing aspiration via compensatory postural techniques, sensory stimuli, voluntary swallow maneuvers, and dietary changes and 2.) exercise to build up strength and coordination to regain full swallowing function without compensation. The effectiveness of different compensatory techniques for a given patient can be assessed during instrumental evaluation. However, little data are available on the long-term effectiveness of various treatment protocols in terms of optimal frequency and duration of treatment.
Five postural techniques (chin-down, chin-up, head turned, head tilted, and lying down) and several postural combinations are currently used for swallow compensation, with reported efficacy in several populations.130 Each posture has a specific effect in terms of flow of food and relationship of oropharyngeal structures and can provide optimal compensation in patients with specific defects in oropharyngeal swallow. For example, the chin-down posture is well suited in patients with a tongue base disorder, and a reclining posture is useful in patients with bilateral pharyngeal damage or reduced laryngeal elevation. Chemical, thermal, and tactile stimulation through changing the taste, volume, temperature, and carbonation of the food (bolus) and even additional pressure on the tongue with a spoon as food is presented have been effectively used to modulate human swallowing behavior.131,132 Recently, taste stimuli,133 tactile thermal oral stimulation,134 and pharyngeal electrical stimulation135 have been shown to modulate swallowing motor pathways, cortical representation of swallowing, and reverse swallowing disability. Like postural techniques, effectiveness of sensory stimuli can be assessed during instrumental evaluation. Some swallow maneuvers such as the supraglottic, super-supraglottic, and effortful swallow and the Mendelsohn maneuver have been used by normal subjects and dysphagic patients to compensate for pharyngeal swallow and are employed in swallow rehabilitation.136-138 All of these voluntary maneuvers result in specific changes in pharyngeal swallow, but few studies have examined optimal protocols for treating various types of dysphagic patients in terms of frequency and duration of treatment. Dietary changes, particularly thickened liquid diets, are commonly used to prevent liquid aspiration in patients with oropharyngeal dysphagia.139 However, results from a recent clinical trial indicate that thickening liquids to various viscosities may not provide greater safety.140
Several range-of-motion exercises are used to boost pharyngeal swallow. However, the efficacy is not immediate. Each exercise program is focused on a particular component of oropharyngeal swallow and takes several weeks (1 to 6) to be effective. For example, tongue-strengthening exercises are aimed at improving oral and pharyngeal transit times and have been reported to be beneficial in dysphagic patients who have had strokes141 and in patients who have been treated for head and neck cancer.142 Likewise, Shaker exercise is aimed at improving hypopharyngeal movement and upper esophageal sphincter opening and has been reported to be effective in preventing aspiration in a small but multi-institutional randomized trial.143 However, further research is needed to identify the subsets of dysphagic patients who can benefit most from various exercises.144
Conclusion
In summary, dysphagia in the elderly is increasingly recognized as an important national healthcare concern with enormous cost. Aging may adversely affect all components of swallowing function. The elderly are at increased risk for development of dysphagia, as illnesses affecting the swallowing mechanism are more common in their population group. Dysphagia evaluation and management are usually a multidisciplinary team effort and are based on careful history, differentiation of oropharyngeal dysphagia from esophageal dysphagia and motor dysphagia from mechanical dysphagia, identifying the underlying cause, ascertaining the degree of risk or presence of silent or overt aspiration, and defining the patients abilities and impairments and the degree to which the impairments can be improved. There are no standard algorithmic approaches for managing elderly patients with dysphagia; rather, goals and plans are individualized to fit given clinical scenarios. Most patients with oropharyngeal dysphagia would benefit from a swallow rehabilitation program even if the underlying pathology was not amenable to treatment. Physicians should inquire about dysphagia in their elderly patients and exert a heightened awareness of life-altering diagnoses and disease-guided therapy with the goal of improving patients’ symptoms and quality of life.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ., III Onset and disappearance of gastrointestinal symptoms and functional gastrointestinal disorders. Am J Epidemiol. 1992;136(2):165–177. doi: 10.1093/oxfordjournals.aje.a116483. [DOI] [PubMed] [Google Scholar]
- 2.Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116(2):455–478. doi: 10.1016/s0016-5085(99)70144-7. [DOI] [PubMed] [Google Scholar]
- 3.Lindgren S, Janzon L. Prevalence of swallowing complaints and clinical findings among 50-79-year-old men and women in an urban population. Dysphagia. 1991;6(4):187–192. doi: 10.1007/BF02493524. [DOI] [PubMed] [Google Scholar]
- 4.Barczi SR, Sullivan PA, Robbins J. How should dysphagia care of older adults differ? Establishing optimal practice patterns. Semin Speech Lang. 2000;21(4):347–361. doi: 10.1055/s-2000-8387. [DOI] [PubMed] [Google Scholar]
- 5.Siebens H, Trupe E, Siebens A, et al. Correlates and consequences of eating dependency in institutionalized elderly. J Am Geriatr Soc. 1986;34(3):192–198. doi: 10.1111/j.1532-5415.1986.tb04202.x. [DOI] [PubMed] [Google Scholar]
- 6. [October 28, 2013]. http://www.census.gov/prod/2010pubs/p25-1138.pdf The next four decades, the older population in the United States: 2010 to 2050. United States Census Bureau.
- 7.Jones B, editor. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. 2nd ed. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 8.Hamdy S, Aziz Q, Rothwell JC, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 9.Masoro EJ. Biology of aging. Current state of knowledge. Arch Intern Med. 1987;147(1):166–169. [PubMed] [Google Scholar]
- 10.Shaker R, Staff D. Esophageal disorders in the elderly. Gastroenterol Clin North Am. 2001;30(2):335–361. doi: 10.1016/s0889-8553(05)70185-0. vii-viii. [DOI] [PubMed] [Google Scholar]
- 11.Humbert IA, Robbins J. Dysphagia in the elderly. Phys Med Rehabil Clin N Am. 2008;19(4):853–866. doi: 10.1016/j.pmr.2008.06.002. ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 13.Nicosia MA, Hind JA, Roecker EB, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 14.Shaw D, Cook I, Gabb M, et al. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol. 1995;268:G389–G396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 15.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50(5):M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 16.Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR Am J Roentgenol. 1991;156(6):1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- 17.Wilson JA, Pryde A, Macintyre CC, Maran AG, Heading RC. The effects of age, sex, and smoking on normal pharyngoesophageal motility. Am J Gastroenterol. 1990;85(6):686–691. [PubMed] [Google Scholar]
- 18.Cook I, Weltman M, Wallace K, et al. Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: a scintigraphic study. Am J Physiol. 1994;266:G972–G977. doi: 10.1152/ajpgi.1994.266.6.G972. [DOI] [PubMed] [Google Scholar]
- 19.Horner J, Massey EW, Riski JE, Lathrop DL, Chase KN. Aspiration following stroke: clinical correlates and outcome. Neurology. 1988;38(9):1359–1362. doi: 10.1212/wnl.38.9.1359. [DOI] [PubMed] [Google Scholar]
- 20.Young EC, Durant-Jones L. Developing a dysphagia program in an acute care hospital: a needs assessment. Dysphagia. 1990;5(3):159–165. doi: 10.1007/BF02412640. [DOI] [PubMed] [Google Scholar]
- 21.Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J (Clin Res Ed). 1987;295(6595):411–414. doi: 10.1136/bmj.295.6595.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drossman D, editor. Rome III: The Functional Gastrointestinal Disorders. 3rd ed. McLean, VA: Degnon Associates, Inc; 2006. [Google Scholar]
- 23.Trate DM, Parkman HP, Fisher RS. Dysphagia. Evaluation, diagnosis, and treatment. Prim Care. 1996;23(3):4l7–432. doi: 10.1016/s0095-4543(05)70338-9. [DOI] [PubMed] [Google Scholar]
- 24.Rothstein RD. A systematic approach to the patient with dysphagia. Hosp Pract. 1995;1997-32(5):169–175. doi: 10.1080/21548331.1997.11443447. [DOI] [PubMed] [Google Scholar]
- 25.Castell DO, Donner MW. Evaluation of dysphagia: a careful history is crucial. Dysphagia. 1987;2(2):65–71. doi: 10.1007/BF02408136. [DOI] [PubMed] [Google Scholar]
- 26.Barloon TJ, Bergus GR, Lu CC. Diagnostic imaging in the evaluation of dysphagia. Am Fam Physician. 1996;53(2):535–546. [PubMed] [Google Scholar]
- 27.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340(11):825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 28.Nayyar AK, Royston C, Bardhan KD. Oesophageal acid-peptic strictures in the histamine H2 receptor antagonist and proton pump inhibitor era. Dig Liver Dis. 2003;35(3):143–150. doi: 10.1016/s1590-8658(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 29.Spechler SJ, Souza RF, Rosenberg SJ, Ruben RA, Goyal RK. Heartburn in patients with achalasia. Gut. 1995;37(3):305–308. doi: 10.1136/gut.37.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Speech-Language-Hearing Association. Guidelines for speech-language pathologists performing videofluoroscopic swallowing studies. ASLHA Suppl. 2004;24:77–92. [Google Scholar]
- 31.Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401–407. doi: 10.1111/j.1532-5415.1995.tb05815.x. [DOI] [PubMed] [Google Scholar]
- 32.Stein MR, Thompson CK, Jr, Sawicki JE, Martel AJ. Esophageal stricture complicat ing Stevens-Johnson syndrome. A case report. Am J Gastroenterol. 1974;62(5):435–439. [PubMed] [Google Scholar]
- 33.Pemberton J. Oesophageal obstruction and ulceration caused by oral potas sium therapy. Br Heart J. 1970;32(2):267–268. doi: 10.1136/hrt.32.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikendall JW, Friedman AC, Oyewole MA, Fleischer D, Johnson LF. Pill-induced esophageal injury. Case reports and review of the medical literature. Dig Dis Sci. 1983;28(2):174–182. doi: 10.1007/BF01315148. [DOI] [PubMed] [Google Scholar]
- 35.Walta DC, Giddens JD, Johnson LF, Kelley JL, Waugh DF. Localized proximal esophagitis secondary to ascorbic acid ingestion and esophageal motor disorder. Gastroenterology. 1976;70(5 pt l):766–769. [PubMed] [Google Scholar]
- 36.Smith V. Association of aspirin ingestion with symptomatic hiatus hernia. South Med J. 1978;71(suppl 1):45–47. doi: 10.1097/00007611-197801001-00012. [DOI] [PubMed] [Google Scholar]
- 37.Eastwood GL, Castell DO, Higgs RH. Experimental esophagitis in cats impairs lower esophageal sphincter pressure. Gastroenterology. 1975;69(1):146–153. [PubMed] [Google Scholar]
- 38.Kikendall J, Johnson L. Pill-induced esophageal injury. In: Castell DO, editor. The Esophagus. 2nd ed. Boston, MA: Little, Brown; 1995. [Google Scholar]
- 39.Colina RE, Smith M, Kikendall JW, Wong RK. A new probable increasing cause of esophageal ulceration: alendronate. Am J Gastroenterol. 1997;92(4):704–706. [PubMed] [Google Scholar]
- 40.Humphries TJ, Castell DO. Pressure profile of esophageal peristalsis in normal humans as measured by direct intraesophageal transducers. Am J Dig Dis. 1977;22(7):64l–645. doi: 10.1007/BF01073085. [DOI] [PubMed] [Google Scholar]
- 41.Evans KT, Roberts GM. Where do all the tablets go? Lancet. 1976;2(7997):1237–1239. doi: 10.1016/s0140-6736(76)91158-2. [DOI] [PubMed] [Google Scholar]
- 42.Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65(2):256–267. doi: 10.1172/JCI109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hey H, Jørgensen F, Sørensen K, Hasselbalch H, Wamberg T. Oesophageal transit of six commonly used tablets and capsules. Br Med J (Clin Res Ed). 1982;285(6356):1717–1719. doi: 10.1136/bmj.285.6356.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCall AJ. Letter: Slow-k ulceration of oesophagus with aneurysmal left atrium. BMJ. 1975;3(5977):230–231. doi: 10.1136/bmj.3.5977.230-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumithran E, Lim KH, Chiam HL. Atrio-oesophageal fistula complicating mitral valve disease. BMJ. 1979;2(6204):1552–1553. doi: 10.1136/bmj.2.6204.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohane TD, Perrault J, Fowler RS. Oesophagitis and oesophageal obstruction from quinidine tablets in association with left atrial enlargement: a case report. Aust Paediatr J. 1978;14(3):191–192. doi: 10.1111/jpc.1978.14.3.191. [DOI] [PubMed] [Google Scholar]
- 47.Whitney B, Croxon R. Dysphagia caused by cardiac enlargement. Clin Radiol. 1972;23(2):147–152. doi: 10.1016/s0009-9260(72)80079-5. [DOI] [PubMed] [Google Scholar]
- 48.Boyce HW., Jr. Dysphagia after open heart surgery. Hosp Pract (Off Ed). 1985;20(9) doi: 10.1080/21548331.1985.11703127. 40, 43, 47 passim. [DOI] [PubMed] [Google Scholar]
- 49.Teplick JG, Teplick SK, Ominsky SH, Haskin ME. Esophagitis caused by oral medication. Radiology. 1980;134(l):23–25. doi: 10.1148/radiology.134.1.7350610. [DOI] [PubMed] [Google Scholar]
- 50.Stoschus B, Allescher HD. Drug-induced dysphagia. Dysphagia. 1993;8(2):154–159. doi: 10.1007/BF02266997. [DOI] [PubMed] [Google Scholar]
- 51.Mason D, Chisholm D, editors. Salivary Glands in Health and Disease. Philadelphia, PA: WB Saunders; 1975. [Google Scholar]
- 52.Kahrilas PJ. Current investigation of swallowing disorders. Baillieres Clin Gastroenterol. 1994;8(4):651–664. doi: 10.1016/0950-3528(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 53.Hendrix TR. Art and science of history taking in the patient with difficulty swallowing. Dysphagia. 1993;8(2):69–73. doi: 10.1007/BF02266982. [DOI] [PubMed] [Google Scholar]
- 54.Logemann J, editor. Evaluation and Treatment of Swallowing Disorders. San Diego, CA: College Hill; 1983. [Google Scholar]
- 55.Bine JE, Frank EM, McDade HL. Dysphagia and dementia in subjects with Parkinsons disease. Dysphagia. 1995;10(3):160–164. doi: 10.1007/BF00260970. [DOI] [PubMed] [Google Scholar]
- 56.Branski D, Levy J, Globus M, Aviad I, Keren A, Chowers I. Dysphagia as a primary manifestation of hyperthyroidism. J Clin Gastroenterol. 1984;6(5):437–440. doi: 10.1097/00004836-198410000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12(1):11–18. doi: 10.1007/pl00009512. discussion 19-20. [DOI] [PubMed] [Google Scholar]
- 58.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatr Logop. 1994;46(1):9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- 59.Poorjavad M, Derakhshandeh F, Etemadifar M, Soleymani B, Minagar A, Maghzi AH. Oropharyngeal dysphagia in multiple sclerosis. Mult Scler. 2010;16(3):362–365. doi: 10.1177/1352458509358089. [DOI] [PubMed] [Google Scholar]
- 60.Dumitru D, editor. Electrodiagnostic Medicine. Philadelphia, PA: Hanley and Belfus; 1995. [Google Scholar]
- 61.Osserman KE, Genkins G. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med. 1971;38(6):497–537. [PubMed] [Google Scholar]
- 62.Khan OA, Campbell WW. Myasthenia gravis presenting as dysphagia: clinical considerations. Am J Gastroenterol. 1994;89(7):1083–1085. [PubMed] [Google Scholar]
- 63.Newson-Davis J. Myasthenia gravis and related syndromes. In: Walton J, Kar-pati G, Hilton-Jones D, editors. Disorders of Voluntary Muscle. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- 64.Oh SJ, Kim DE, Kuruoglu R, Bradley RJ, Dwyer D. Diagnostic sensitivity of the laboratory tests in myasthenia gravis. Muscle Nerve. 1992;15(6):720–724. doi: 10.1002/mus.880150616. [DOI] [PubMed] [Google Scholar]
- 65.Tymms KE, Webb J. Dermatopolymyositis and other connective tissue diseases: a review of 105 cases. J Rheumatol. 1985;12(6):1140–1148. [PubMed] [Google Scholar]
- 66.Plotz PH, Dalakas M, Leff RL, Love LA, Miller FW, Cronin ME. Current concepts in the idiopathic inflammatory myopathies: polymyositis, dermatomyositis, and related disorders. Ann Intern Med. 1989;111(2):143–157. doi: 10.7326/0003-4819-111-2-143. [DOI] [PubMed] [Google Scholar]
- 67.Sweatman MC, Chambers L. Disordered oesophageal motility in thyrotoxic myopathy. Postgrad Med J. 1985;61(717):619–620. doi: 10.1136/pgmj.61.717.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cook IJ, Dodds WJ, Dantas RO, et al. Timing of videofluoroscopic, mano-metric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4(1):8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 69.Kendall KA, McKenzie S, Leonard RJ, Goncalves MI. Walker A Timing of events in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15(2):74–83. doi: 10.1007/s004550010004. [DOI] [PubMed] [Google Scholar]
- 70.Martin-Harris B, Brodsky M, Price C, et al. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Phys. 2003;94(5):1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 71.Van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of glottic closure during swallowing: a combined electromyographic and endoscopic analysis. Ann Otol Rhinol Laryngol. 2005;114(6):478–487. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- 72.Castell JA, Castell DO, Schultz AR, Georgeson S. Effect of head position on the dynamics of the upper esophageal sphincter and pharynx. Dysphagia. 1993;8(1):1–6. doi: 10.1007/BF01351470. [DOI] [PubMed] [Google Scholar]
- 73.Steele CM, Huckabee ML. The influence of orolingual pressure on the timing of pharyngeal pressure events. Dysphagia. 2007;22(1):30–36. doi: 10.1007/s00455-006-9037-4. [DOI] [PubMed] [Google Scholar]
- 74.Green JR, Wang YT. Tongue-surface movement patterns during speech and swallowing. J Acoust Soc Am. 2003;113(5):2820–2833. doi: 10.1121/1.1562646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43(5):1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 76.Logemann JA, Pauloski BR, Rademaker AW, Kahrilas PJ. Oropharyngeal swallow in younger and older women: videofluoroscopic analysis. J Speech Lang Hear Res. 2002;45(3):434–445. doi: 10.1044/1092-4388(2002/034). [DOI] [PubMed] [Google Scholar]
- 77.Leonard R, Belafsky PC, Rees CJ. Relationship between fluoroscopic and manometric measures of pharyngeal constriction: the pharyngeal constriction ratio. Ann Otol Rhinol Laryngol. 2006;115(12):897–901. doi: 10.1177/000348940611501207. [DOI] [PubMed] [Google Scholar]
- 78.Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22(3):251–265. doi: 10.1007/s00455-006-9074-z. [DOI] [PubMed] [Google Scholar]
- 79.Cerenko D, McConnel FM, Jackson RT. Quantitative assessment of pharyngeal bolus driving forces. Otolaryngol Head Neck Surg. 1989;100(1):57–63. doi: 10.1177/019459988910000109. [DOI] [PubMed] [Google Scholar]
- 80.Daniels SK, Schroeder MF, DeGeorge PC, Corey DM, Rosenbek JC. Effects of verbal cue on bolus flow during swallowing. Am J Speech Lang Pathol. 2007;16(2):140–147. doi: 10.1044/1058-0360(2007/018). [DOI] [PubMed] [Google Scholar]
- 81.Johnsson F, Shaw D, Gabb M, Dent J, Cook I. Influence of gravity and body position on normal oropharyngeal swallowing. Am J Physiol. 1995;269(5 pt 1):G653–G658. doi: 10.1152/ajpgi.1995.269.5.G653. [DOI] [PubMed] [Google Scholar]
- 82.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103(1):128–136. doi: 10.1016/0016-5085(92)91105-d. [DOI] [PubMed] [Google Scholar]
- 83.Aviv JE, Martin JH, Keen MS, Debell M, Blitzer A. Air pulse quantification of supraglottic and pharyngeal sensation: a new technique. Ann Otol Rhinol Laryngol. 1993;102(10):777–780. doi: 10.1177/000348949310201007. [DOI] [PubMed] [Google Scholar]
- 84.Leow LP, Huckabee ML, Sharma S. Tooley TP The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses. 2007;32(2):119–128. doi: 10.1093/chemse/bjl037. [DOI] [PubMed] [Google Scholar]
- 85.Pelletier CA, Dhanaraj GE. The effect of taste and palatability on lingual swallowing pressure. Dysphagia. 2006;21(2):121–128. doi: 10.1007/s00455-006-9020-0. [DOI] [PubMed] [Google Scholar]
- 86.Martin-Harris B, Jones B. The videofluorographic swallowing study. Phys Med Rehabil Clin N Am. 2008;19(4):769–785. doi: 10.1016/j.pmr.2008.06.004. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warnecke T, Teismann I, Oelenberg S, et al. The safety of fiberoptic endoscopic evaluation of swallowing in acute stroke patients. Stroke. 2009;40(2):482–486. doi: 10.1161/STROKEAHA.108.520775. [DOI] [PubMed] [Google Scholar]
- 88.Dziewas R, Warnecke T, Olenberg S, et al. Towards a basic endoscopic assessment of swallowing in acute stroke—development and evaluation of a simple dysphagia score. Cerebrovasc Dis. 2008;26(1):4l–47. doi: 10.1159/000135652. [DOI] [PubMed] [Google Scholar]
- 89.Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing comparer. Laryngoscope. 2007;117(10):1723–1727. doi: 10.1097/MLG.0b013e318123ee6a. [DOI] [PubMed] [Google Scholar]
- 90.Warnecke T, Ritter MA, Kroger B, et al. Fiberoptic endoscopic dysphagia severity scale predicts outcome after acute stroke. Cerebrovasc Dis. 2009;28(3):283–289. doi: 10.1159/000228711. [DOI] [PubMed] [Google Scholar]
- 91.Langmore SE. Evaluation of oropharyngeal dysphagia: which diagnostic tool is superior? Curr Opin Otolaryngol Head Neck Surg. 2003;ll(6):485–489. doi: 10.1097/00020840-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 92.Logemann JA, Rademaker AW, Pauloski BR, Ohmae Y, Kahrilas PJ. Normal swallowing physiology as viewed by videofluoroscopy and videoendoscopy. Folia Phoniatr Logop. 1998;50(6):311–319. doi: 10.1159/000021473. [DOI] [PubMed] [Google Scholar]
- 93.Aviv JE. Prospective, randomized outcome study of endoscopy versus modified barium swallow in patients with dysphagia. Laryngoscope. 2000;110(4):563–574. doi: 10.1097/00005537-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 94.Lieberman DA, De Garmo PL, Fleischer DE, Eisen GM, Helfand M. Patterns of endoscopy use in the United States. Gastroenterology. 2000;118(3):619–624. doi: 10.1016/s0016-5085(00)70269-1. [DOI] [PubMed] [Google Scholar]
- 95.Spechler SJ. AGA technical review on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology. 1999;117(1):233–254. doi: 10.1016/s0016-5085(99)70573-1. [DOI] [PubMed] [Google Scholar]
- 96.Ott DJ, Kelley TF, Chen MY, Gelfand DW, Wu WC. Use of a marshmallow bolus for evaluating lower esophageal mucosal rings. Am] Gastroenterol. 1991;86(7):817–820. [PubMed] [Google Scholar]
- 97.Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc. 2005;61(7):804–808. doi: 10.1016/s0016-5107(05)00297-x. [DOI] [PubMed] [Google Scholar]
- 98.Ott DJ. Radiographic techniques and efficacy in evaluating esophageal dysphagia. Dysphagia. 1990;5(4):192–203. doi: 10.1007/BF02412687. [DOI] [PubMed] [Google Scholar]
- 99.Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology. 1994;107(6):1865–1884. doi: 10.1016/0016-5085(94)90835-4. [DOI] [PubMed] [Google Scholar]
- 100.Pandolfino JE, Kahrilas PJ. American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128(1):209–224. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49(1):145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gambitta P, Indriolo A, Grosso C, Pirone Z, Colombo P, Arcidiacono R. Role of oesophageal manometry in clinical practice. Dis Esophagus. 1999;12(1):4l–46. doi: 10.1046/j.1442-2050.1999.00013.x. [DOI] [PubMed] [Google Scholar]
- 103.Lindgren S. Endoscopic dilatation and surgical myectomy of symptomatic cervical esophageal webs. Dysphagia. 1991;6(4):235–238. doi: 10.1007/BF02493534. [DOI] [PubMed] [Google Scholar]
- 104.Shaw DW, Cook IJ, Jamieson GG, Gabb M, Simula ME, Dent J. Influence of surgery on deglutitive upper oesophageal sphincter mechanics in Zenker’s diverticulum. Gut. 1996;38(6):806–811. doi: 10.1136/gut.38.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatlebakk JG, Castell JA, Spiegel J, Paoletti V, Katz PO, Castell DO. Dilatation therapy for dysphagia in patients with upper esophageal sphincter dysfunction— manometric and symptomatic response. Dis Esophagus. 1998;11(4):254–259. doi: 10.1093/dote/11.4.254. [DOI] [PubMed] [Google Scholar]
- 106.Mason RJ, Bremner CG, DeMeester TR, et al. Pharyngeal swallowing disorders: selection for and outcome after myotomy. Ann Surg. 1998;228(4):598–608. doi: 10.1097/00000658-199810000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Curtis DJ, Cruess DF, Berg T. The cricopharyngeal muscle: a videorecording review. AJR Am J Roentgenol. 1984;l42(3):497–500. doi: 10.2214/ajr.142.3.497. [DOI] [PubMed] [Google Scholar]
- 108.Jones B, Ravich WJ, Donner MW, Kramer SS, Hendrix TR. Pharyngoesoph-ageal interrelationships: observations and working concepts. Gastrointest Radiol. 1985;10(3):225–233. doi: 10.1007/BF01893105. [DOI] [PubMed] [Google Scholar]
- 109.Sokol EM, Heitmann P, Wolf BS, Cohen BR. Simultaneous cineradiographic and manometric study of the pharynx, hypopharynx, and cervical esophagus. Gastroenterology. 1966;51(6):960–974. [PubMed] [Google Scholar]
- 110.Dantas RO, Cook IJ, Dodds WJ, Kern MK, Lang IM, Brasseur JG. Biomechanics of cricopharyngeal bars. Gastroenterology. 1990;99(5):1269–1274. doi: 10.1016/0016-5085(90)91149-z. [DOI] [PubMed] [Google Scholar]
- 111.Cruse JP, Edwards DA, Smith JF, Wyllie JH. The pathology of a cricopharyngeal dysphagia. Histopathology. 1979;3(3):223–232. doi: 10.1111/j.1365-2559.1979.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 112.Saffouri MH, Ward PH. Surgical correction of dysphagia due to cervical osteophytes. Ann Otol Rhinol Laryngol. 1974;83(1):65–70. doi: 10.1177/000348947408300111. [DOI] [PubMed] [Google Scholar]
- 113.Stuart D. Dysphagia due to cervical osteophytes. A description of five patients and a review of the literature. Int Orthop. 1989;13(2):95–99. doi: 10.1007/BF00266369. [DOI] [PubMed] [Google Scholar]
- 114.Kodama M, Sawada H, Udaka F, Kameyama M, Koyama T. Dysphagia caused by an anterior cervical osteophyte: case report. Neuroradiology. 1995;37(1):58–59. doi: 10.1007/BF00588521. [DOI] [PubMed] [Google Scholar]
- 115.Welsh LW, Welsh JJ, Chinnici JC. Dysphagia due to cervical spine surgery. Ann Otol Rhinol Laryngol. 1987;96(1 pt 1):112–115. doi: 10.1177/000348948709600125. [DOI] [PubMed] [Google Scholar]
- 116.Halama AR. Surgical treatment of oropharyngeal swallowing disorders. Acta Otorhinolaryngol Belg. 1994;48(2):217–227. [PubMed] [Google Scholar]
- 117.Curtis DJ, Cruess DF, Crain M, Sivit C, Winters C, Jr, Dachman AH. Lateral pharyngeal outpouchings: a comparison of dysphagic and asymptomatic patients. Dysphagia. 1988;2(3):156–161. doi: 10.1007/BF02424934. [DOI] [PubMed] [Google Scholar]
- 118.Pace-Balzan A, Habashi SM, Nassar WY. View from within: radiology in focus lateral pharyngeal diverticulum. J Laryngol Otol. 1991;105(9):793–795. [PubMed] [Google Scholar]
- 119.Wortmann RL. Inflammatory diseases of muscle and other myopathies. In: Kelley WN, Harris ED, Ruddy S, Sledge CB, editors. Text book of Rheumatology. 5th ed. Philadelphia, PA: WB Saunders Co; 1997. [Google Scholar]
- 120.Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. A’Engl J Med. 1991;325(21):1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- 121.Baijens LW, Speyer R. Effects of therapy for dysphagia in Parkinson’s disease: systematic review. Dysphagia. 2009;24(1):91–102. doi: 10.1007/s00455-008-9180-1. [DOI] [PubMed] [Google Scholar]
- 122.Coates C, Bakheit AM. Dysphagia in Parkinson’s disease. Eur Neurol. 1997;38(1):49–52. doi: 10.1159/000112902. [DOI] [PubMed] [Google Scholar]
- 123.Johnston BT, Li Q, Castell JA, Castell DO. Swallowing and esophageal function in Parkinson’s disease. Am J Gastroenterol. 1995;90(10):174l–1746. [PubMed] [Google Scholar]
- 124.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson’s disease. Age Ageing. 2006;35(6):614–618. doi: 10.1093/ageing/afl105. [DOI] [PubMed] [Google Scholar]
- 125.Nóbrega AC, Rodrigues B, Melo A. Silent aspiration in Parkinson’s disease patients with diurnal sialorrhea. Clin Neurol Neurosurg. 2008;110(2):117–119. doi: 10.1016/j.clineuro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 126.Tjaden K. Speech and swallowing in Parkinson’s disease. Top Geriatr Rehabil. 2008;24(2):115–126. doi: 10.1097/01.TGR.0000318899.87690.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110(2):383–392. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 128.Fuh JL, Lee RC, Wang SJ, et al. Swallowing difficulty in Parkinson’s disease. Clin Neurol Neurosurg. 1997;99(2):106–112. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 129.Warnecke T, Teismann I, Zimmermann J, Oelenberg S, Ringelstein EB, Dziewas R. Fiberoptic endoscopic evaluation of swallowing with simultaneous Tensilon application in diagnosis and therapy of myasthenia gravis. J Neurol. 2008;255(2):224–230. doi: 10.1007/s00415-008-0664-6. [DOI] [PubMed] [Google Scholar]
- 130.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2nd ed. Austin, TX: Pro-Ed Publishers; 1998. [Google Scholar]
- 131.Hamdy S, Jilani S, Price V, Parker C, Hall N, Power M. Modulation of human swallowing behaviour by thermal and chemical stimulation in health and after brain injury. Neurogastroenterol Motil. 2003;15(1):69–77. doi: 10.1046/j.1365-2982.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- 132.Lazzara G, Lazarus C, Logemann J. Impact of thermal stimulation on the triggering of the swallowing reflex. Dysphagia. 1986;l(1):73–77. [Google Scholar]
- 133.Mistry S, Rothwell JC, Thompson DG, Hamdy S. Modulation of human cortical swallowing motor pathways after pleasant and aversive taste stimuli. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G666–G671. doi: 10.1152/ajpgi.00573.2005. [DOI] [PubMed] [Google Scholar]
- 134.Teismann IK, Steinsträter O, Warnecke T, et al. Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. 2009;10(1):71. doi: 10.1186/1471-2202-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jayasekeran V, Singh S, Tyrrell P, et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010;138(5):1737–1746,1746.e2. doi: 10.1053/j.gastro.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 136.Kahrilas PJ, Logemann JA, Gibbons P. Food intake by maneuver; an extreme compensation for impaired swallowing. Dysphagia. 1992;7(3):155–159. doi: 10.1007/BF02493449. [DOI] [PubMed] [Google Scholar]
- 137.Fujiu M, Logemann J, Pauloski B. Increased postoperative posterior pharyngeal wall movement in patients with anterior oral cancer: preliminary findings and possible implications for treatment. Am J Speech Lang Pathol. 1995;4(1):24–30. [Google Scholar]
- 138.Fujiu M, Logemann J. Effect of a tongue holding maneuver on posterior pharyngeal wall movement during deglutition. Am J Speech Lang Pathol. 1996;5(1):23–30. [Google Scholar]
- 139.Groher ME, McKaig TN. Dysphagia and dietary levels in skilled nursing facilities. J Am Geriatr Soc. 1995;43(5):528–532. doi: 10.1111/j.1532-5415.1995.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 140.Logemann JA, Gensler G, Robbins J, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J Speech Lang Hear Res. 2008;51(1):173–183. doi: 10.1044/1092-4388(2008/013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Robbins J, Kays SA, Gangnon RE, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 142.Lazarus C, Logemann JA, Pauloski BR, et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637. doi: 10.1002/hed.20577. [DOI] [PubMed] [Google Scholar]
- 143.Logemann JA, Rademaker A, Pauloski BR, et al. A randomized study comparing the Shaker exercise with traditional therapy: a preliminary study. Dysphagia. 2009;24(4):403–4ll. doi: 10.1007/s00455-009-9217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shaker R, Kern M, Bardan E, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272(6 pt 1):G1518–G1522. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]