Abstract
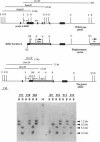
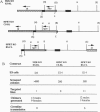
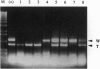
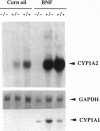
Cytochrome P450 1A2 (CYP1A2) is a predominantly hepatic enzyme known to be important in the metabolism of numerous foreign chemicals of pharmacologic, toxicologic, and carcinogenic significance. CYP1A2 substrates include aflatoxin B1, acetaminophen, and a variety of environmental arylamines. To define better the developmental and metabolic functions of this enzyme, we developed a CYP1A2-deficient mouse line by homologous recombination in embryonic stem cells. Mice homozygous for the targeted Cyp1a2 gene, designated Cyp1a2(-/-), are completely viable and fertile; histologic examination of 15-day embryos, newborn pups, and 3-week-old mice revealed no abnormalities. No CYP1A2 mRNA was detected by Northern blot analysis. Moreover, mRNA levels of Cyp1a1, the other gene in the same subfamily, appear unaffected by loss of the Cyp1a2 gene. Because the muscle relaxant zoxazolamine is a known substrate for CYP1A2, we studied the Cyp1a2(-/-) genotype by using the zoxazolamine paralysis test: the Cyp1a2(-/-) mice exhibited dramatically lengthened paralysis times relative to the Cyp1a2(+/+) wild-type animals, and the Cyp1a2(+/-) heterozygotes showed an intermediate effect. Availability of a viable and fertile CYP1A2-deficient mouse line will provide a valuable tool for researchers wishing to define the precise role of CYP1A2 in numerous metabolic and pharmacokinetic processes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. A., Lang N. P., Young J. F., Caporaso N. E., Vineis P., Hayes R. B., Teitel C. H., Massengill J. P., Lawsen M. F., Kadlubar F. F. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992 Jun;2(3):116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cobb R. R., Stoming T. A., Whitney J. B., 3rd The aryl hydrocarbon hydroxylase (Ah) locus and a novel restriction-fragment length polymorphism (RFLP) are located on mouse chromosome 12. Biochem Genet. 1987 Jun;25(5-6):401–413. doi: 10.1007/BF00554549. [DOI] [PubMed] [Google Scholar]
- Dey A., Westphal H., Nebert D. W. Cell-specific induction of mouse Cyp1a1 mRNA during development. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7446–7450. doi: 10.1073/pnas.86.19.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Dorin J. R., Dickinson P., Alton E. W., Smith S. N., Geddes D. M., Stevenson B. J., Kimber W. L., Fleming S., Clarke A. R., Hooper M. L. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature. 1992 Sep 17;359(6392):211–215. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., Fujii-Kuriyama Y. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P., Pineau T., Hilbert D. M., McPhail T., Lee S. S., Kimura S., Nebert D. W., Rudikoff S., Ward J. M., Gonzalez F. J. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995 May 5;268(5211):722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Evolution of the P450 gene superfamily: animal-plant 'warfare', molecular drive and human genetic differences in drug oxidation. Trends Genet. 1990 Jun;6(6):182–186. doi: 10.1016/0168-9525(90)90174-5. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Shimada T., Raney K. D., Yun C. H., Meyer D. J., Ketterer B., Harris T. M., Groopman J. D., Kadlubar F. F. Elucidation of catalytic specificities of human cytochrome P450 and glutathione S-transferase enzymes and relevance to molecular epidemiology. Environ Health Perspect. 1992 Nov;98:75–80. doi: 10.1289/ehp.929875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann L. J., Sheen Y. Y., Bigelow S. W., Nebert D. W. Trout P450IA1: cDNA and deduced protein sequence, expression in liver, and evolutionary significance. DNA. 1988 Jul-Aug;7(6):379–387. doi: 10.1089/dna.1.1988.7.379. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Gonzalez F. J., Kozak C. A., Nebert D. W. Regional linkage analysis of the dioxin-inducible P-450 gene family on mouse chromosome 9. Biochem Biophys Res Commun. 1985 Jul 16;130(1):396–406. doi: 10.1016/0006-291x(85)90430-9. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Hyde S. C., Gill D. R., Higgins C. F., Trezise A. E., MacVinish L. J., Cuthbert A. W., Ratcliff R., Evans M. J., Colledge W. H. Correction of the ion transport defect in cystic fibrosis transgenic mice by gene therapy. Nature. 1993 Mar 18;362(6417):250–255. doi: 10.1038/362250a0. [DOI] [PubMed] [Google Scholar]
- Kimura S., Donovan J. C., Nebert D. W. Expression of the mouse P(1)450 gene during differentiation without foreign chemical stimulation. J Exp Pathol. 1987 Winter;3(1):61–74. [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- Li H., Witte D. P., Branford W. W., Aronow B. J., Weinstein M., Kaur S., Wert S., Singh G., Schreiner C. M., Whitsett J. A. Gsh-4 encodes a LIM-type homeodomain, is expressed in the developing central nervous system and is required for early postnatal survival. EMBO J. 1994 Jun 15;13(12):2876–2885. doi: 10.1002/j.1460-2075.1994.tb06582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Nebert D. W. Drug metabolism. Growth signal pathways. Nature. 1990 Oct 25;347(6295):709–710. doi: 10.1038/347709a0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Drug-metabolizing enzymes in ligand-modulated transcription. Biochem Pharmacol. 1994 Jan 13;47(1):25–37. doi: 10.1016/0006-2952(94)90434-0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., McKinnon R. A. Cytochrome P450: evolution and functional diversity. Prog Liver Dis. 1994;12:63–97. [PubMed] [Google Scholar]
- Nebert D. W. Pharmacogenetics: an approach to understanding chemical and biologic aspects of cancer. J Natl Cancer Inst. 1980 Jun;64(6):1279–1290. doi: 10.1093/jnci/64.6.1279. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Mol Endocrinol. 1991 Sep;5(9):1203–1214. doi: 10.1210/mend-5-9-1203. [DOI] [PubMed] [Google Scholar]
- O'Neal W. K., Hasty P., McCray P. B., Jr, Casey B., Rivera-Pérez J., Welsh M. J., Beaudet A. L., Bradley A. A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet. 1993 Oct;2(10):1561–1569. doi: 10.1093/hmg/2.10.1561. [DOI] [PubMed] [Google Scholar]
- Ono S., Hatanaka T., Hotta H., Tsutsui M., Satoh T., Gonzalez F. J. Chlorzoxazone is metabolized by human CYP1A2 as well as by human CYP2E1. Pharmacogenetics. 1995 Jun;5(3):143–150. doi: 10.1097/00008571-199506000-00002. [DOI] [PubMed] [Google Scholar]
- Ozols J., Heinemann F. S., Johnson E. F. Amino acid sequence of an analogous peptide from two forms of cytochrome P-450. J Biol Chem. 1981 Nov 25;256(22):11405–11408. [PubMed] [Google Scholar]
- Pasco D. S., Boyum K. W., Merchant S. N., Chalberg S. C., Fagan J. B. Transcriptional and post-transcriptional regulation of the genes encoding cytochromes P-450c and P-450d in vivo and in primary hepatocyte cultures. J Biol Chem. 1988 Jun 25;263(18):8671–8676. [PubMed] [Google Scholar]
- Pineau T., Fernandez-Salguero P., Lee S. S., McPhail T., Ward J. M., Gonzalez F. J. Neonatal lethality associated with respiratory distress in mice lacking cytochrome P450 1A2. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5134–5138. doi: 10.1073/pnas.92.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Glover E., Taylor B. A. The murine Ah locus: a new allele and mapping to chromosome 12. Mol Pharmacol. 1987 Oct;32(4):471–478. [PubMed] [Google Scholar]
- Ratcliff R., Evans M. J., Cuthbert A. W., MacVinish L. J., Foster D., Anderson J. R., Colledge W. H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993 May;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Robinson J. R., Nebert D. W. Genetic expression of aryl hydrocarbon hydroxylase induction. Presence or absence of association with zoxazolamine, diphenylhydantoin, and hexobarbital metabolism. Mol Pharmacol. 1974 May;10(3):484–493. [PubMed] [Google Scholar]
- Schweikl H., Taylor J. A., Kitareewan S., Linko P., Nagorney D., Goldstein J. A. Expression of CYP1A1 and CYP1A2 genes in human liver. Pharmacogenetics. 1993 Oct;3(5):239–249. doi: 10.1097/00008571-199310000-00003. [DOI] [PubMed] [Google Scholar]
- Silver G., Krauter K. S. Expression of cytochromes P-450c and P-450d mRNAs in cultured rat hepatocytes. 3-Methylcholanthrene induction is regulated primarily at the post-transcriptional level. J Biol Chem. 1988 Aug 25;263(24):11802–11807. [PubMed] [Google Scholar]
- Small K. M., Potter S. S. Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 1993 Dec;7(12A):2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- Tang B. K., Zhou Y., Kadar D., Kalow W. Caffeine as a probe for CYP1A2 activity: potential influence of renal factors on urinary phenotypic trait measurements. Pharmacogenetics. 1994 Jun;4(3):117–124. [PubMed] [Google Scholar]
- Tuteja N., Gonzalez F. J., Nebert D. W. Developmental and tissue-specific differential regulation of the mouse dioxin-inducible P1-450 and P3-450 genes. Dev Biol. 1985 Nov;112(1):177–184. doi: 10.1016/0012-1606(85)90131-9. [DOI] [PubMed] [Google Scholar]