Summary
In the phase III AZA-001 trial, low-dose cytarabine (LDara-C), the most widely used low-dose chemotherapy in patients with higher-risk myelodysplastic syndrome (MDS) who are ineligible for intensive treatment, was found to be associated with poorer survival compared with azacitidine. This analysis further compared the efficacy and the toxicity of these two drug regimens. Before randomization, investigators preselected patients to receive a conventional care regimen, one of which was LDara-C. Of 94 patients preselected to LDara-C, 45 were randomized to azacitidine and 49 to LDara-C. Azacitidine patients had significantly more and longer haematologicalal responses and increased red blood cell transfusion independence. Azacitidine prolonged overall survival versus LDara-C in patients with poor cytogenetic risk, presence of −7/del(7q), and French-American-British subtypes refractory anaemia with excess blasts (RAEB) and RAEB in transformation. When analyzed per patient year of drug exposure, azacitidine treatment was associated with fewer grade 3–4 cytopenias and shorter hospitalisation time than LDara-C in these higher-risk MDS patients.
Keywords: azacitidine, higher-risk myelodysplastic syndromes, low-dose cytarabine (ara-C), myelodysplastic syndromes, survival
The myelodysplastic syndromes (MDS) are bone marrow stem cell disorders characterized by ineffective haematopoiesis leading to blood cytopenias, and by a high risk of progression to acute myeloid leukaemia (AML). Patients with higher-risk MDS have a median survival of around 1 year (Greenberg et al, 1997; Kurzrock, 2002), and prolonging their survival is the primary goal of therapy. Allogeneic stem cell transplantation (SCT) remains the only curative treatment for higher-risk MDS, but is rarely feasible in elderly patients while intensive anthracycline–cytarabine chemotherapy (IC) is usually restricted to younger patients (Alessandrino et al, 2002).
Low-dose cytarabine (LDara-C) is widely used, especially in Europe, to treat patients with MDS or AML unable to receive IC or allogeneic SCT. Responses [complete remission (CR), partial remission (PR), or haematological improvement (HI)] with LDara-C are achieved in 10–20% of patients (Fenaux et al, 1990; Hellstrom-Lindberg et al, 1992; Miller et al, 1992; Detourmignies et al, 1993; Nair et al, 1998; Bowen et al, 2003; Burnett et al, 2007). Median CR duration ranges from 9 to 11 months and most responses are shorter than 18 months (Hellstrom-Lindberg et al, 1992; Miller et al, 1992; Detourmignies et al, 1993; Visani et al, 2004). Still, responders seem to benefit from LDara-C and even a few prolonged remissions have been observed, especially in AML (Detourmignies et al, 1993). No survival advantage of LDara-C over best supportive care (BSC) was detected in an early randomized trial, but after only one cycle of LDara-C (Miller et al, 1992). Recently, elderly patients with AML or higher-risk MDS treated in many cases with more than one cycle of LDara-C, had a survival advantage over BSC (Burnett et al, 2007). Moreover, combining LDara-C with other drugs, including arsenic trioxide and clofarabine, in elderly patients with AML and higher-risk MDS, can improve response (Faderl et al, 2008; Roboz et al, 2008).
As previously reported, in the randomized, phase III AZA-001 study in higher-risk MDS, the demethylating agent azacitidine was associated with a significant overall survival (OS) advantage over conventional care regimens (CCR), which included LDara-C, BSC only, or IC (Fenaux et al, 2009). After enrollment but before randomization to treatment, patients were preselected, based on the treating physician’s clinical judgement, to receive one of the three CCR. Azacitidine significantly improved OS versus LDara-C in the subset of AZA-001 patients preselected to receive LDara-C. Here, we report previously unpublished efficacy and safety data that support differences between azacitidine and LDara-C therapy that may have contributed to the OS benefit with azacitidine.
Methods
Patient eligibility and AZA-001 study design are reported in detail elsewhere (Fenaux et al, 2009). The trial was registered with http://clinicaltrials.gov/ct2/show/NCT00071799 (number NCT00071799).
Efficacy and safety assessments
Efficacy assessments and analyses were intention to treat (ITT) in the subgroup of patients preselected to receive LDara-C and then randomized to either azacitidine or LDara-C. Safety analyses included all patients who received ≥1 dose of study drug and had ≥1 post-dose safety assessment. The primary efficacy endpoints analyzed were median OS and the proportions of patients alive at 2-years, both based on Kaplan–Meier methods. Exploratory OS analyses assessed treatment effect in predefined subgroups based on the French-American-British (FAB) subtype [refractory anaemia with excess blasts (RAEB), RAEB in transformation (RAEB-t)], International Prognostic Scoring System (IPSS) cytogenetic risk (good, intermediate, poor), and presence of −7/del(7q). Secondary endpoints included duration of any haematological response: CR, PR, or HI. Haematologic response rates with azacitidine versus LDara-C were previously reported (Fenaux et al, 2009). Other secondary endpoints included red blood cell (RBC) transfusion independence (TI, no RBC transfusions during a 56 consecutive day period); hospitalisation days due to adverse events; changes from baseline haemoglobin (Hb), absolute neutrophil count (ANC) and platelet levels; and occurrence of adverse events.
Statistical methods
Overall survival was defined as time from randomization to death from any cause. Living patients were censored at last follow-up. Randomization and analyses were stratified using FAB subtype and IPSS risk group. Time-to-event curves were plotted using unadjusted Kaplan–Meier methods. Hazard ratios (HR) and associated 95% confidence intervals (CI) are from a Cox proportional hazards regression model, stratified by IPSS and FAB subtype, adjusting for baseline Eastern Cooperative Oncology Group (ECOG) status, RBC transfusions, presence of −7/del 7(q), lactate dehydrogenase (LDH) and Hb. Treatment by investigator selection was included in the model. Exploratory OS analyses of sub-cohorts defined by IPSS cytogenetic risk group, presence of −7/del(7q), or FAB subtype were not powered to make inferential comparisons. However, hazard ratios are provided.
Rate of hospitalisation due to adverse events was calculated as the number of days in hospital during treatment divided by patient-years of follow-up. Relative risk (RR) represents the ratio of the azacitidine rate to the LDara-C rate. Plots of mean Hb, ANC and platelet levels used last observation carried forward (LOCF). Adverse events were graded by National Cancer Institute’s Common Toxicity Criteria, Version 2.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf).
Results
Patients
During the preselection of treatment phase of the AZA-001 trial, 94 patients were preselected by investigators to receive LDara-C, then randomized to either azacitidine (n = 45) or LDara-C (n = 49). Baseline characteristics were well balanced between treatment groups (Table 1). Five patients allocated to LDara-C did not receive the study drug and were included in the ITT efficacy analyses but excluded from safety analyses. Cytogenetic data were missing for one azacitidine patient and one LDara-C patient.
Table I.
Baseline characteristics.
| AZA | LDara-C | |
|---|---|---|
| Parameter | N = 45 | N = 49 |
| Median age, years (range) | 69·0 (42–82) | 71·0 (56–85) |
| Age categories, n (%) | ||
| <65 years | 14 (31) | 7 (14) |
| 65–74 years | 21 (47) | 28 (57) |
| ≥75 years | 10 (22) | 14 (29) |
| Male gender – n (%) | 39 (87) | 35 (71) |
| FAB Classification – n (%) | ||
| RAEB | 27 (60) | 25 (51) |
| RAEB-t | 15 (33) | 19 (39) |
| CMML | 1 (2·2) | 1 (2·0) |
| AML | 1 (2·2) | 0 |
| MPD | 0 | 1 (2·0) |
| Indeterminate | 1 (2·2) | 3 (6·1) |
| IPSS – n (%) | ||
| Intermediate-1 | 1 (2·2) | 2 (4·1) |
| Intermediate-2 | 22 (49) | 21 (43) |
| High | 19 (42) | 21 (43) |
| Indeterminate | 2 (4·4) | 4 (8·2) |
| Not applicable★ | 1 (2·2) | 1 (2·0) |
| ECOG Status – n (%) | ||
| Grade 0 | 21 (47) | 29 (59) |
| Grade 1 | 21 (47) | 17 (35) |
| Grade 2 | 1 (2) | 2 (4) |
| Missing | 2 (4) | 1 (2) |
| # RBC transfusions – median (range) during the 56 d prior to study enrollment | 2·0 (0–9) | 1·0 (0–10) |
| Hb (g/l) – median (range) | 96 (71–122) | 97 (54–143) |
| Platelet count (109/l) – median (range) | 42 (7–252) | 84 (18–437) |
| ANC (109/l) – median (range) | 0·8 (0–7·9) | 1·3 (0·2–18·7) |
FAB, French-American- British; RAEB, refractory anaemia with excess blasts; RAEB-t, RAEB in transformation; CMML, chronic myelo-monocytic leukaemia; AML, acute myeoid leukaemia; MPD, myelo-proliferative disease; IPSS, International Prognostic Scoring System; ECOG, Eastern Cooperative Oncology Group; RBC, red blood cell; Hb, haemoglobin; ANC, absolute neutrophil count.
IPSS classification was not applicable for patients who were classified as AML or MPD (per the FAB classification) because the IPSS classification was not developed for those patients.
Treatment
Azacitidine was administered for a median of nine cycles (range 1–27). Median azacitidine cycle length was 28 d (range 22–189), with 52% of patients receiving azacitidine in 28-d cycles and 87% remaining on the 75 mg/m2 per day dose with no reductions throughout the study. LDara-C was administered for a median of 4·5 cycles (range 1–15). The median cycle length was 35 d (range 27–69), and 29% of patients received LDara-C in 4-week cycles. Median treatment period was 13 months (range 1–32) for azacitidine versus 5 months (range 0–17) for LDara-C.
Overall survival (OS)
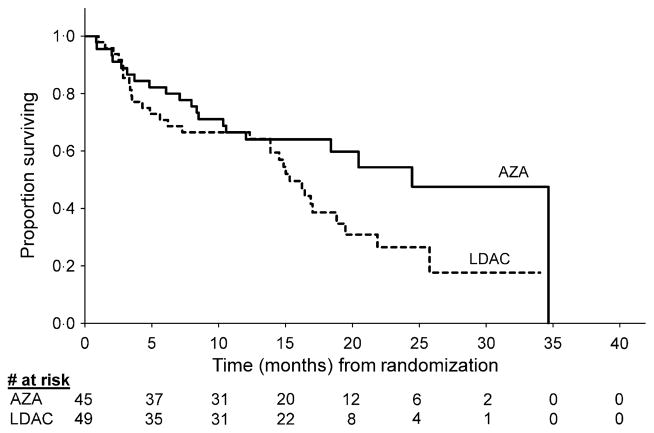
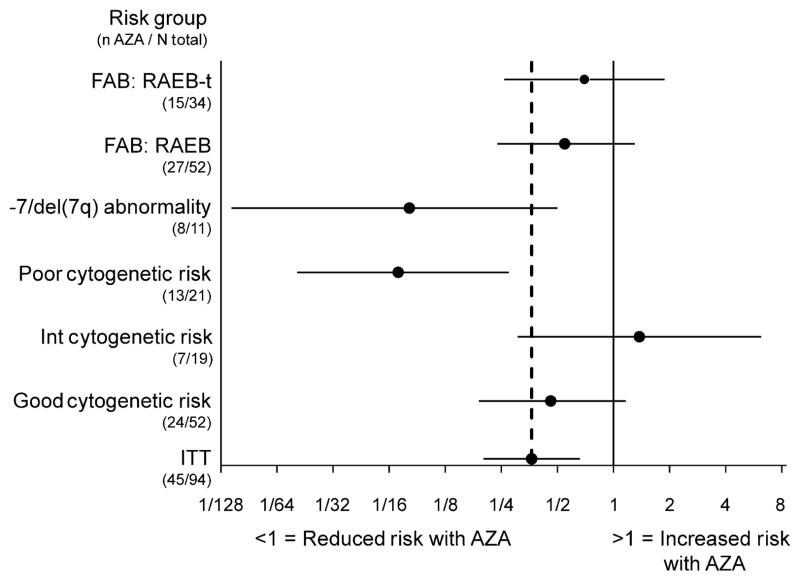
As reported previously, median OS was 24·5 months (95% CI: 8·4, 34·7) with azacitidine compared with 15·3 months (95% CI: 4·9, 25·8) with LDara-C, P = 0·0006 (Fenaux et al, 2009). At 2 years, 54% and 27% of patients receiving azacitidine and LDara-C respectively, remained alive (Fig 1). In patients with good-risk cytogenetics, median OS was not reached (NR) at 21·1 months (95% CI: 10·6, NR) in the azacitidine group (n = 24) versus 19·5 months (95% CI: 14·9, NR) in the LDara-C group (n = 28) (HR = 0·46; 95% CI: 0·19, 1·15) (Fig 2). Median OS for patients with intermediate-risk cytogenetics was 20·5 months (95% CI: 7·1, NR) for the azacitidine group (n = 7) versus 17·0 months (95% CI: 13·9, 21·9) for the LDara-C group (n = 12) (HR = 1·38; 95% CI: 0·31, 6·19). In patients with poor-risk cytogenetics, median OS was 24·5 months (95% CI: 8·4, 34·7) in the azacitidine group (n = 13) compared with 2·9 months (95% CI: 2·5, 4·9) in the LDara-C group (n = 8), (HR = 0·07; 95% CI: 0·02, 0·27). In particular, patients with −7 or del 7q, had a median survival with azacitidine (n = 8) of 21·4 months (95% CI 3·7, 24·5) versus 3·5 months (95% CI: 2·9, 4·9) for LDara-C (n = 3), (HR = 0·08; 95% CI: 0·01, 0·50). In patients with RAEB (n = 52), median OS was 34·7 months (95% CI 8·4, 34·7) versus 15·0 months (95% CI: 7·3, 21·9) with azacitidine (n = 27) versus with LDara-C (n = 25) respectively, (HR = 0·32; 95% CI: 0·14, 0·74). In patients with RAEB-t (n = 34), median OS was 21·4 months (95% CI: 10·6, NR) versus 16·4 months (95% CI; 14·5, 25·8) respectively, with azacitidine (n = 15) versus LDara-C (n = 19; HR = 0·42, 95% CI: 0·16, 1·10).
Fig 1.
Unadjusted Kaplan–Meier estimated overall survival: azacitidine (AZA) versus low dose cytarabine (LDara-C).
Fig 2.
Hazard ratio and 95% CI for overall survival by FAB subtype and cytogenetic risk (the horizontal axis has a logarithmic scale). FAB, French-American-British; RAEB, refractory anaemia with excess blasts; RAEB-tm, RAEB in transformation; ITT, intention to treat; AZA, azacitidine.
Haematological response duration and transfusion independence (TI)
As reported, overall rate of haematological response and improvement rates were significantly higher with azacitidine versus LDara-C (84% vs. 37%, respectively) (Fenaux et al, 2009). Further analysis showed that the median duration of any haematological response (CR, PR, or HI) was significantly longer with azacitidine: 20·9 months (95% CI: 15·1, NR) versus 7·0 months (95% CI: 2·5, NR) with LDara-C (HR = 0·43, 95% CI 0·12, 1·50). More baseline RBC transfusion-dependent patients became TI with azacitidine (n = 14/31, 45·2%) versus LDara-C (n = 4/30, 13·3%, OR = 5·4, 95% CI 1·5, 19·0), and more azacitidine-treated patients who were RBC TI at baseline remained TI (13/14, 92·9% versus 9/19, 47·4% with LDara-C, OR = 14·4, 95% CI 1·6, 133·6). Few patients were platelet transfusion-dependent at baseline (11 azacitidine and 12 LDara-C) and there was no difference in the proportions of these patients achieving platelet TI. However, a significantly higher proportion of azacitidine-treated patients who were platelet TI at baseline (n = 34, LDara-C n = 37) retained platelet TI (88·2% vs. 45·9%, OR = 8·8, 95% CI 2·6, 30·1).
Safety
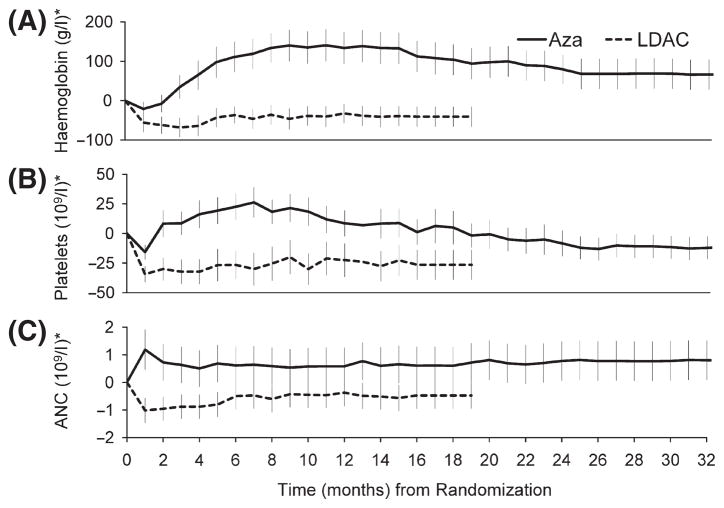
Patients treated with LDara-C received fewer median treatment cycles, and consequently, had less drug exposure than patients receiving azacitidine. Treatment discontinuation due to adverse events was seen at similar frequency in the two groups: five (10%) with LDara-C and four (9%) with azacitidine. Neutropenia, thrombocytopenia and anaemia were the most common grade 3 and 4 adverse events occurring in this analysis. Neutropenia was reported in 40 (89%) patients receiving azacitidine and 39 (89%) patients receiving LDara-C, thrombocytopenia in 42 (93%) and 42 (96%) and anaemia in 29 (64%) and 34 (77%), respectively. Further, a change from grade 0–2 at baseline to grade 3–4 during treatment was observed in 14/18 (78%) patients in the azacitidine group and 19/24 (79%) in the LDara-C group for neutropenia; in 17/20 (85%) and 29/30 (97%) for thrombocytopenia; and in 25/40 (63%) and 28/37 (76%) respectively, for anaemia (Fenaux et al, 2009). The rates of these haematological events per patient-year of exposure to azacitidine or LDara-C were 3·0 vs. 4·7 respectively, for neutropenia (RR = 0·65; 95% CI 0·51, 0·83), 3·3 vs. 5·1 for thrombocytopenia (RR = 0·66; 95% CI 0·52, 0·83), and 1·6 vs. 3·5 for anaemia (RR = 0·46; 95% CI 0·33, 0·62). Changes from baseline mean Hb, platelet and ANC levels are shown in Fig 3. Other commonly reported grade 3 or 4 adverse events included pyrexia, observed in one (2%) patient and five (11%) patients in the azacitidine and LDara-C groups respectively, with per-patient-year rates of 0·02 vs. 0·24, RR = 0·09; 95% CI 0·00, 0·64), and pneumonia, observed in four (9%) and five (11%) of patients, with per-patient-year rates of 0·09 vs. 0·20 (RR = 0·46; 95% CI 0·11, 1·80).
Fig 3.
Mean (±SE) changes from baseline for haemoglobin (A), platelets (B) and absolute neutrophil count (C). *‘Last observation carried forward’ methodology used for both azacitidine and LDara-C groups.
Rate of days in hospital due to an adverse event was significantly lower in the azacitidine group compared with the LDara-C group: 18·4 vs. 27·4 hospital days per patient-year of exposure respectively, (RR = 0·67; 95% CI 0·65, 0·80).
Discussion
LDara-C remains a widely used low-dose chemotherapy regimen in higher-risk MDS, especially in Europe. Thus, a closer look at previously unpublished outcomes that may have contributed to the OS advantage with azacitidine versus LDara-C noted in the AZA-001 trial (Fenaux et al, 2009) is warranted. The overall OS advantage at 2-years with azacitidine (54% vs. 27% with LDara-C) is especially important in patients with higher-risk MDS. Similarly, the survival advantage seen in patients with poor-risk cytogenetics, particularly −7/del(7q), is consistent with effects in all patients with −7/del(7q) in the AZA-001 trial and support the significant activity of azacitidine previously reported in MDS patients with this cytogenetic abnormality (Raj et al, 2006, 2007; Mufti et al, 2008). Moreover, these OS findings add further evidence supporting the poor results obtained with LDara-C in other studies of patients with AML and MDS with unfavourable karyotype (Fenaux et al, 1990; Hellstrom-Lindberg et al, 1992; Burnett et al, 2007). OS was also longer with azacitidine in patients with favourable karyotype, who accounted for more than half of the patients (Fig 2), while the results in patients with intermediate karyotype were difficult to assess due to small patient numbers. Median OS in both FAB subgroups, RAEB and RAEB-t, was also notably longer with azacitidine compared with LDara-C.
The prolonged OS advantage observed with azacitidine compared with LDara-C is probably related to significantly higher response rates with azacitidine (Fenaux et al, 2009) and to the significantly longer duration of these haematological responses reported here. The significant increase in RBC TI with azacitidine after baseline dependence also may have mediated the significant survival advantage.
This analysis also strongly suggests that azacitidine treatment was better tolerated than LDara-C. Of all azacitidine doses received, over half were administered at the protocol-specified 4-week cycle compared with only 29% of LDara-C doses. These findings suggest azacitidine was less toxic and better tolerated, with blood lineage nadirs recovering more rapidly than with LDara-C (Fig 3). Further, when analyzed per patient year of drug exposure, azacitidine was associated with lower rates of anaemia, thrombocytopenia and neutropenia versus LDara-C. Lower rates of neutropenia with azacitidine were supported by significantly fewer infections requiring intravenous antimicrobial treatment (Fenaux et al, 2009). Finally, the lower incidence of cytopenias may account for the lower proportion of hospital days per patient-year of drug exposure due to adverse events observed with azacitidine versus LDara-C, and contributed to the better survival obtained with azacitidine. It could be argued that some of the survival benefit obtained with azacitidine in the present study was due to the longer treatment duration compared with LDara-C. In the study reported by Miller et al (1992) comparing LDara-C and BSC, only one cycle of LDara-C was administered, with no survival advantage observed. Conversely, in the recent British MRC study (Burnett et al, 2007) in elderly patients with AML, LDara-C significantly improved survival and response versus hydroxycarbamide, with 55% of patients receiving more than one cycle of LDara-C. In AZA-001, the protocol mandated that LDara-C dosing was to continue until intolerable toxicity or disease progression. The median number of LDara-C cycles was less than the median number of azacitidine cycles administered, primarily due to the lower overall response rate (Fenaux et al, 2009), shorter duration of responses, and longer median cycle lengths (caused by longer time to blood lineage nadir recovery) with LDara-C. Nevertheless, patients in this study received more LDara-C cycles than in other large studies in MDS (Miller et al, 1992; Detourmignies et al, 1993; Zwierzina et al, 2005; Burnett et al, 2007). In spite of this, LDara-C was inferior to azacitidine for prolonging OS.
Differences between azacitidine and LDara-C effects on OS emerged at approximately 6 months (Fig 1), at which time, mortality in the azacitidine group began to level off while mortality continued steadily in the LDara-C group. This finding probably reflects differences in drug activities. Azacitidine reduces abnormal DNA hypermethylation implicated in the progression of the disease and, after several dosing cycles, appears to restore normal transcription of tumour suppressor genes (Silverman et al, 2002; Gore et al, 2006). In contrast, although in vitro data suggest LDara-C may induce apoptosis by differentiation induction, most evidence suggests its clinical effects are more strongly related to cytotoxic activity (Housset et al, 1982; Leyden et al, 1984; Cheson & Simon, 1987; Visani et al, 2004).
This more detailed analysis of AZA-001 trial results, focusing on the azacitidine and LDara-C comparison, suggests that the OS advantage seen with azacitidine over LDara-C is present in subgroups of higher-risk MDS patients defined by FAB classification and IPSS cytogenetic risk, and shows that the OS advantage results not only from higher response rates with azacitidine, but also from haematological responses of significantly longer duration. The survival advantage appears to be strongly supported by significantly increased TI and a significantly lower rate of grade 3–4 haematological adverse events. Finally, the reduced rate of hospitalisation days due to adverse events with azacitidine also supports a survival advantage. Decreases in health-care utilisation with azacitidine are also important. These results further support the use of azacitidine as first-line therapy in higher-risk MDS patients for whom allogeneic SCT and intensive chemotherapy are not indicated.
Acknowledgments
The study was funded by Celgene Corporation. The paper was written by Pierre Fenaux and other co-authors. Sheila Truten, BS and Neil Malone, MA, provided editorial assistance and Indrasiri Fernando, PhD, provided statistical support during manuscript development. ST is employed by Medical Communication Company, Wynnewood, PA; NM and IF are employed by Celgene Corporation, Summit, NJ.
References
- Alessandrino EP, Amadori S, Barosi G, Cazzola M, Grossi A, Liberato LN, Locatelli F, Marchetti M, Morra E, Rebulla P, Visani G, Tura S. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica. 2002;87:1286–1306. [PubMed] [Google Scholar]
- Bowen D, Culligan D, Jowitt S, Kelsey S, Mufti G, Oscier D, Parker J UK MDS Guidelines Group. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. British Journal of Haematology. 2003;120:187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, Wheatley K National Cancer Research Institute Haematological Oncology Study Group Adult Leukemia Working Party. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Simon R. Low-dose ara-C in acute nonlymphocytic leukemia and myelodysplastic syndromes: a review of 20 years’ experience. Seminars in Oncology. 1987;14:126–133. [PubMed] [Google Scholar]
- Detourmignies L, Wattel E, Lai JL, Bauters F, Fenaux P. Is there a role for low-dose cytarabine arabinoside in de novo acute myeloid leukemia in the elderly? Annals of Hematology. 1993;66:235–240. doi: 10.1007/BF01738471. [DOI] [PubMed] [Google Scholar]
- Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z, Borthakur G, Verstovsek S, Thomas DA, Kwari M, Kantarjian HM. A randomized study of clofarabine plus low-dose cytarabine as front-line therapy for patients age 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Lai JL, Gardin C, Bauters F. Cytogenetics are a predictive factor of response to low-dose ara-C in acute myelogenous leukemia (AML) in the elderly. (Letter) Leukemia. 1990;4:312. [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR International Vidaza High-risk MDS Survival Study Group. Efficacy of azacitidine compared with conventional care regimens in higher-risk myelodysplastic syndromes: results of a randomised, phase III study. The Lancet Oncology. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, Grever M, Galm O, Dauses T, Karp JE, Rudek MA, Zhao M, Smith BD, Manning J, Jiemjit A, Mays A, Zwiebel J, Murgo A, Weng LJ, Herman GJ. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Research. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2098. [PubMed] [Google Scholar]
- Hellstrom-Lindberg E, Robert KH, Gahrton G, Lindberg G, Forsblom AM, Kock Y, Ost A. A predictive model for the clinical response to low dose ara-C: a study of 102 patients with myelodysplastic syndromes or acute leukemia. British Journal of Haematology. 1992;81:503–511. doi: 10.1111/j.1365-2141.1992.tb02982.x. [DOI] [PubMed] [Google Scholar]
- Housset M, Daniel MT, Degos L. Small doses of ara-C in the treatment of acute myeloid leukemia: differentiation of myeloid leukemia cells? British Journal of Haematology. 1982;51:125–129. doi: 10.1111/j.1365-2141.1982.tb07297.x. [DOI] [PubMed] [Google Scholar]
- Kurzrock R. Myelodysplastic syndrome overview. Seminars in Hematology. 2002;39:18–25. doi: 10.1053/shem.2002.35981. [DOI] [PubMed] [Google Scholar]
- Leyden M, Manoharan A, Boyd A, Cheng ZM, Sullivan J. Low dose cytosine arabinoside: partial remission of acute myeloid leukaemia without evidence of differentiation induction. British Journal of Haematology. 1984;57:301–307. doi: 10.1111/j.1365-2141.1984.tb02899.x. [DOI] [PubMed] [Google Scholar]
- Miller KB, Kyungmann K, Morrison FS, Winter JN, Bennett JM, Neiman RS, Head DR, Cassileth PA, O’Connell MJ. The evaluation of low-dose cytarabine in the treatment of myelodysplastic syndromes: a phase-III intergroup study. Annals of Hematology. 1992;65:162–168. doi: 10.1007/BF01703109. [DOI] [PubMed] [Google Scholar]
- Mufti GJ, Fenaux P, Hellstrom-Lindberg E, Santini V, List AF, Gore S, Seymour JF, Silverman LR, Backstrom J, Beach CL. Treatment of high-risk MDS patients (pts) with −7/del(7q) with azacitidine (AZA) versus conventional care regimens (CCR): effects on overall survival (OS) Journal of Clinical Oncology. 2008;26:380s, Abstract 7033. [Google Scholar]
- Nair R, Nair CN, Advani SH. All trans retinoic acid with low-dose cytosine arabinoside in the treatment of myelodysplastic syndrome. Leukemia & Lymphoma. 1998;29:187–192. doi: 10.3109/10428199809058394. [DOI] [PubMed] [Google Scholar]
- Raj K, John A, Ho A, Thomas NSB, Mufti GJ. Early and sustained response to azacitidine occurs in high risk MDS patients with monosomy 7: responses correlate with increased apoptosis not CDKN2B demethylation. British Journal of Haematology. 2006;133:61–62. Abstract 111. [Google Scholar]
- Raj K, John A, Ho A, Chronis C, Khan S, Samuel J, Pomplun S, Thomas NSB, Mufti GJ. CDKN2B methylation status and isolated chromosome 7 abnormalities predict responses to treatment with 5-azacytidine. Leukemia. 2007;21:1937–1944. doi: 10.1038/sj.leu.2404796. [DOI] [PubMed] [Google Scholar]
- Roboz GJ, Ritchie EK, Curcio T, Provenzano J, Carlin R, Samuel M, Wittenberg B, Mazumdar M, Christos PJ, Mathew S, Allen-Bard S, Feldman EJ. Arsenic trioxide and low-dose cytarabine in older patients with untreated acute myeloid leukemia, excluding acute promyelocytic leukemia. Cancer. 2008;113:2504–2511. doi: 10.1002/cncr.23855. [DOI] [PubMed] [Google Scholar]
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF. Randomized controlled trial of azacitidine in patients with myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. Journal of Clinical Oncology. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- Visani G, Malagola M, Piccaluga PP, Isidori A. Low-dose ara-C for myelodysplastic syndromes: is it still a current therapy? Leukemia & Lymphoma. 2004;45:1531–1538. doi: 10.1080/10428190310001653727. [DOI] [PubMed] [Google Scholar]
- Zwierzina H, Suciu S, Loeffler-Ragg J, Neuwirtova R, Fenaux P, Becsak M, Harousseau J, Nuessler V, Cermak J, Solbu G, Willemze R, de Witte T, Amadori S EORTC Leukemia Cooperative Group. Low-dose cytosine arabinoside (LD-AraC) vs LD-AraC plus granulocyte/macrophage colony stimulating factor vs LD-AraC plus interleukin-3 for myelodysplastic syndrome patients with a high risk of developing acute leukemia: final results of a randomized phase III study (06903) of the EORTC Leukemia Cooperative Group. Leukemia. 2005;19:1929–1933. doi: 10.1038/sj.leu.2403934. [DOI] [PubMed] [Google Scholar]