Abstract
Importance
It remains unclear whether vitamin D insufficiency, which is common in individuals with multiple sclerosis (MS), has an adverse effect on MS outcomes.
Objectives
To determine whether serum concentrations of 25-hydroxyvitamin D (25(OH))D, a marker of vitamin D status, predict disease activity and prognosis in patients with a first event suggestive of multiple sclerosis (MS) (clinically isolated syndrome [CIS]).
Design, Setting, and Participants
BENEFIT was a randomized trial originally designed to evaluate the impact of early- versus delayed-interferon beta-1b (IFNB-1b) treatment in patients with CIS. Serum 25(OH)D concentrations were measured at baseline, 6, 12, and 24 months. 465 of the 468 patients randomized had at least one 25(OH)D measurement, and 334 patients had both 6 and 12 months (seasonally asynchronous) measurements. Patients were followed for 5 years clinically and by MRI.
Main Outcomes and Measures
MRI: New active lesions, increased T2 lesion volume, and brain volume. Clinical: MS relapses and disability (EDSS).
Results
Higher 25(OH)D levels predicted reduced MS activity and slower rate of progression. A 50 nmol/L (20 ng/mL) increment in average serum 25(OH)D levels within the first 12 months predicted a 57% lower rate of new active lesions (p=0·0009), 57% lower relapse rate (p=0·03), 25% lower yearly increase in T2 lesion volume (p=0·00004), and 0·41% lower yearly loss in brain volume (p=0·07) from month 12 to 60. Similar associations were found between 25(OH)D measured up to 12 months and MS activity or progression from month 24 to 60. In analyses using dichotomous 25(OH)D levels, values >= 50 nmol/L (20 ng/mL) up to 12 months predicted lower disability (EDSS = −0·17; p=0·004) during the subsequent 4 years.
Conclusion and Relevance
Among MS patients mainly treated with IFNB-1b, low 25(OH)D levels early in the disease course are a strong risk factor for long term MS activity and progression.
Introduction
Multiple sclerosis (MS) is a common cause of neurological disability in young adults.1 Most patients experience bouts of inflammatory demyelination (relapsing-remitting MS) followed years later by treatment-resistant disease progression and brain atrophy.2 A higher MS risk in individuals with low vitamin D intake 3 or low circulating 25-dihydroxyvitamin D (25(OH)D) 4–7 as well as an inverse correlation between vitamin D status and MS activity have been reported 8–11 and suggest that vitamin D is related to the disease process that leads to and perpetuates MS. Previous clinical studies, however, were conducted among patients with variable disease duration and could not determine whether low vitamin D is a consequence of MS activity 12, or whether vitamin D levels early in the disease course contribute to predict long term progression and disability. Because the prevalence of vitamin D insufficiency (25(OH)D<50 nmol/L [20 ng/mL]) is high13, supplementation could potentially benefit a large proportion of MS patients.
Therefore, we determined whether vitamin D status early in the disease process influences long term disease course among participants in the Betaferon/Betaseron in Newly Emerging multiple sclerosis For Initial Treatment (BENEFIT) trial.
Methods
Study population and study design
BENEFIT was originally designed to compare early- versus delayed-interferon beta-1b (IFNB-1b; Betaseron®, Berlin, Germany) treatment in patients with a diagnosis of clinically isolated syndrome (CIS). Between February 2002 and June 2003, patients from 18 European countries, Israel and Canada were enrolled in 98 centers. Patients presenting with a first episode of neurological dysfunction highly suggestive of MS with a minimum of two clinically silent lesions on MRI were randomized in a 5:3 ratio to receive either IFNB-1b 250 μg (n = 292) or placebo (n = 176) subcutaneously every other day for 2 years, or until a second clinical event occurred and the diagnosis of clinically definite MS (CDMS) could be established. All patients were then eligible to enter a prospectively planned follow-up phase with open-label IFNB-1b up to a maximum of 5 years after randomization. Details of the study results and design have been published elsewhere. 14–16
Measurement of 25(OH)D
25(OH)D was measured in serum samples collected at baseline, 6, 12, and 24 months. Samples were shipped to the central laboratory within 3 days of being drawn and then maintained at −20°C until further analysis. Only samples with a minimum of 2 mL serum were used for this study, resulting in 465 patients (out of 468 enrolled) with at least one 25(OH)D measurement, 417 with two or more, 396 with three or more, and 303 with all four measurements. Serum 25(OH)D was measured using an enzyme-linked immunosorbent assay (ELISA) (Immunodiagnostic Systems Inc. Fountain Hills, AZ). The average intra-assay coefficient of variation (CV) derived from blind quality control samples was 4·4% and the average inter-assay CV was 11·7%. As expected, serum 25(OH)D levels varied by season (Supplementary Figure 1). Baseline level of 25(OH)D was strongly correlated with levels at 12 (spearman correlation, r = 0·61) and 24 months (r=0·60), and moderately correlated with the opposite season levels at 6 months (r=0·30). Because the primary purpose of the study was to estimate the effects of long term average 25(OH)D levels, seasonal variations were removed as previously described. 4
Statistical analyses
Serum 25(OH)D was treated as a time-dependent variable, using at each time point the average of all previous values. Because the seasonally synchronous baseline, 12 month, and 24 month samples could provide a biased estimate of the year-around vitamin D status, most analyses were restricted to patients having 25(OH)D measured at both 6 and 12 months. The 12 month level was preferred to the baseline because the latter had to happen within 60 days after start of the CIS and thus could have been affected by the acute inflammatory process. To minimize the possibility that lower 25(OH)D levels were a consequence of MS severity rather than its cause, we also related the cumulative average 25(OH)D levels at 12 months with outcomes between 12 and 60 months or between 24 and 60 months (i.e. leaving a one-year lag between 25(OH)D measurement and assessment of MS activity or progression). Three sets of analyses were planned a priori, each with a different specification of serum 25(OH)D levels: i) continuous to determine the overall linear trend; ii) quintiles to explore the dose-response; iii) categorical with the following predefined intervals: < 25 nmol/L; 25-<50 nmol/L; 50-<75 nmol/L; and >=75 nmol/L; because of sparse data in the extreme groups, these categories were collapsed to <50 nmol/L vs >= 50 nmol/L.
Three outcome categories were analysed using clinical and MRI assessments: time to a definite diagnosis of MS, MS activity, and MS progression. A specially trained evaluating physician conducted all standardized neurological evaluations and determined the Expanded Disability Status Scale (EDSS).17 Relapses were assessed and defined in accordance with established guidelines. 18 MRIs were conducted every three months in the first year, and then at 18, 24, 36, 48 and 60 months. All MRIs were quality controlled and centrally evaluated by the Image Analysis Center in Amsterdam (lead by FB). Definite diagnosis of MS was determined by clinical and MRI criteria (McDonald MS [MDMS])19 and by purely clinical criteria (modified Poser criteria, 1983, referred to as CDMS). 18 MS-activity was assessed as the number of relapses and the number of new active lesions on brain MRI (defined as new T2 lesions, new Gd+ lesions or enlarging T2 lesions). MRI markers of progression were the percent change of T2 lesion volume and the percent change of brain volume. The change in brain volume was measured in the Magnetic Resonance Image Analysis Center in Basel (lead by EWR) using the Structural Image Evaluation using Normalization of Atrophy (SIENA) method20 focusing on the central cerebral volume. Due to rigorous criteria with respect to scan quality and brain coverage ~20% of the images were excluded from brain volume analysis. Considering that the baseline MRI was obtained at a time of acute inflammation (the CIS) with a high probability of partial spontaneous resolution of MRI pathology in the first year after the CIS 21 and considering the possible effect of resolution of edema and cellular infiltrates on atrophy measurements (pseudoatrophy) after initiation of antiinflammatory MS therapies 22, the month 12 visit was used as the primary reference point for the analysis of the percent change in T2 volume and brain volume. Clinically, progression was assessed by changes of the EDSS over time. Because spontaneous recovery of neurological deficits due to the CIS was expected after the baseline visit 16 (Table e1), month 6 was used as the primary reference point for analysis of the EDSS outcome.
MDMS and CDMS were analyzed using a Cox proportional hazards regression model. Because many conversions occurred during the first 6 months, these results are strongly dependent on 25(OH)D levels measured close to the time of the first clinical event, which may not accurately reflect a patient’s average vitamin D status. 4 Therefore, we also examined the relation between 25(OH)D levels and rate of conversion to MS using as baseline the 6 month or 12 month visit. Other outcomes, including relapse rate, number of new active MRI lesions, change in T2 lesion volume, percent brain loss, and change in EDSS were analyzed using a generalized mixed effects model treating patient as a random effect. Relapse rate was modeled as a binary variable indicating whether a relapse occurred on a given day, number of new active MRI lesions was modeled as a count variable, and the other outcomes were modeled as continuous variables. The within subject data were adjusted for any serial correlation that was present.
All the analyses were adjusted for sex, age at baseline, initial group of randomization (IFNB-1b or placebo), baseline T2 lesion score (the logarithm of the number of T2 lesions), and type of CIS (monofocal vs multifocal). Further adjustments for baseline body mass index (BMI) and use of steroids for the initial clinical event did not materially change the results and were therefore omitted. Because no significant interactions were present between 25(OH)D levels and either randomization group (initial-placebo or initial IFNB-1b) or sex in any of the above analyses only aggregate results are shown (results by treatment group are included as supplementary material). Of note, patients randomized to initial treatment had a mean exposure of 1387 days to IFNB-1b, whereas patients randomized to initial placebo were exposed for 498 days to placebo and for 1016 days to IFNB-1b (means). P values were not corrected for multiple comparisons.
Results
Patient characteristics and 25(OH)D
Patients with higher 25(OH)D levels tended to have a lower age, a lower BMI, a lower number of T2 lesions and a higher brain volume at the CIS, but were otherwise similar to those with lower levels (Table 1). Patient characteristics in the subgroup with month 6 and 12 measurements did not differ from these results (see Table e1). The distribution of 25(OH)D levels by visit and by season is shown in figures e1 and e2).
Table 1.
Selected characteristics of BENEFIT participants by average of baseline, 6M, and 12M season-adjusted 25(OH)D level
| Quintiles of 25(OH)D | Low vs. high 25(OH)D | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Low (<50 nmol/L) | High (≥50 nmol/L) | |
|
|
|||||||
| N patientsa | 93 | 93 | 92 | 93 | 93 | 251 | 213 |
| 25(OH)D, nmol/L | |||||||
| range | 19–36 | 36–45 | 45–52 | 52–61 | 61–98 | 19–50 | 50–98 |
| median | 31 | 40 | 48 | 56 | 69 | 39 | 60 |
| Age, at recruitment mean (SD) | 30·4 (7.9) | 32·7 (7·3) | 29·3 (7·3) | 30·5 (7·0) | 30·8 (7·5) | 31·3 (7·6) | 30·1 (7·2) |
| Female, % | 69·9 | 77·4 | 65·2 | 71·0 | 71·0 | 71·7 | 70·0 |
| Randomized to IFNB-1b, % | 65·6 | 62·4 | 53·3 | 57 | 73·1 | 59·8 | 65·3 |
| Monofocal onset, % | 53·8 | 54·8 | 48·9 | 51·6 | 52·7 | 52·6 | 52·1 |
| Number of T2 lesions at baseline, median (Q1–Q3) | 21 (10–37) | 19 (9–45) | 16 (7–31) | 15 (7–36) | 16 (6–44) | 20 (8–38) | 15 (7–37) |
| T2 volume at baseline, mm3 median (Q1–Q3) | 2091 (598·0–5165·0) | 1883 (772·0–5184·0) | 1860 (706·3–3227·5) | 1711 (506·0–4340·0) | 1942 (764·0–5536·0) | 1903 (629·5–4549·0) | 1871 (621·0–4602·0) |
| Central brain volume at baseline, cm3 median (Q1–Q3) | 1045 (1026–1072) | 1040 (1017–1065) | 1051 (1019–1080) | 1047 (1028–1078) | 1070 (1032–1085) | 1045 (1017–1072) | 1059 (1029–1085) |
| BMI, mean (SD) | 25·2 (5·5) | 24·2 (4·5) | 24·4 (4.0) | 23·8 (3.8) | 23·9 (4·4) | 24·6 (4·8) | 24·0 (4·0) |
| Steroids for 1st clinical event, % | 80·6 | 64·5 | 71·7 | 74·2 | 64·5 | 72·9 | 69·0 |
| Time to last EDSS, mean (days) | 1558·5 | 1692·3 | 1637·6 | 1574·2 | 1691·8 | 1632·9 | 1629·6 |
| Time to last MRI, mean (days) | 1483·1 | 1609·7 | 1578·7 | 1512·1 | 1615·0 | 1552·1 | 1570·2 |
N= 464 patients, because one patient only had 25(OH)D at 24 months.
Conversion to definite MS
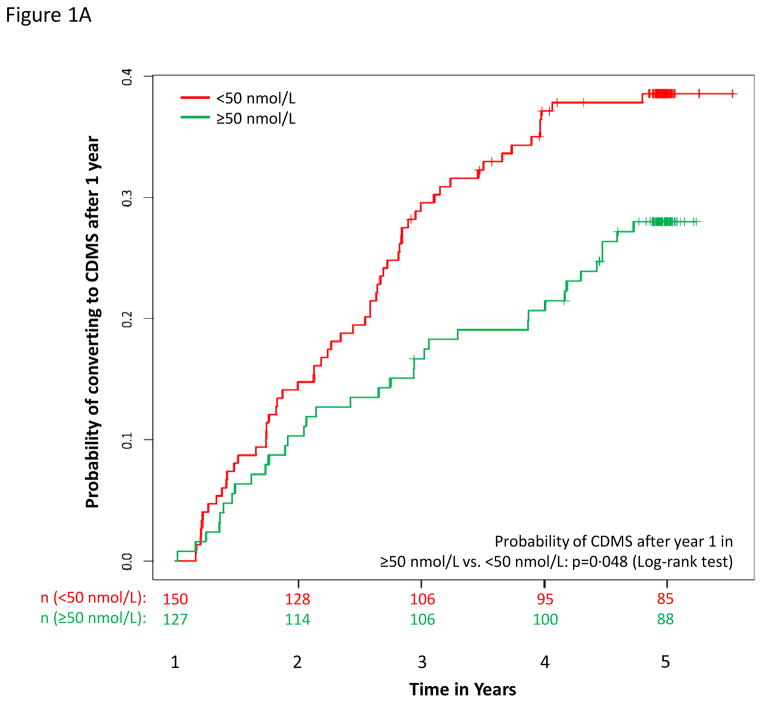
During the 5-years of follow-up, 377 (81·3 %) patients converted to MDMS and 216 (46·6 %) converted to CDMS. The hazard of conversion decreased with increasing serum 25(OH)D, more strongly after 6 months and among patients with both 6 and 12 months 25(OH)D, among whom the hazard of conversion decreased by over 50% for a 50 nmol/L (20 ng/mL) increase in 25(OH)D (Table e2). Results by treatment group are in Table e2 and by quintiles in Figure e3. Mean serum 25(OH)D levels at 12 months contributed to predict subsequent conversions to MDMS (p = 0·018)and CDMS (p = 0·048, Figure 1a).
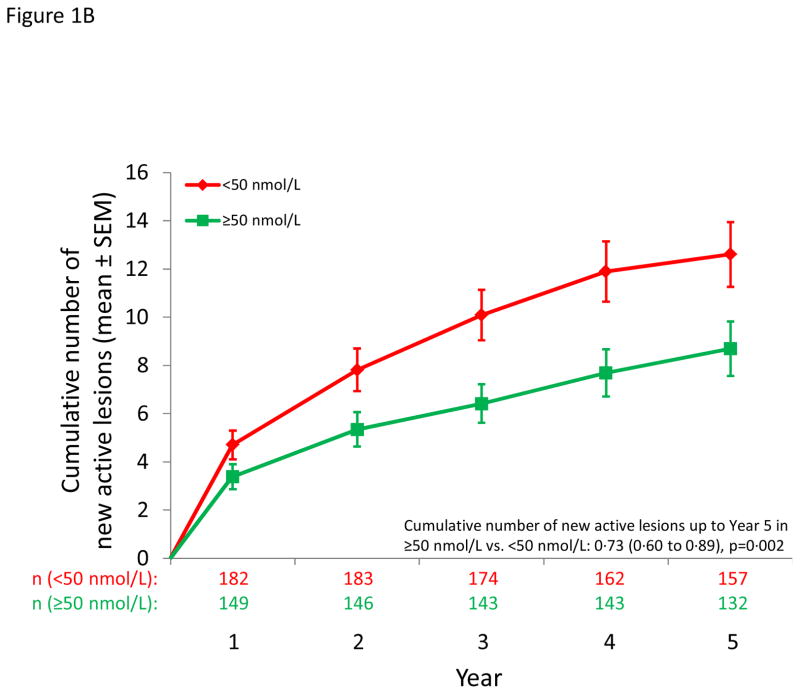
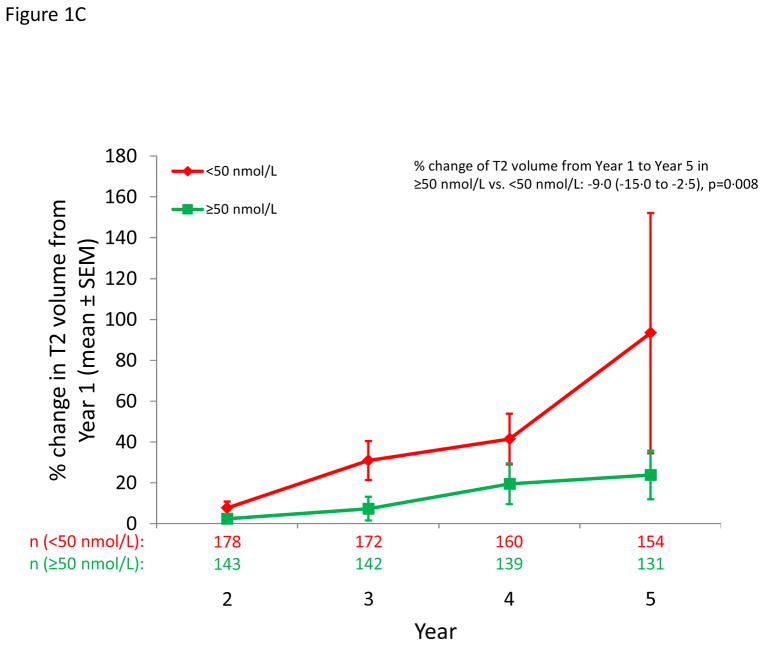
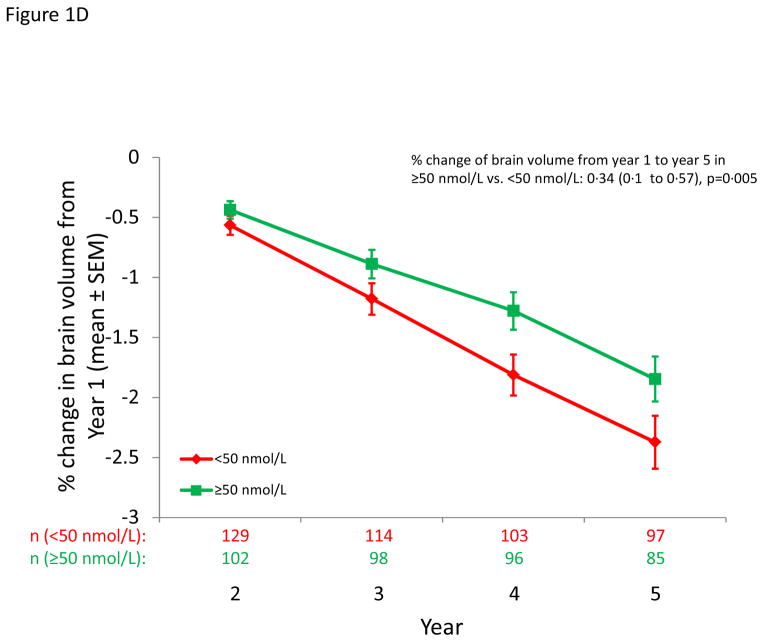
Figure 1.
MS outcomes according to dichotomous serum 25(OH)D levels. A: Probability of conversion to CDMS after 12 months; B) Cumulative number of new active lesions on brain MRI; C: % change in T2 lesion volume from year 1 to year 5 on brain MRI; D: % change in brain volume from year 1 to year 5.
MS activity
New active MRI lesions
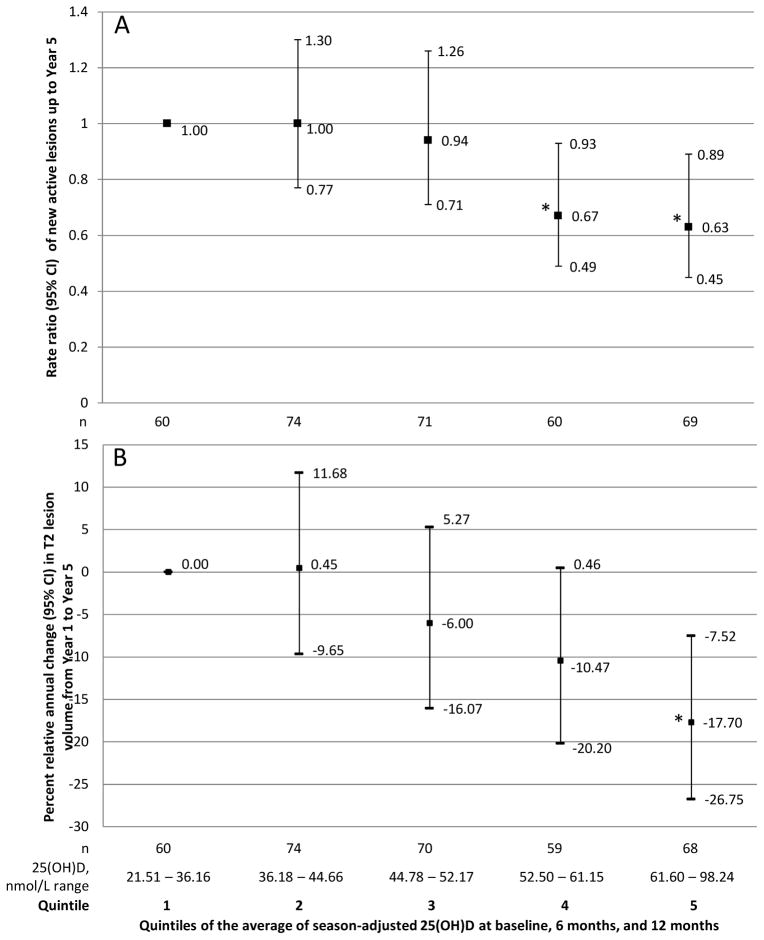
The rate of occurrence of new active lesions decreased with increasing serum 25(OH)D (Table 2); this inverse association was particularly strong for patients with both 6 and 12 month 25(OH)D, among whom a 50 nmol/l increase in the average 25(OH)D up to 12 months predicted a 57% lower rate between 12 and 60 months and a 63% lower rate between 24 and 60 months (Table 2). Significant inverse associations were also observed in categorical analyses (dichotomous, Figure 1B, quintiles Figure 2A). Results by treatment group are in Table e3.
Table 2.
Rate ratios (RR) for new active lesions on brain MRI and MS relapses according to a 50 nmol/L increment in serum 25(OH)D levels.
| Period | All patients (N=454) | No missing 6 or 12M 25(OH)D (N=334) | ||||||
|
| ||||||||
| Baseline to 60M | Baseline to 60M | 12M to 60M | 24M to 60M | |||||
|
| ||||||||
| 25(OH)D | Cumulative average - Updated to 24M | Cumulative average - Updated to 24M | Cumulative average – updated to 12M | Cumulative average – updated to 12M | ||||
|
| ||||||||
| RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | |
| New active lesions | 0·61 (0·44–0·83) | 0·002 | 0·54 (0·37–0·78) | 0·001 | 0·43 (0·26–0·70) | 0·0009 | 0·37 (0·21–0·63) | 0·0004 |
| Relapses | 0·73 (0·46–1·16) | 0·19 | 0·66 (0·38–1·17) | 0·16 | 0·43 (0·20–0·92) | 0·03 | 0·41 (0·16–1·06) | 0·06 |
RR adjusted for age, sex, treatment, time of follow-up, T2 lesion score at baseline, and type of onset.
Figure 2.
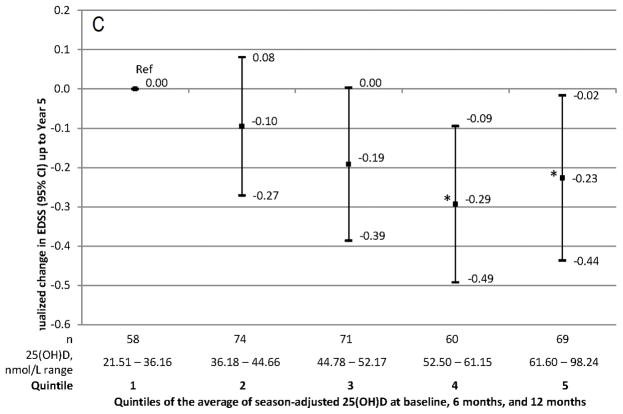
MRI evidence of MS activity (panels A and B) and progression (panel C). A: Rate ratio of new active lesions on brain MRI up to year 5. B: Change of T2 lesion volume on brain MRI from year 1 to year 5 by quintiles of serum 25(OH)D. C: Annualized change in EDSS from 6 months to year 5 by quintiles of serum 25(OH)D.
Relapses
On average, patients in BENEFIT experienced 0.2 relapses per year. Overall, the relapse rate decreased by 27% (not significant) for a 50 nmol/L increment in 25(OH)D (Table 2). This association was stronger among patients with both 6 and 12 months 25(OH)D; in this group, a significantly lower relapse rate with increasing serum 25(OH)D was observed after 12 months. No significant associations were found in analyses based on categorical 25(OH)D level (Figure e3).
Progression of MS
Change of T2 volume
Higher levels of 25(OH)D were associated with less T2 lesion volume accumulation over time; the relative decrease in T2 lesion volume for a 50 nmol/L increase in 25(OH)D was 20% per year (p=0·00002) (Table 3). Restriction to patients with both 6 month and 12 month 25(OH)D tended to strengthen the results. Results by treatment group are in Table e4. Results by dichotomous 25(OH)D are shown in Figure 1C, and those by quintiles in Figure 2B.
Table 3.
Percent annual change in cerebral T2 lesion volume and brain volume, and change of EDSS for a 50 nmol/L increment in serum 25(OH)D.
| Patients | All (N=418) | No missing 6M or 12M 25(OH)D (N=331) | ||||
|
| ||||||
| Period | 12M to 60M | 12M to 60M | 24M to 60M | |||
|
| ||||||
| 25(OH)D | Cumulative average -Updated to 24M | Cumulative average – updated to 12M | Cumulative average – updated to 12M | |||
|
| ||||||
| % change (95% CI) | p | % change (95% CI) | p | % change (95% CI) | p | |
| T2 lesion volume | −20 (−28 to −11) | 0·00002 | −25 (−34 to −14) | 0·00004 | −27 (−37 to −16) | 0·00003 |
| Patients | All (N=324) | No missing 6M or 12M 25(OH)D (N=252) | ||||
|
| ||||||
| Period | 12M to 60M | 12M to 60M | 24M to 60M | |||
|
| ||||||
| 25(OH)D | Cumulative average -updated to 24M | Cumulative average – updated to 12M | Cumulative average – updated to 12M | |||
|
| ||||||
| % change (95% CI) | p | % change (95% CI) | p | % change (95% CI) | p | |
| Brain volume | 0·27 (−0·07 to 0·62) | 0·12 | 0·41 (−0·03 to 0·85) | 0·07 | 0·48 (−0·03 to 1·00) | 0·07 |
| Patients | All (N=434) | No missing 6M or 12M 25(OH)D (N=332) | ||||
|
| ||||||
| Period | 6M to 60M | 6M to 60M | 24M to 60M | |||
|
| ||||||
| 25(OH)D | Cumulative average -updated to 24 M | Cumulative average – updated to 12M | Cumulative average – updated to 12M | |||
|
| ||||||
| change (95% CI) | p | change (95% CI) | p | change (95% CI) | p | |
| EDSS | −0·16 (−0·37 to 0·04) | 0·11 | −0·18 (−0·44 to 0·09) | 0·19 | −0·20 (−0·49 to 0·10) | 0·19 |
All analyses adjusted for age, sex, treatment, time of follow-up, T2 lesion score at baseline, and type of onset.
Change of brain volume
A 50 nmol/L (20 ng/mL) increase in 25(OH)D was associated with a 0·27% lower rate of brain loss (p=0·12) (Table 3). In analyses excluding patients missing the 6 or 12 month 25(OH)D levels, the overall 25(OH)D-related annual difference in brain volume loss for a 50 nmol/L (20 ng/mL) increase in 25(OH)D was 0·41% (p=0·07). Results by treatment group are in Table e4. In analyses with dichotomous 25(OH)D, the percent loss in brain volume was lower among patients with 25(OH)D concentrations at 12 months >=50 nmol/L (20 ng/mL) as compared to those <50 nmol/L (20 ng/mL) (0·34%; p=0·005; Figure 1D). Analyses by quintiles revealed an unexpected J-shaped relation (higher brain volume in the lowest quintile, (Figure e4).
Change in EDSS
A 50 nmol/L increase in 25(OH)D levels was associated with a reduction of 0·16 steps in the average EDSS (p=0·11). Results restricted to patients with measured 6 and 12 month 25(OH)D were similar (Table 3). The annualized change in EDSS was lower among patients with serum 25(OH)D >=50 nmol/L as compared with those < 50 nmol/L (−0·17; p=0·004), as well as in patients in the highest quintiles compared to those in the lowest quintile (Figure 2C).
Discussion
In this large prospective investigation we found that average serum 25(OH)D levels in the first 12 months following a CIS strongly predicted MS activity and progression over the subsequent 4 years. By the end of the follow-up at 60 months, those patients with serum 25(OH)D concentrations >=50 nmol/L had a four times lower change in T2 lesion volume, a two-fold lower rate of brain atrophy, and lower disability than those below 50 nmol/L. Although associations were generally stronger for MRI than for clinical outcomes, the latter were still remarkable considering the overall low rate of relapses (0·2/year) and small EDSS change (median change 0·0) in BENEFIT. Moreover, the profound association of 25(OH)D levels with MRI measures of disease activity and progression is of particular clinical relevance for CIS patients, in which the prognostic value of MRI pathology for the development of long-term disability has been demonstrated. 23,24 It is also noteworthy that the inverse relation of 25(OH)D levels with development of MS, relapses and MRI measures was observed in a population being treated with IFNB-1b which had a considerable impact on these outcomes in the present study. 14,16,21
Distinctive strengths of this study include the longitudinal design, recruitment of all patients at the time of CIS, repeated measurement of serum 25(OH)D levels, large number of participants, standardized treatment (early vs late IFNB-1b), and rigorous clinical and MRI assessment of all patients during a 5 year period. 16 Because serum 25(OH)D levels strongly depend on time spent outdoors, which can in turn be affected by MS activity, reverse causation could have explained the cross-sectional or short term inverse associations between serum 25(OH)D concentrations and MS activity previously reported. 9–12,25,26 The robustness of our results in analyses leaving a one-year lag-time between the last serum 25(OH)D measurement and assessment of MS outcomes provides evidence that low vitamin D was not a consequence of the disease process itself, but rather its predictor.
Our study also has some limitations. First, almost all patients in our study were whites of European ancestry, thus limiting generalizations to individuals of other races or ethnicities. Second, the majority of participants were eventually treated with IFNB-1b – although uniform treatment is an important advantage, our results may not apply to patients treated with different drugs. Third, although a clear dose-response was observed for the most sensitive MRI outcomes, there was no evidence of a ceiling effect, and it is thus possible that the potential benefits of vitamin D are fully reached only at serum 25(OH)D concentrations above the still moderate level observed in the highest quintile of participants in BENEFIT (median: 69 nmol/L or 27.6 ng/mL).
The combined results of previous epidemiological studies relating serum 25(OH)D levels to MS risk4–6 and those of the present investigation imply that either serum 25(OH)D levels directly affect the disease process before and after the onset of symptoms, or act as a prognostic marker. The inverse association between vitamin D and MS outcomes could be explained if both were affected by a common factor or confounder. Among healthy individuals, the main predictors of 25(OH)D levels, besides vitamin D intake, are exposure to UV light, BMI, and genetic factors. 27–29 UV light has some immunosuppressive effects not mediated by vitamin D 30,31, which could contribute to the associations reported here, but the inverse association between vitamin D intake and MS risk3 and the beneficial effects of vitamin D in animal models of MS 32,33 favor a role of vitamin D itself. Differences in BMI are also unlikely to explain our results, because adjustment for this factor was inconsequential. Finally, genetic effects 28,29 are too small to account for the strong inverse associations reported here. 34 As in all observational studies, a role of unknown factors cannot be excluded. Results of three small double blind randomized trials have been inconsistent35–37, but these studies were not powered to determine the efficacy of vitamin D on MS outcomes.
The results of our study reveal a robust prognostic value of vitamin D levels measured early in the MS course, and converge with previous epidemiological and biological evidence supporting a protective effect of vitamin D on the disease process underlying MS38, and thus the importance of correcting vitamin D insufficiency, which affects about 50% of MS patients in Europe39 and 20% in the U.S.11,40 Further investigations, however, are needed to determine the optimal levels of vitamin D and whether results apply to different races or ethnicities, to patients with the secondary or primary progressive course of MS, or in combination with drugs other than IFNB-1b.
In summary, in this large longitudinal study among patients with CIS randomized to early versus late treatment with IFNB-1b, we found that higher serum 25(OH)D levels robustly predicted a lower degree of MS activity, MRI lesion load, brain atrophy, and clinical progression over the 5 years of follow-up. Although controlled studies currently underway41 may provide more definitive answers as to the therapeutic value of further increasing already adequate vitamin D levels, our results suggest that identification and correction of vitamin D insufficiency has an important role in the early treatment of MS.
Supplementary Material
Acknowledgments
Dr. Ascherio had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would like to acknowledge the BENEFIT participants, Robert Ristuccia for technical support, and Leslie Unger for administrative support.
Funding: National Institute of Neurological Diseases and Stroke and National Multiple Sclerosis Society.
Financial Disclosures
A Ascherio has received honoraria for speaking at scientific symposia by Roche, Sanofi Aventis, Serono, and Almirall.
KL Munger has no disclosures.
R White has served as a consultant for and received financial compensation from Bayer Schering Pharma.
K Köchert is a statistical consultant paid by Bayer Pharma AG.
KC Simon has no conflicts of interest to disclose.
CH Polman has received personal compensation from Biogen Idec, Bayer Schering Pharma AG, Teva Pharmaceutical Industries, Merck Serono, Novartis Pharmaceutical Corporation, GlaxoSmithKline, Actelion Pharmaceuticals Ltd, UCB, and Roche for consulting services. The VU University Medical Center received financial support for research activities from Bayer Schering, Biogen Idec, Merck Serono, Teva, Novartis, GSK, and Roche.
MS Freedman has received personal compensation and research support from Teva Pharmaceutical Industries, Merck Serono, Bayer Schering Pharma AG, Biogen-Dompé, and Genmab.
H-P Hartung has received honoraria for consulting and speaking at symposia from Bayer-Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, Teva, and Sanofi Aventis, with approval by the rector of Heinrich-Heine University.
DH Miller has received honoraria and compensation through payments to University College London (UCL) Institute of Neurology, for advisory committee and/or consultancy advice in multiple sclerosis studies from Biogen Idec, GlaxoSmithKline, and Bayer Schering, and for work as editor of Journal of Neurology. DM has received research grant support through UCL Institute of Neurology, for performing central MRI analysis of MS trials from GlaxoSmithKline, Biogen Idec, and Novartis.
X Montalban has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Bayer, Biogen Idec, EMD, Genentech, Genzyme, Merck Serono, Neurotec, Novartis, Sanofi-Aventis, Teva Pharmaceuticals and Almirall.
G Edan has received honoraria for lectures or consulting from Biogen Idec, Merck Serono and sanofi-aventis, received personal compensation for serving on the BENEFIT scientific advisory board, and for speaking from Bayer Schering Pharma AG. GE has also received research support from Serono: grant to University Hospital to support a research program on MRI in MS and from Teva: grant to support a research program on anti-IBF neutralizing antibodies.
F Barkhof has received compensation for consultancy from Bayer-Schering Pharma, Biogen-Idec, Merck Serono, Novartis, Sanofi Aventis, Genzyme, Roche, Teva and has received research support from the Dutch Foundation for MS research (an NGO).
D Pleimes is a salaried employee of Bayer Pharma AG/Bayer HealthCare Pharmaceuticals. DP owns stock in Bayer AG, the owner of Bayer Pharma AG/Bayer HealthCare Pharmaceuticals
E W Radü Has received honoraria for serving as speaker at scientific meetings and consultant for Novartis, Biogen Idec, Merck Serono, Bayer Schering. He has received financial support for research activities from Actelion, Basilea Pharmaceutica Ltd, Biogen Idec, Merck Serono and Novartis.
R Sandbrink is a salaried employee of Bayer Pharma AG/Bayer HealthCare Pharmaceuticals. RS owns stock in Bayer AG, the owner of Bayer Pharma AG/Bayer HealthCare Pharmaceuticals.
L Kappos has lectured at medical conferences or in public and received honoraria which have been exclusively used for funding research for his department. Research and the clinical operations (nursing and patient care services) of the MS Center in Basel have been supported by nonrestricted grants from Acorda Therapeutics, Inc., Actelion Pharmaceuticals Ltd, Allozyne, BaroFold, Bayer HealthCare Pharmaceuticals, Bayer Schering Pharma, Bayhill, Biogen Idec, Boehringer Ingelheim, Eisai, Inc., Elan, Genmab, GlaxoSmithKline, Merck Serono, MediciNova, Novartis, sanofi-aventis, Santhera Pharmaceuticals, Shire plc, Roche, Teva, UCB, Wyeth and by grants from the Swiss MS Society, the Swiss National Research Foundation, the European Union, the Gianni Rubatto Foundation, Novartis, and Roche Research Foundations.
C Pohl is a salaried employee of Bayer Pharma AG/Bayer HealthCare Pharmaceuticals. CP owns stock in Bayer AG, the owner of Bayer Pharma AG/Bayer HealthCare Pharmaceuticals.
References
- 1.Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007 Oct;62(4):335–346. doi: 10.1002/ana.21163. [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006 Mar 2;354(9):942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 3.Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 4.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006 Dec 20;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 5.Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012 Nov 20;79(21):2140–2145. doi: 10.1212/WNL.0b013e3182752ea8. [DOI] [PubMed] [Google Scholar]
- 6.Banwell B, Bar-Or A, Arnold DL, et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol. 2011 May;10(5):436–445. doi: 10.1016/S1474-4422(11)70045-X. [DOI] [PubMed] [Google Scholar]
- 7.Martinelli VDCG, Colombo B, Dalla Libera D, Rubinacci A, Filippi M, Furlan R, Comi G. Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Multiple Sclerosis Journal. 2013 doi: 10.1177/1352458513494959. [DOI] [PubMed] [Google Scholar]
- 8.Simpson S, Jr, Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010 Aug;68(2):193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 9.Runia TF, Hop WCJ, De Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79(3):261–266. doi: 10.1212/WNL.0b013e31825fdec7. [DOI] [PubMed] [Google Scholar]
- 10.Løken-Amsrud KI, Holmøy T, Bakke SJ, et al. Vitamin D and disease activity in multiple sclerosis before and during interferon beta treatment. Neurology. 2012;79(3):267–273. doi: 10.1212/WNL.0b013e31825fdf01. [DOI] [PubMed] [Google Scholar]
- 11.Mowry EM, Waubant E, McCulloch CE, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012 Aug;72(2):234–240. doi: 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ascherio A, Marrie RA. Vitamin D in MS: A vitamin for 4 seasons. Neurology. 2012 Jul 17;79(3):208–210. doi: 10.1212/WNL.0b013e31825fe131. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006 Oct 10;67(7):1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- 15.Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007 Aug 4;370(9585):389–397. doi: 10.1016/S0140-6736(07)61194-5. [DOI] [PubMed] [Google Scholar]
- 16.Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009 Nov;8(11):987–997. doi: 10.1016/S1474-4422(09)70237-6. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Poser C, Paty D, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 19.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuro Image. 2002 Sep;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 21.Barkhof F, Polman CH, Radue EW, et al. Magnetic resonance imaging effects of interferon beta-1b in the BENEFIT study: integrated 2-year results. Arch Neurol. 2007 Sep;64(9):1292–1298. doi: 10.1001/archneur.64.9.1292. [DOI] [PubMed] [Google Scholar]
- 22.Zivadinov R, Reder AT, Filippi M, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008 Jul 8;71(2):136–144. doi: 10.1212/01.wnl.0000316810.01120.05. [DOI] [PubMed] [Google Scholar]
- 23.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008 Mar;131(Pt 3):808–817. doi: 10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- 24.Tintore M, Rovira A, Rio J, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology. 2006 Sep 26;67(6):968–972. doi: 10.1212/01.wnl.0000237354.10144.ec. [DOI] [PubMed] [Google Scholar]
- 25.Mowry EM, Krupp LB, Milazzo M, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. 2010 May;67(5):618–624. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 26.Stewart N, Simpson S, Jr, van der Mei IA, et al. Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology. 2012;79(3):254–260. doi: 10.1212/WNL.0b013e31825fded9. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand KA, Giovannucci E, Liu Y, et al. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr. 2012 Nov;108(10):1889–1896. doi: 10.1017/S0007114511007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn J, Stolzenberg-Solomon R, Simon K, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas RM, Ponsonby AL. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006 Sep;92(1):140–149. doi: 10.1016/j.pbiomolbio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Becklund BR, Severson KS, Vang SV, Deluca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci U S A. 2010;107(14):6418–6423. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. Faseb J. 2001 Dec;15(14):2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 33.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005 Sep 15;175(6):4119–4126. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 34.Simon KC, Munger KL, Kraft P, Hunter DJ, De Jager PL, Ascherio A. Genetic predictors of 25-hydroxyvitamin D levels and risk of multiple sclerosis. J Neurol. 2011 Mar 23;258:1676–1682. doi: 10.1007/s00415-011-6001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein MS, Liu Y, Gray OM, et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011 Oct 25;77(17):1611–1618. doi: 10.1212/WNL.0b013e3182343274. [DOI] [PubMed] [Google Scholar]
- 36.Kampman MT, Steffensen LH, Mellgren SI, Jorgensen L. Effect of vitamin D3 supplementation on relapses, disease progression and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012 Feb 21;18(8):1144–1151. doi: 10.1177/1352458511434607. [DOI] [PubMed] [Google Scholar]
- 37.Soilu-Hanninen M, Aivo J, Lindstrom BM, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon beta-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:565–571. doi: 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 38.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012 Nov 5;8(11):602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007 Mar;85(3):860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 40.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002 May;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 41.Smolders J, Hupperts R, Barkhof F, et al. Efficacy of vitamin D(3) as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: A Phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011 Dec 15;311(1–2):44–49. doi: 10.1016/j.jns.2011.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.