Abstract
The effects of hyperbaric oxygen (HBO) on mouse skin two-stage chemical carcinogenesis were examined. Six-week-old inbred CD-1 female mice were divided into the following five groups: group 1, normoxia and application of 25 nmol 7,12-dimethylbenz[a]anthracene (DMBA) and 8.5 nmol 12-O-tetradecanoylphorbol-13-acetate (TPA) (n=19); group 2, HBO and DMBA/TPA (n=21); group 3, HBO and DMBA/acetone (n=3); group 4, normoxia and acetone (n=3); and group 5, non-treatment group (n=5). HBO was started at the same time as DMBA. Mice were euthanized at 23 weeks after the start of the experiment. Mice in group 2 showed the occurrence of tumors at 8 weeks after the beginning of the experiment, while the occurrence of tumors in mice in group 1 was observed beginning at 9 weeks. There was a difference in occurrence among low-grade papillomas, high-grade papillomas and SCCs in both groups 1 and 2 by the χ2-test at end of the experiment (p<0.05). The Ki-67 labeling indices of tumors revealed that the percentages of positive cells in low-grade papillomas in groups 1 and 2 were 15.27 ± 2.54% and 29.67 ± 2.82%, respectively (p<0.01). The results suggested that the tumors in group 2, which was treated with HBO, were more progressive than those in group 1, which was not treated with HBO. In this study, HBO accelerated tumor cell proliferation and advanced tumor progression in skin carcinogenesis by DMBA/TPA.
Keywords: hyperbaric oxygen, skin, neoplasm, two-stage chemical carcinogenesis, mice
Introduction
In general, hypoxia within tumor tissues plays a significant negative role in the treatment of malignant neoplasms, because the angiogenesis, evasion of apoptosis and increased glycolytic rate are all adaptations made by tumors in the hypoxic microenvironment1, 2. To improve therapeutic efficacy, recent efforts have been concentrated on the concept of eliminating the hypoxic state of tumors in order to remove the driving force behind these adaptations. Hyperbaric oxygen (HBO) therapy has been considered to control the hypoxia of the tumor microenvironment and possibly improve treatment outcome. HBO therapy refers to breathing pure (100%) oxygen under increased atmospheric pressure3,4,5. This potential capacity is believed to reflect an increase O2 level in tumor cells and conquer hypoxic situation by increased amount of dissolved oxygen in the tissue. HBO may elevate blood levels of active oxygen, which would generate free radicals and cause cellular DNA damage in tissues6. However, the effect of utilizing HBO for cancer treatments has not been clarified yet.
HBO has been reported to increase tumor radiosensitivity both in basic and clinical studies7. HBO has been used as combination treatment with chemotherapy and radiation therapy for malignant tumors8. In our University Hospital, HBO therapy has been used for wound healing, recovery of radiation-injured tissues and cancer treatment in neurosurgery and radiation oncology8. However, many clinicians and researchers do not yet recognize HBO therapy as an effective mechanism of cancer treatment. It still remains controversial in cancer treatment9, 10.
Therefore, the role and modifying mode of HBO with regard to tumors need to be analyzed. In this study, we examined the modification effects on tumors developed under an HBO environment in skin two-stage chemical carcinogenesis using 7,12-dimethylbenz[a]anthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA)11.
Materials and Methods
Animals, chemicals and HBO
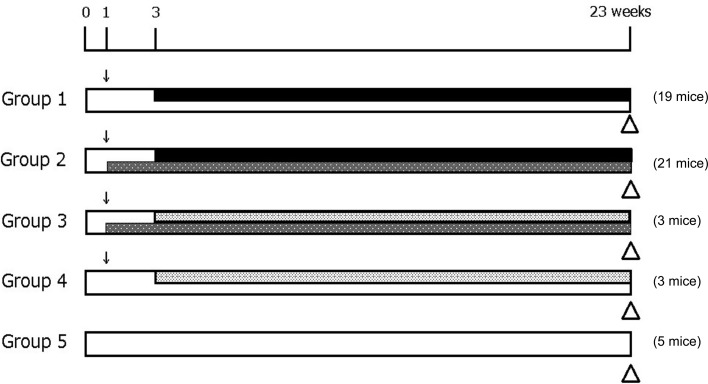
A total of 51 six-week-old inbred CD-1 female mice (Japan SLC, Hamamatsu, Japan) were housed in cages with access freely to pelleted diet (CE-2, CLEA Japan, Inc., Japan) and drinking water and exposed to a 12-hour light-dark cycle during the experimental period. Mice were divided into the following five groups: group 1, normoxia and DMBA/TPA (n=19); group 2, HBO and DMBA/TPA (n=21); group 3, HBO and DMBA/acetone (n=3); group 4, normoxia and acetone (n=3); and group 5, non-treatment group (n=5) (Fig. 1). Animal care and experiments were approved by the University of the Ryukyus Animal Ethics Committee and carried out in accordance with the guidelines for animal experimentation of the University of the Ryukyus. For two-stage chemical carcinogenesis, the dorsal skin of mice was shaved using surgical clippers. After a 1-week quarantine period, 25 nmol DMBA (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.2 ml acetone per mouse was topically applied to mice once except in group 5. After 2 weeks, we began twice-weekly applications of 8.5 nmol TPA (EMD Chemicals, San Diego, CA, USA) in 0.2 ml acetone per mouse in groups 1 and 2 (Fig. 1), and this was continued until the end of the experiment. Acetone was applied to mice in groups 3 and 4 instead of TPA.
Fig. 1.
Experimental design. ↓, DMBA; triangle, sacrifice; black bar, TPA; light gray bar, acetone; gray bar, HBO.
After DMBA was applied, mice in groups 2 and 3 were placed in a hyperbaric chamber (Barotec Hanyuda Co., Ltd., Tokyo, Japan) to be exposed to HBO. HBO was administered at a pressure of 2.2 ATA (atmospheres absolute) for 90 minutes. A minimum of 15 minutes of pressurization and depressurization was allowed for animals to adjust to the changes in pressure. HBO was administered 5 days a week. Mice were euthanized under deep anesthesia at 23 weeks from the start of the experiment (Fig. 1).
Measurement of tumor growth
Skin was examined for the presence of tumors, and the size and location of tumors were recorded. We counted the number and multiplicity of skin tumors in each mouse. Tumor size was measured externally by caliper at sacrifice. The volume of the tumor was calculated as:
V=4/3π (a)2(b), where (a) is the minor and (b) is the major axis (mm) of the tumor.
Histological analysis
Histopathologically, the skin tumors in groups 1 and 2 that were larger than 3.5 mm in diameter were examined by hematoxylin and eosin (HE) staining. According to the criteria of Conti et al.12, papillomas were judged based on two categories: low-grade papilloma, which is a well-differentiated hyperplastic lesion with no atypical cells or with very few atypical cells in the basal layer, and high-grade papilloma, which is a lesion with more than two-thirds of the thickness of the epithelium occupied by atypical cells. For inflammation, the induced inflammation state was divided into persistent and active; persistent: it appears almost lymphocyte infiltration in tumoral stroma with slightly edema; active: it appears predominantly neutrophil infiltration with lymphocytes in tumoral stroma, with increased and dilated vessels.
Immunohistochemical analysis
In order to measure cell proliferation in the skin tumor, the Ki-67 labeling index (LI) was determined. Immunohistochemical staining was performed as described in our previous study13. The embedded tissues were cut into 4-μm sections and then stained using anti-Ki-67 antibody (Dako, Carpinteria, CA, USA) and an LSAB Kit (Dako). Five hot spots within each tumor were selected, and the number of positive cells (dense brown precipitate restricted to the nuclei) in 500 cells for each tumor was counted to determine the Ki-67 LI, which was defined as the proportion of positive cells. The histopathological diagnosis and Ki-67 LI evaluation were confirmed by multiple pathologists.
Statistical analysis
Data obtained in this study are presented as means ± SEM (standard error of the mean). We used InStat (GraphPad Software, La Jolla, CA, USA) for data analysis. Welch’s t test or the χ2-test was used to determine the significance of differences between groups. P values of <0.05 were considered significant.
Results
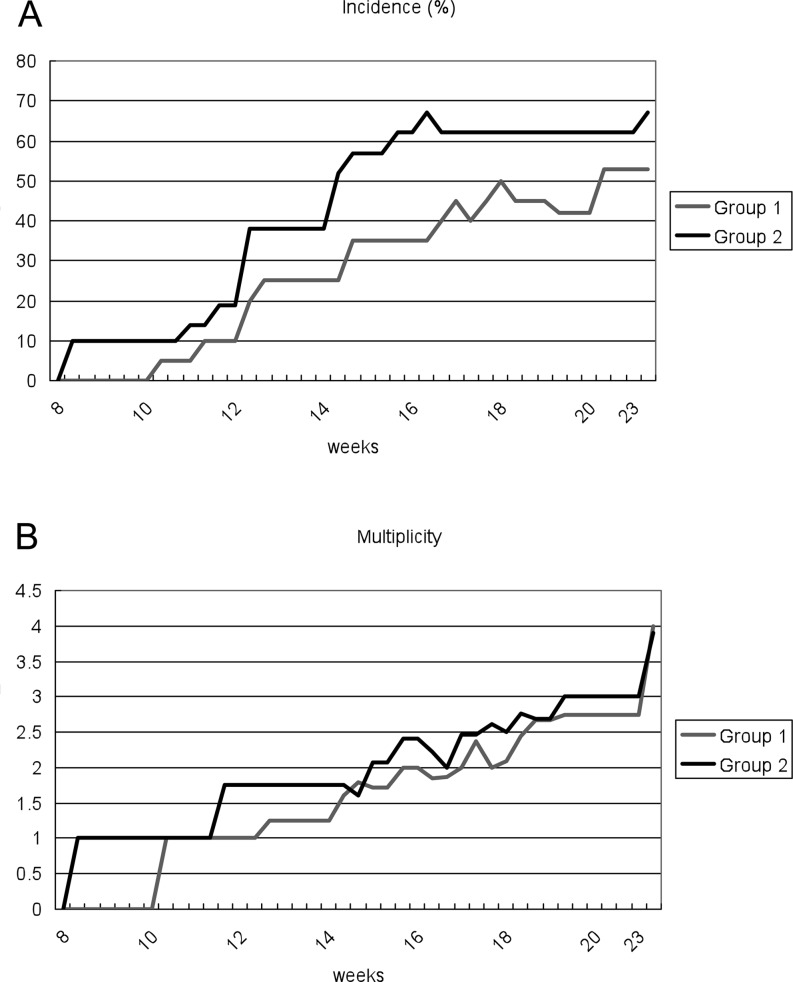
All mice survived throughout the experimental period. There were no significant differences in the initial or final body weights between mice in all groups. The appearance of tumors in group 2 occurred at 8 weeks after the beginning of the experiment, whereas they began to appear in group 1 at 9 weeks. At 12 weeks, the incidences of tumors in groups 1 and 2 were 20% and 38%, respectively (Fig. 3). Ten of 19 mice in group 1 and 14 of 21 mice in group 2 had macroscopic tumors on the surface of dorsal skin at the end of the experiment (Fig. 2 and Table 1). Final incidences of tumors in groups 1 and 2 were 53% and 67%, respectively (Table 1). The final multiplicities of tumors in groups 1 and 2 were 3.30 ± 0.87 and 3.35 ± 0.64, respectively (Table 1). There were no significant differences in tumor incidence and multiplicity between groups 1 and 2. Although the average volume (21.75 ± 9.03 mm3) of tumors in group 2 was greater than that in group 1 (13.81 ± 4.63 mm3), there was no significant difference between these groups (Table 1). No effects on the skin were observed in groups 3, 4 and 5. In addition, none of the other organs were affected by HBO in any group.
Fig. 3.
Incidence (A) and multiplicity (B) of skin tumor in groups 1 and 2.
Fig. 2.
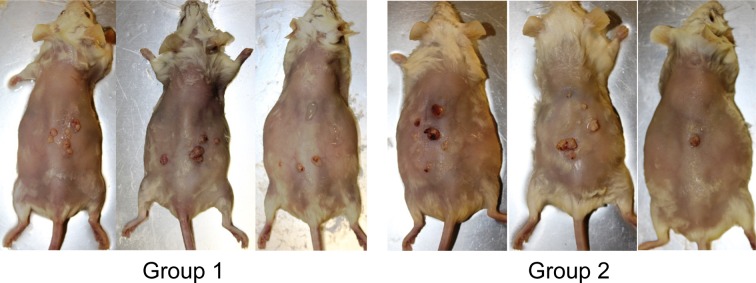
Representative images of the gross appearance of the skin tumors in groups 1 and 2 at the end of the experiment (23 weeks).
Table 1. Summary of Macroscopic Results in Groups 1 and 2 at the End of the Experiment (23 weeks).
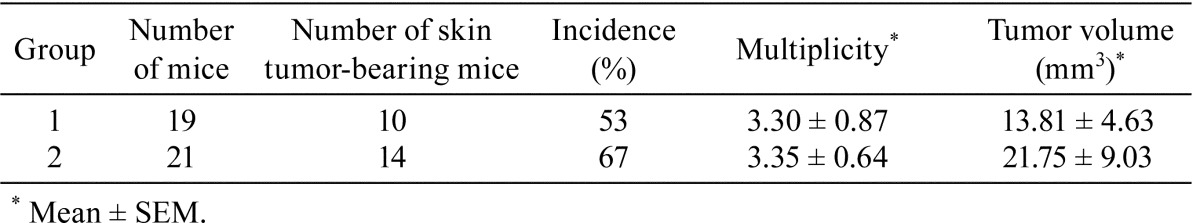
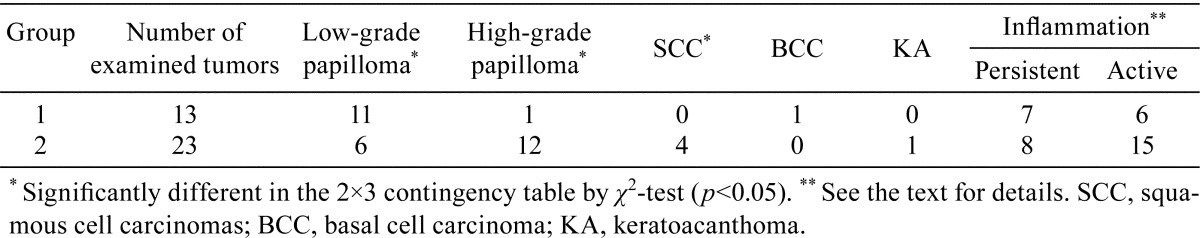
Histopathologically, the skin tumors larger than 3.5 mm in diameter in group 1 included 11 low-grade papillomas, 1 high-grade papilloma and 1 basal cell carcinoma (BCC), while there were 6 low-grade papillomas, 12 high-grade papillomas, 4 squamous cell carcinomas (SCCs) and 1 keratoacanthoma (KA) in group 2 (Table 2). There was the difference in the occurrence of tumors showing low-grade and high-grade papillomas and SCCs between these groups according to the χ2-test (p<0.05, Table 2). Compared with the stromal inflammation reactions of the tumors in group 1, those in group 2 tended to be more associated with leukocyte infiltration and edema in the stroma, without statistical significance (Table 2).
Table 2. Histopathological Findings of Tumors in Groups 1 and 2.
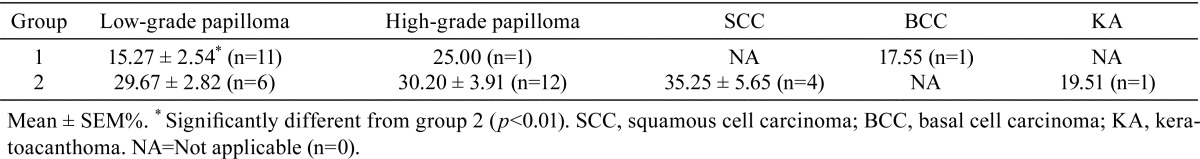
Concerning the effect of HBO on cell proliferation, the Ki-67 LI was analyzed in groups 1 and 2. The Ki-67 LIs for low-grade papilloma, high-grade papilloma, SCC, BCC and KA are summarized in Table 3. The Ki-67 LIs for low-grade papilloma in groups 1 and 2 were 15.27 ± 2.54% and 29.67 ± 2.82%, respectively, and there was a significant difference in Ki-67 LI for low-grade papillomas between groups 1 and 2 according to the Welch’s t test (p<0.01). However, there was no significant difference in the Ki-67 LI for high-grade papillomas between these two groups.
Table 3. Ki-67 Labeling Indices of Tumors in Groups 1 and 2.
Discussion
To the best of our knowledge, this study is the first report to examine an effect of HBO on a mouse skin two-stage chemical carcinogenesis model in vivo. We tried to compare the tumorigenesis and proliferative state in the chemical carcinogenesis model between HBO and normoxia groups. In the past, many similar experiments were performed to culture cells but there were a few studies in vivo2, 7, 14, 15. In the clinical study of advanced epithelial tumors of the head and neck, treatment with HBO markedly suppressed local tumor growth and significantly suppressed remote metastasis of a tumor to the lung7, 10. HBO has been applied to clinical practice16; however, the effect of HBO on tumors has not been clarified. HBO therapy has been used in clinical medicine in combination with radiotherapy17,18,19 or chemotherapy20 for cancer treatment, but no obvious answer has been reported concerning the efficacy of HBO alone against tumors21. In this study, the experiment was designed to examine the effect on tumor cells actually in an environment similar to a living body in a mouse chemical carcinogenesis model. The results showed that the tumor volume in group 2 was greatly increased compared with that of group 1; that is, HBO hastened the growth of tumors, although there was no statistical difference (Fig. 2 and Table 1). Pande et al.22 also reported a similar result, i.e., there was accelerated growth and progression of tumors after HBO therapy. Furthermore, McMillan et al.15 reported that HBO appears to have a stimulatory effect during the proliferative phase of carcinoma in hamster cheek pouch carcinogenesis.
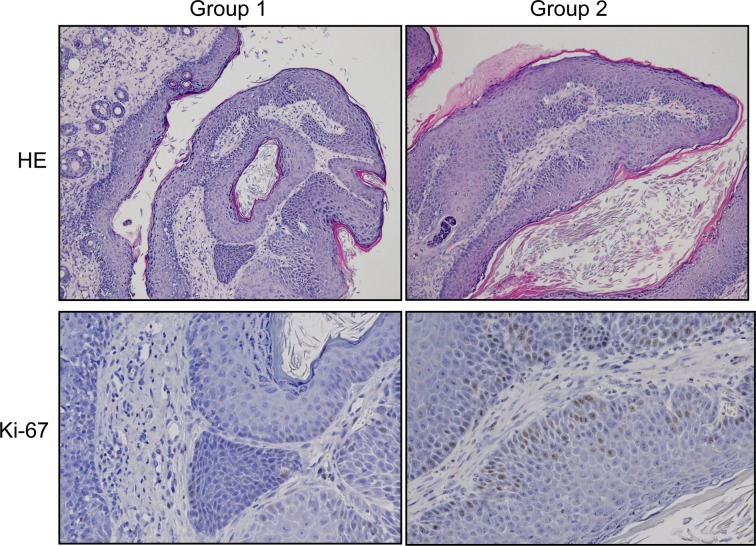
Histopathologically, the appearance of the tumors in group 2 was more progressive or aggressive than that in group 1 (Table 2). This suggested that the HBO treatment under the present conditions had a proliferative and aggressive affect on tumor cells. We also found that the cell proliferation of low-grade papillomas in group 2 with HBO was higher than that in group 1 without HBO, although there was no statistical difference in cell proliferation of high-grade papillomas between groups 1 and 2 (Fig. 4 and Table 3). It seems that HBO influences cell growth. Generally, HBO is often used in combination with radiation therapy8. The combination of HBO and radiation therapy is particularly effective for local tumor control according to the results of a trial of the British Medical Research Council23. The effectiveness of the combination of chemotherapy and HBO has also been reported by Stuhr et al.24 and Kalns et al.25. The results of the present study, which showed that HBO increased the Ki-67 LI in tumor cells, confirm their conclusions concerning one of mechanisms of HBO effectiveness in the combination therapy by irradiation against cancers, because irradiation is much effective to mitotic cells (Ki-67-positive cells).
Fig. 4.
Representative images of the low grade papillomas from the histopathological and immunohistochemical findings in both groups 1 and 2. Original magnification, 10× for HE stain and 20× for Ki-67 immunohistochemistry.
Additionally, HBO is known to induce DNA damage in humans and experimental animals6. In the present study, there is a possibility that the oxidative stress resulting from HBO therapy influenced the initiation phase in tumorigenesis, but it is complex to distinguish the DNA damage in lesions affected with HBO from those by DMBA and TPA used in this model. Further studies are needed.
In conclusion, we found that the HBO accelerated tumor development and enhanced tumor growth in a mouse skin chemical carcinogenesis model. Since there are several inconsistent reports regarding the effect of HBO, further investigations about the combined effect of HBO with radiotherapy or chemotherapy on tumor development are necessary.
Acknowledgments
The authors greatly acknowledge the financial support from the project for “Development of new diagnostic methods and treatment methods and understanding of local characteristics and treatment resistance mechanisms of intractable malignant tumors in Okinawa.” This research was also supported in part by a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Daruwalla J, and Christophi C. The effect of hyperbaric oxygen therapy on tumour growth in a mouse model of colorectal cancer liver metastases. Eur J Cancer. 42(18): 3304–3311 2006. [DOI] [PubMed] [Google Scholar]
- 2.Daruwalla J, and Christophi C. Hyperbaric oxygen therapy for malignancy: a review. World J Surg. 30(12): 2112–2131 2006. [DOI] [PubMed] [Google Scholar]
- 3.Tibbles PM, and Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 334(25): 1642–1648 1996. [DOI] [PubMed] [Google Scholar]
- 4.Neuman TS, and Thom SR. Physiology and Medicine of Hyperbaric Oxygen Therapy. Elsevier Health Sciences, Philadelphia. 2008 [Google Scholar]
- 5.Leach RM, Rees PJ, and Wilmshurst P. ABC of oxygen—Hyperbaric oxygen therapy. BMJ. 317(7166): 1140–1143 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennog C, Hartmann A, Frey G, and Speit G. Detection of DNA damage after hyperbaric oxygen (HBO) therapy. Mutagenesis. 11(6): 605–609 1996. [DOI] [PubMed] [Google Scholar]
- 7.Feldmeier J, Carl U, Hartmann K, and Sminia P. Hyperbaric oxygen: does it promote growth or recurrence of malignancy? Undersea and Hyperb Med. 30(1): 1–18 2003. [PubMed] [Google Scholar]
- 8.Ogawa K, Kohshi K, Ishiuchi S, Matsushita M, Yoshimi N, and Murayama S. Old but new methods in radiation oncology: hyperbaric oxygen therapy. Int J Clin Oncol. 18(3): 364–370 2013. [DOI] [PubMed] [Google Scholar]
- 9.Johnson R, and Lauchlan S. Epidermoid carcinoma of the cervix treated by 60°C therapy and hyperbaric oxygen. Proceedings of the third international congress on hyperbaric medicine. Vol. 648-652. 1966
- 10.Van den Brenk HA, Madigan JP, and Kerr RC. An analysis of the progression and development of metastases in patients receiving x-radiation in hyperbaric oxygen. Clin Radiol. 18(1): 54–61 1967. [DOI] [PubMed] [Google Scholar]
- 11.Abel EL, Angel JM, Kiguchi K, and DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 4(9): 1350–1362 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti CJ, Slaga TJ, and Klein-Szanto AJP. Skin Tumors: Experimental and Clinical Aspects. Raven Press, New York, 1989 [Google Scholar]
- 13.Sunagawa N, Inamine M, Morioka T, Chiba I, Morita N, Aoki Y, Suzui M, and Yoshimi N. Inhibitory effect of rice bran-derived crude glycosphingolipid on colon preneoplastic biomarker lesions induced by azoxymethane in male F344 rats. Mol Med Rep. 2(1): 45–49 2009. [DOI] [PubMed] [Google Scholar]
- 14.Moen I, Øyan AM, Kalland KH, Tronstad KJ, Akslen LA, Chekenya M, Sakariassen PO, Reed RK, and Stuhr LE. Hyperoxic treatment induces mesenchymal-to-epithelial transition in a rat adenocarcinoma model. PLoS One. 4(7): e6381 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillan T, Calhoun KH, Mader JT, Stiernberg CM, and Rajaraman S. The effect of hyperbaric oxygen therapy of oral mucosal carcinoma. Laryngoscope. 99(3): 241–244 1989. [DOI] [PubMed] [Google Scholar]
- 16.Thom SR. Hyperbaric oxygen— its mechanisms and efficacy. Plastic and reconstructive surgery. 127(Suppl 1): 131S–141S 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampson NB, Holm JR, Wreford-Brown CE, and Feldmeier J. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 118(15): 3860–3868 2012. [DOI] [PubMed] [Google Scholar]
- 18.Gupta P, Sahni T, Jadhav GK, Manocha S, Aggarwal S, and Verma S. A retrospective study of outcomes in subjects of head and neck cancer treated with hyperbaric oxygen therapy for radiation induced psteoradionecrosis of mandible at a tertiary care centre: an Indian experience. Indian Journal of Otolaryngology and Head & Neck Surgery. 65: S140–S143 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen S, Kilian C, Phelps J, and Whelan HT. The use of hyperbaric oxygen for treating delayed radiation injuries in gynecologic malignancies: a review of literature and report of radiation injury incidence. Supportive Care in Cancer. 20(10): 2467–2472 2012. [DOI] [PubMed] [Google Scholar]
- 20.Kawasoe Y, Yokouchi M, Ueno Y, Iwaya H, Yoshida H, and Komiya S. Hyperbaric oxygen as a chemotherapy adjuvant in the treatment of osteosarcoma. Oncology Reports. 22(5): 1045–1050 2009. [DOI] [PubMed] [Google Scholar]
- 21.Wenwu L, Xuejun S, Hengyi T, and Kan L. Hyperbaric oxygen and cancer: more complex than we expected. Targeted oncology. 8: 79-81. 2013 [DOI] [PubMed] [Google Scholar]
- 22.Pande S, Sengupta A, Srivastava A, Gude RP, and Ingle A. Re-evaluate the effect of hyperbaric oxygen therapy in cancer - a preclinical therapeutic small animal model study. PLoS One. 7(11): e48432 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson ER, Halnan KE, Dische S, Saunders MI, Cade IS, McEwen JB, Wiernik G, Perrins DJ, and Sutherland I. Hyperbaric oxygen and radiotherapy: a Medical Research Council trial in carcinoma of the cervix. Br J Radiol. 51(611): 879–887 1978. [DOI] [PubMed] [Google Scholar]
- 24.Stuhr LE, Iversen VV, Straume O, Maehle BO, and Reed RK. Hyperbaric oxygen alone or combined with 5-FU attenuates growth of DMBA-induced rat mammary tumors. Cancer Lett. 210(1): 35–40 2004. [DOI] [PubMed] [Google Scholar]
- 25.Kalns J, Krock L, and Piepmeier E, Jr . The effect of hyperbaric oxygen on growth and chemosensitivity of metastatic prostate cancer. Anticancer Res. 18(1A): 363–367 1998. [PubMed] [Google Scholar]